Abstract
Alternative fuels are being explored to mitigate the effects of petroleum-based fuels. Pyrolysis oil from waste tires is a promising alternative fuel; however, it contains very high concentrations of benzothiophene (BT) which are beyond the allowable sulfur limits of Taiwan and the Philippines. Mixing-assisted oxidative desulfurization (MAOD) is a method that removes sulfur from fuel oils by utilizing high-shear mixing and oxidants. In this paper, the oxidation of BT in a model fuel was studied to determine optimal process conditions. Crude Fe(VI) prepared from sludge was used as the oxidant. Using the Box–Behnken design under response surface methodology, the significance of the following independent variables was studied: mixing speed (4400–10 800 rpm), phase transfer agent (PTA) amount (100 to 300 mg), Fe(VI) concentration (400–6000 ppm), and mixing temperature (40 to 60 °C). The results from a comprehensive statistical analysis showed the increase of sulfur conversion with high levels of Fe(VI) concentrations and PTA amounts together with low levels of agitation speeds and temperatures. The BT to BT sulfone conversions from experimental runs ranged from 17% to 64%. The optimum sulfur conversion of 88% for the BT model fuel was reached at the maximum levels of Fe(VI) concentration and mixing speed, along with the minimum levels of PTA concentration and temperature. The optimal MAOD variables were applied to a high-sulfur pyrolysis oil sample, which resulted in a sulfur reduction of 55%. The produced fuel oil meets the sulfur requirements of Taiwan and the Philippines for industrial heating oils. Therefore, the findings of the study support the effectiveness of sludge-derived Fe(VI) in the MAOD of BT in the model fuel and pyrolysis oil under mild process conditions.
1. Introduction
Fossil fuel-derived oils have been conventionally used to accompany the increasing demand for heat and power generation, especially in transportation and industries. However, due to the rapid depletion of materials and the negative environmental effects of fossil fuels, researchers are exploring alternative fuels with lower environmental impacts than fossil oil sources. These alternative fuels include pyrolysis oil from biomass, waste plastic, and waste tires. In this study, pyrolysis is a process wherein waste tires are subjected to high temperatures (above 300 °C) in the absence of oxygen to facilitate main chain degradation and cross-linking disconnection.1 Main chain degradation occurs in C–C bonds and is accompanied by hydrogen transfer. Meanwhile, the bond fragments with sulfur radicals recombine and form new bonds.2 After the heating process, char and volatile products are produced. The volatile products then undergo condensation, which separates gaseous products from pyrolysis oil.3 Pyrolysis oil is reported to be useful due to its similar characteristics to diesel, particularly in density and calorific value, after undergoing treatment.4 Although pyrolysis oil is not applied as a transportation fuel, it has shown promising applications in industrial usage, specifically as a boiler fuel and heavy oil generator. It also serves as potential feedstock for the production of carbon black.5 However, a disadvantage of utilizing pyrolysis oil from waste tires is the high amount of sulfur compounds, especially benzothiophene (BT).6
Exposure to sulfur compounds can cause several problems, which include respiratory problems in humans and growth problems in plants. Environmentally, the release of these compounds in the atmosphere may strengthen acid rain and reduce atmospheric visibility.7 Due to these potential issues, laws have been set by governments to limit the sulfur concentration in fuels for industrial usage. In 2020, Taiwan set its sulfur limit for heating oils to 5 000 ppm.8 In the Philippines, special-grade industrial fuel oils have a sulfur limit of 10 000 ppm.9 To reduce the sulfur concentration in fuel, the process of hydrodesulfurization (HDS) is commonly used because it is efficient in removing acyclic and aliphatic sulfur compounds, particularly thiols, sulfides, and disulfides. However, HDS can also decrease the lubricating properties of fuels as it involves the removal and hydrogenation of selected aromatics.10 Furthermore, HDS was found to be ineffective in converting sterically hindered heterocyclic thiophenic compounds, such as BT and dibenzothiophene (DBT), and their alkyl derivatives.11
With this, alternative desulfurization processes were introduced, one of which is oxidative desulfurization (ODS). ODS promotes the oxidation of aromatic compounds to their resulting sulfones and/or sulfoxides with the aid of an oxidant. The advantage of ODS over HDS is its use of mild operating conditions, such as atmospheric pressure and temperatures below 100 °C.12 Following the oxidation stage, oxidized sulfur compounds can be easily extracted through adsorption, extraction of solvent, or distillation, as they are significantly more polar than hydrocarbons.13 Phase transfer agents (PTAs) are also added to the reaction mixture to aid the transfer of the materials between the two phases. Various catalysts are also studied to improve the efficiency of the process. Common heterogeneous catalysts used in the ODS process include transition metal oxides, polyoxometalates, and ionic liquids.14 Metal–organic frameworks are also widely studied for their high surface area, thermal stability, and excellent recyclability.15
Modifications to the ODS process have been made to improve the rate of sulfur removal under optimal conditions. A common modification is ultrasound-assisted oxidative desulfurization (UAOD). UAOD utilizes ultrasound irradiation to increase cavitation and provide more sites for reaction, promoting mass transfer and accelerating oxidation.16 However, in a commercialized setting, the UAOD method would demand high energy and numerous devices, such as amplifiers and sono-reactors.17 Mixing-assisted oxidative desulfurization (MAOD) is a modification in ODS that utilizes high shear mixing to enhance contact between the two phases by creating small droplets through molecular diffusion and achieving sulfur conversion.18
Commonly used oxidants in ODS include hydrogen peroxide,19 oxygen gas,10 and ferrate.20 H2O2 is particularly utilized since it is cheap, easily available, and environmentally friendly.21 A study in 2014 utilized H2O2 and compared the capability of the sulfur conversion of UAOD and MAOD.17 The study findings indicated that both systems achieved a conversion rate of 99% for DBT and 98% for BT. The optimized conditions for the MAOD reaction were found to be 10 000 rpm mixing speed, 70 °C reaction temperature, and 30 min reaction time. Oxygen gas has also been studied due to its wide availability and low cost. Noncatalytic ODS of kerosene and diesel fractions was also optimized using oxygen gas in the presence of water. In this process, a bubble reactor was maintained at 180 to 200 °C and 2.5 to 3.0 MPa. The study revealed that water had a positive effect in balancing the oxidation intensity for the hydrocarbon medium but had no effect on the removal of sulfur compounds from the oil fractions. The mercaptan sulfur removal degree reported in the study was 62%.10 Meanwhile, Fe(VI) or potassium ferrate (K2FeO4) is known for its high stability, selectivity, and a high reduction potential of 2.2 V in an acidic medium and is considered environmentally friendly.22 One study explored the effectiveness of commercial Fe(VI) as an oxidant in the MAOD of sulfur compounds and real diesel oil.20 The researchers optimized the process by varying agitation speed (7600 to 14 000 rpm), temperature (50 to 70 °C), and mixing time (10 to 30 min). The optimal parameters were determined to be 12 198 rpm, 52.22 °C, and 15.42 min for BT, and 8,704 rpm, 51.26 °C, and 14.43 min for DBT. Confirmatory runs resulted in mean conversion rates of 84.35% for BT and 93.68% for DBT in model fuels. The same parameters were applied to diesel oil wherein sulfur conversion reached 58.03% and 93.15% using BT and DBT optimal conditions, respectively. The study also highlighted the impact of the temperature on the system, finding that higher temperatures favor the formation of Fe(VI) complexes. However, increasing the temperature to 70 °C may lead to reduced oxidation activity due to the low thermostability of Fe(VI) at this temperature.
Despite the effectiveness of commercial Fe(VI), its availability is very limited and costly. Therefore, different methods for synthesizing Fe(VI) from various sources are being explored. One method is called the wet oxidation method, wherein raw materials containing Fe(III) are oxidized using hypochlorite and hydroxide solutions.23 One potential raw material for this method is drinking water treatment sludge (DWTS). In the drinking water treatment process, Fe is used an oxidant and a disinfectant, particularly in sludge dewatering, anaerobic fermentation, sludge minimization, and pollutant removal.23 The produced DWTS contains high concentrations of Fe(III) compounds, which can be used to derive Fe(VI). A recent study reported the effect of Fe(VI) derived from DWTS as an oxidant in the UAOD of organosulfur compounds and optimized sulfur conversion by varying ferrate concentration (100 to 300 ppm), PTA concentration (100 to 300 mg), organic to aqueous phase ratio (10:30 to 30:10), and sonication time (10 to 30 min). The applied optimum parameter values of 100 ppm ferrate, 100.01 mg PTA, 30:10 organic to aqueous phase ratio, and 13.32 min resulted in 43.91% BT conversion in a model fuel.11
Previous studies have established that increasing various variables, such as ferrate concentration, PTA concentration, agitation speed, and mixing temperature, does not necessarily translate to an increase in sulfur conversion. For instance, excessive concentrations of ferrate and PTA may produce a low sulfur conversion due to the nature of compounds and steric hindrance.18 Additionally, in the frame of MAOD, the increase in agitation speed increases the rate of mass transfer; however, elevated mixing speeds may also cause the formation of whirlpools, which slow down the rate of desulfurization.24
A recent paper by the authors has synthesized Fe(VI) from drinking-water treatment sludge (DWTS) and applied it in the MAOD of DBT. The results showed that the maximum DBT conversion in model fuel reached 99.6%. Furthermore, the high levels of Fe(VI) concentration and low levels of PTA concentration and agitation speed favored sulfur oxidation. The application of the optimized parameters in pyrolysis oil desulfurization reached 53.2%.25
From previous studies, the desulfurization of BT was found to be harder to achieve than DBT due to its lower electron density, which translated to a rate constant that is eight times slower.26 Thus, this paper is a continuation of the previous study, as the effect of using Fe(VI) from drinking-water treatment sludge in the MAOD of BT has not been explored.
The novelties of this study are as follows:
-
1
usage of Fe(VI) derived from DWTS to oxidize BT in an MAOD system;
-
2
optimization of Fe(VI) concentration, PTA concentration, mixing speed, and mixing temperature to achieve the maximum BT conversion using the Box–Behnken design under the response surface methodology (BBD-RSM); and
-
3
application of the optimal variables in the MAOD of a high-sulfur pyrolysis oil sample and comparison of its performance with the optimal variables obtained from the MAOD of DBT.
2. Methods
2.1. Materials
The model sulfur compound BT (98% purity) was purchased from Alfa Aesar (Taiwan). Glacial acetic acid, tetraoctylammonium bromide (TOAB; 98% purity), toluene (0.99 mass fractions), sodium hydroxide pellets (≥99%), and potassium hydroxide pellets (≥99%) were obtained from Sigma-Aldrich (USA). Nitric acid (69%) and sodium hypochlorite (6–12%) were acquired from Merck (USA) and Nihon Shiyaku (Japan), respectively. DWTS was sourced from the Changhua No. 3 Water Purification Plant located in Changhua City, Taiwan. The pyrolysis oil from waste tires was procured from Kao Hsing Chang Company, Taiwan.
2.2. Oxidant Preparation
The method of K2FeO4 preparation was adapted from the study by Arcega et al.11 A sludge sample weighing 8.5 g was dissolved in 8.5 mL of 2 M HNO3. The mixture was stirred for 1.5 h at 100 rpm. In another flask, a mixture of 1:2 NaOH:NaOCl was vigorously stirred in a cold-water bath. The combination of the predissolved sludge and the NaOH–NaOCl mixture was continuously stirred at 400 rpm for 1 h. After this, 250 mL of saturated KOH was added, and the solution was stirred for 40 min at 400 rpm. This was followed by centrifugation at 4 000 rpm for 20 min. The top dark purple liquid was extracted and prepared for the MAOD experiment. The concentration of the Fe(VI) product was tested by using ultraviolet–visible spectroscopy at 510 nm.
2.3. MAOD Experiment
The experiment was conducted in a 400 mL beaker in which predetermined concentrations of 50 mL of the BT model fuel, 50 mL of the K2FeO4 oxidant, and PTA were added. To maintain the optimal conditions, glacial acetic acid was added dropwise until pH of 5 was achieved and the temperature was controlled using a heating mantle. The mixture was agitated for 30 min in a T 25 digital ULTRA-TURRAX mixer under varying Fe(VI) concentrations, PTA concentrations, mixing speed, and mixing temperature. The pH and temperature of the system were closely monitored to ensure that controlled conditions were maintained. After the completion of the reaction, the mixture underwent a cooling phase and was subsequently centrifuged at 4 000 rpm for 20 min. The model fuel was extracted for instrumental analysis.
2.4. Statistical Analysis
The experimental design under BBD-RSM is presented in Table 1. The four independent variables tested were the following: ferrate concentration (X1: 400 ppm to 600 ppm), PTA concentration (X2: 100 to 300 mg per 50 mL–1 model fuel), mixing speed (X3: 4 400 rpm to 10 800 rpm), and mixing temperature (X4: 40 to 60 °C).
Table 1. Box–Behnken Design Variables for the Study.
| Levels |
|||
|---|---|---|---|
| Factors | Low (−1) | Medium (0) | High (+1) |
| Fe(VI) concentration (ppm), X1 | 400 | 500 | 600 |
| PTA concentration (mg/50 mL model fuel), X2 | 100 | 200 | 300 |
| Mixing speed (rpm), X3 | 4,400 | 7,600 | 10,800 |
| Mixing temperature (°C), X4 | 40 | 50 | 60 |
The general form of the quadratic equation to optimize the process is given by eq 1.
| 1 |
where y is percentage sulfur conversion, X1 to Xk are the four operating variables, and β1 to βk are unknown variable coefficients. The experimental results were investigated by analysis of variance (ANOVA) and Design Expert v.22.
2.5. Instrumental Analysis
The BT model fuel was quantitatively analyzed using a gas chromatograph with a sulfur chemiluminescence detector (GC-SCD, Agilent Gas Chromatograph, 7890A, CA, USA) to ensure precision in measuring sulfur concentrations. The column was stabilized for 1 h prior to its usage to ensure constant temperature throughout the equipment. The column temperature upon starting the analysis was set at 150 °C for 1 min, and heating was done at 20 °C min–1 for 4 min until the temperature reached 220 °C. The inlet was operated with 18.243 psi and 28.2 mL min–1 total flow in split mode with a 20:1 split ratio. The column utilized in the analysis was an Agilent 19091S–433 with a flow of 1.2 mL min–1. A total sulfur analyzer (Horiba SLFA-2100) was used to measure sulfur concentrations of the pyrolysis oil sample, before and after desulfurization.
3. Results and Discussion
3.1. Effects of Fe(VI) Concentration and PTA Concentration
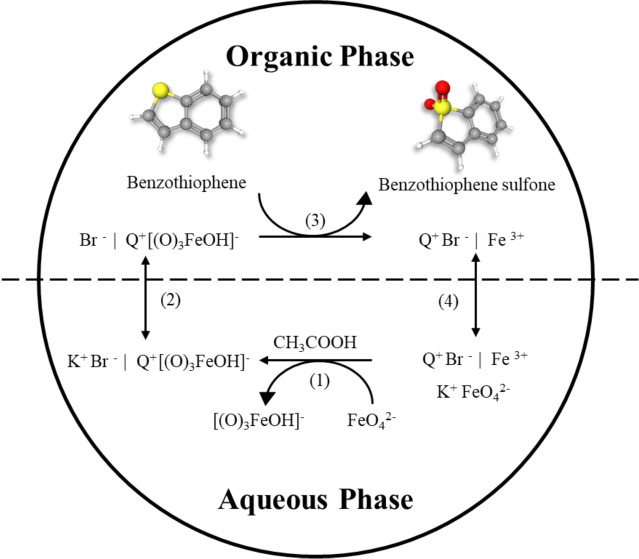
A proposed mechanism for oxidation involves the formation of a more reactive complex, [O3Fe(OH)]−, from Fe(VI) and acetic acid. This complex bonds with the quaternary ammonium cation (Q+), forming Q+[O3Fe(OH)]−.18 This compound creates an emulsion that enables the oxidation of BT to its sulfoxide and sulfone forms. Throughout the mixing process, the oxidant exhibits an evident color change from purple to brown, indicating the reduction of Fe(VI) to Fe(III). However, due to current limitations, detailed characterization of the oxidant and stability of the complex was reserved for further experimentation.
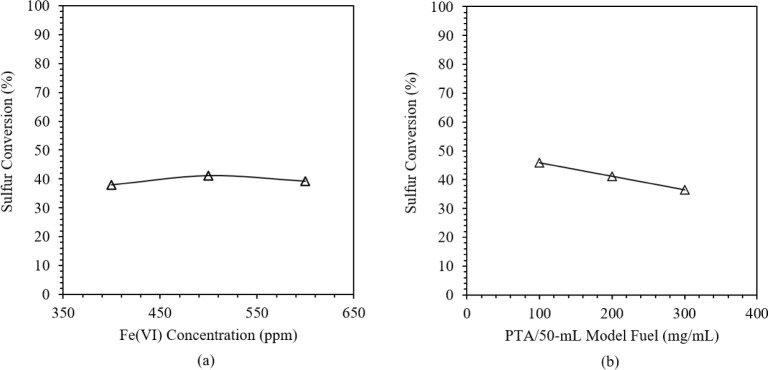
Figure 1 presents the effects of (a) Fe(VI) Concentration and (b) PTA concentration on BT conversion in a model fuel. It was observed that using 400 to 500 ppm Fe(VI) concentration resulted in an increase in oxidation. This increase is attributed to the presence of more complex Fe(VI) ions in acidic media, such as monoprotonated Fe(VI), which are responsible for oxidizing BT.27 However, increasing the Fe(VI) concentration to 600 ppm resulted in a lower sulfur conversion. Previous studies reported similar results and attributed it to the basic shift in the system pH due to the high Fe(VI) concentration, as more ions are present to react with H+ and water.18 Meanwhile, it is observed that BT oxidation decreased from 45.8% to 36.4% with an increase in the PTA amount from 100 to 300 mg. This is attributed to the formation of thicker and more turbid layers as the level of PTA increases. From a similar ODS study using commercial Fe(VI), it was reported that the high concentration of alkyl groups from the PTA may cause steric hindrance in the system, preventing the oxidation of BT.18 Additionally, brominated byproducts are formed in the MAOD of BT due to the reaction of TOAB with BT. These unwanted byproducts are caused by side reactions that may compete with the desired oxidation reaction of BT to BTO.
Figure 1.
Effects of (a) Fe(VI) concentration and (b) PTA concentration on % S conversion of BT.
In comparison to the previous desulfurization study, it is notable that BT desulfurization resulted in a significantly lower sulfur conversion. In application to DBT, the same system produced the maximum sulfur conversion of 97.3% and 98.7% in the one-factor analysis using Fe(VI) concentration and PTA concentration, respectively.25 This significant contrast is due to the selectivity of oxidative desulfurization to DBT and BT. The reactivity of the refractory compounds is associated with their electron density. A previous study reported that DBT has a higher electron density than BT, with a reported oxidation rate constant of 0.0460 L mol–1 min–1, eight times that of BT.26 This implies that DBT undergoes oxidation much faster and explains the lack of brominated byproducts in the MAOD of DBT.28
3.2. Effects of Mixing Speed and Mixing Temperature
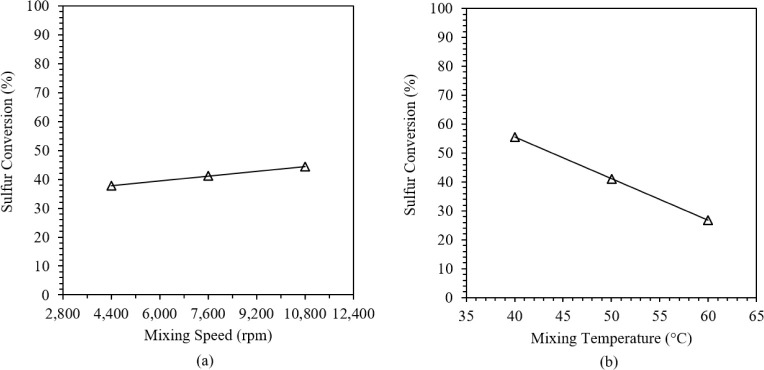
The effects of the (a) mixing speed and (b) mixing temperature on BT conversion are illustrated in Figure 2. An increasing trend was observed for BT conversion where results increased from 37.8% to 44.5% and from 4 400 rpm to 10 800 rpm. The mass transfer resistance in the system is reduced as the agitation speed increases to 10 800 rpm and more Fe(VI) ions are transferred in the interface to react with the sulfur compounds, translating to a higher sulfur conversion.24 On the other hand, a decreasing trend of BT oxidation was observed for an increase in temperature, wherein sulfur conversion lowered from 55.5% to 26.8% and from 40 to 60 °C. The low sulfur conversions at increasing temperatures may be attributed to the increased reaction of Fe(VI) with water, which produces Fe(III) and O2 and reduces its high oxidation potential.29
Figure 2.
Effects of (a) mixing speed and (b) mixing temperature on the % S conversion of BT.
Figure 3 presents the reaction products in the BT model fuel after undergoing MAOD, as identified using GC-SCD. At the peak times of 3.622 and 4.976 s, the compounds benzothiophene, and 3-bromo and 2-bromothianapthene 1,1-dioxide were observed. The formation of these brominated minor products is caused by the reaction of BT with TOAB which was used as the PTA in the MAOD process. As temperature increases, there is also a higher collision frequency between PTA and BT, which can increase the rate of unwanted side reactions in the system. As a result, this decreases the reaction of BT into BTO, translating to a lower %S conversion. A similar ODS study reported the presence of the same brominated byproducts using H2O2 as an oxidant and TOAB as the PTA over different polyoxometalate catalysts under a temperature range of 30 to 70 °C.30
Figure 3.
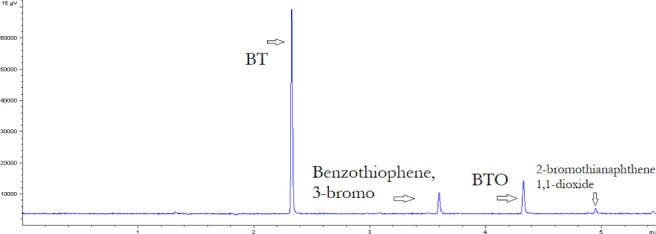
Reaction products after the MAOD of BT.
3.3. Statistical Analysis
A total of 27 runs were performed for the study under BBD-RSM. As presented in Table 3, BT sulfur conversions were observed from 16.5 to 63.7%. The fit summary of linear, two-factor, quadratic, and cubic models is presented in Table 2. The coefficient of determination, expressed as R2, refers to the statistical evaluation of the model’s quality. The resulting adjusted R2 and predicted R2 metrics are indicative of the goodness of fit within the generated model equation. The results in Table 3 indicate a high R2 of 0.9083 for the quadratic model, as determined through the ANOVA test. The model’s capacity for prediction is supported by a predicted R2 value of 0.7754, demonstrating its effectiveness in calculating sulfur reduction based on the Fe(VI) concentration, PTA concentration, mixing speed, and mixing temperature. The quadratic model was also determined to be the best fit for the sulfur conversion data with a probability value (p-value) of 0.0063 and a significant lack of fit derived from the Fisher variance ratio (F-value) of 0.500 in comparison with the cubic, linear, and two-factor interaction models.
Table 3. Fit Summary for BT Models Using BBD-RSM.
| Source | Sequential p-value | Lack of Fit p-value | Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|---|
| Linear | 0.0011 | 0.1150 | 0.4650 | 0.3018 | |
| 2FI | 0.0011 | 0.2626 | 0.7904 | 0.6393 | |
| Quadratic | 0.0063 | 0.5010 | 0.9083 | 0.7754 | Suggested |
| Cubic | 0.4910 | 0.4195 | 0.9149 | –0.1072 | Aliased |
Table 2. BBD-RSM Variables with Corresponding Percent Conversions.
| Fe(VI) Concentration (ppm) | PTA (mg/50 mL model fuel) | Agitation Speed (rpm) | Mixing Temperature (°C) | BT Percent Conversion (%) |
|---|---|---|---|---|
| 500 | 100 | 4,400 | 50 | 40.0 |
| 400 | 200 | 7,600 | 40 | 43.7 |
| 400 | 200 | 4,400 | 50 | 54.1 |
| 600 | 200 | 4,400 | 50 | 21.9 |
| 500 | 200 | 4,400 | 60 | 24.4 |
| 600 | 200 | 7,600 | 60 | 16.5 |
| 500 | 300 | 4,400 | 50 | 33.3 |
| 500 | 200 | 7,600 | 50 | 42.6 |
| 500 | 200 | 7,600 | 50 | 50.0 |
| 500 | 300 | 7,600 | 40 | 61.6 |
| 500 | 200 | 4,400 | 40 | 49.0 |
| 500 | 100 | 7,600 | 60 | 44.2 |
| 600 | 200 | 10,800 | 50 | 53.1 |
| 400 | 100 | 7,600 | 50 | 32.2 |
| 500 | 200 | 10,800 | 60 | 30.1 |
| 600 | 300 | 7,600 | 50 | 20.9 |
| 600 | 100 | 7,600 | 50 | 49.6 |
| 500 | 300 | 10,800 | 50 | 46.9 |
| 500 | 100 | 7,600 | 40 | 54.1 |
| 400 | 300 | 7,600 | 50 | 32.1 |
| 400 | 200 | 7,600 | 60 | 25.4 |
| 500 | 300 | 7,600 | 60 | 17.3 |
| 500 | 200 | 7,600 | 50 | 47.8 |
| 500 | 100 | 10,800 | 50 | 48.2 |
| 400 | 200 | 10,800 | 50 | 20.8 |
| 500 | 200 | 10,800 | 40 | 63.7 |
| 600 | 200 | 7,600 | 40 | 57.9 |
The coefficients of independent variables (β1 to βk), based on the independent variable responses defined in Table 1 (X1, X2, X3, and X4), were determined using the experimental data of sulfur conversion. The quadratic response surface model that predicts %S conversion is presented in eq 2. A positive sign in the model equation implies that sulfur conversion is increased by increasing the levels of the independent variables. Meanwhile, a negative sign denotes the reduction of sulfur conversion as a result of increasing the tested variables.
| 2 |
The ANOVA of the BT quadratic model is presented in Table 4. This analysis determines the level of importance of the individual process variables to validate the generated quadratic equation. The residual values show the degree of unexplained variation in the response. Meanwhile, the lack of fit assesses the extent to which model predictions differ from the observed values. The indicated pure error measures the differences between replicate runs, while the row of corrected total sum of squares (correlation total) defines the overall variation around the mean of the observations. For each process variable and interaction, the sum of squares, degrees of freedom, mean square, F-value, and p-value are listed. The sum of squares defines the sum of squared differences between the overall average and the variance explained by the source in each row. Meanwhile, the degrees of freedom pertain to the number of estimated parameters used to compute the sum of squares. The mean square, also known as variance, is computed by dividing the sum of squares by the degrees of freedom. The F-value and p-value are also utilized to assess the significance levels of a specific process variable and its interactive variables with respect to the sulfur conversion parameter in the context of the MAOD system. A high F-value implies that the variability of the response can be explained by the quadratic model, indicating the significance of the coefficient term. Meanwhile, a p-value of less than 0.05 indicates that the model and the coefficient terms are statistically significant.31 The ANOVA for the reduced quadratic model is presented in Table 5. The results show that the significant terms in the model were X2, X3, X4, X1X2, X1X3, X1X4, X2X4, and X12. These variables are identified to have a strong significance as supported by their p-values (<0.0500). Moreover, the specific process variables of X4 and X1X3 were found to be extremely significant in strongly influencing the sulfur conversion response based on their high F-values (X4 = 145.85 and X1X3 = 61.46). The lack of fit F-value of 1.34 and p-value (0.5010) also imply that the lack of fit has no significant relationship with the pure error.
Table 4. ANOVA for the BT Quadratic Model.
| Source | Sum of Squares | d f | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 5033.52 | 14 | 359.54 | 19.39 | <0.0001 | significant |
| X1-Fe(VI) Concentration | 11.21 | 1 | 11.21 | 0.6048 | 0.4518 | not significant |
| X2-PTA | 263.20 | 1 | 263.20 | 14.20 | 0.0027 | significant |
| X3-Agitation Speed | 134.00 | 1 | 134.00 | 7.23 | 0.0197 | significant |
| X4-Temperature | 2468.20 | 1 | 2468.20 | 133.11 | <0.0001 | significant |
| X1 X2 | 204.49 | 1 | 204.49 | 11.03 | 0.0061 | significant |
| X1 X3 | 1040.06 | 1 | 1040.06 | 56.09 | <0.0001 | significant |
| X1 X4 | 133.40 | 1 | 133.40 | 7.19 | 0.0200 | significant |
| X2 X3 | 7.29 | 1 | 7.29 | 0.3932 | 0.5424 | not significant |
| X2 X4 | 295.84 | 1 | 295.84 | 15.96 | 0.0018 | significant |
| X3 X4 | 20.25 | 1 | 20.25 | 1.09 | 0.3166 | not significant |
| X12 | 440.04 | 1 | 440.04 | 23.73 | 0.0004 | significant |
| X22 | 34.91 | 1 | 34.91 | 1.88 | 0.1951 | not significant |
| X32 | 19.68 | 1 | 19.68 | 1.06 | 0.3232 | not significant |
| X42 | 14.01 | 1 | 14.01 | 0.7557 | 0.4017 | not significant |
| Residual | 222.50 | 12 | 18.54 | |||
| Lack of Fit | 193.62 | 10 | 19.36 | 1.34 | 0.5010 | not significant |
| Pure Error | 28.88 | 2 | 14.44 | |||
| Cor Total | 5256.03 | 26 |
Table 5. ANOVA for a Reduced BT Quadratic Model.
| Source | Sum of Squares | d f | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 4951.41 | 8 | 618.93 | 36.57 | <0.0001 | significant |
| X2-PTA | 263.20 | 1 | 263.20 | 15.55 | 0.0010 | significant |
| X3-Agitation Speed | 134.00 | 1 | 134.00 | 7.92 | 0.0115 | significant |
| X4-Temperature | 2468.20 | 1 | 2468.20 | 145.85 | <0.0001 | significant |
| X1 X2 | 204.49 | 1 | 204.49 | 12.08 | 0.0027 | significant |
| X1 X3 | 1040.06 | 1 | 1040.06 | 61.46 | <0.0001 | significant |
| X1 X4 | 133.40 | 1 | 133.40 | 7.88 | 0.0116 | significant |
| X2 X4 | 295.84 | 1 | 295.84 | 17.48 | 0.0006 | significant |
| X12 | 412.21 | 1 | 412.21 | 24.36 | 0.0001 | significant |
| Residual | 304.61 | 18 | 16.92 | |||
| Lack of Fit | 275.73 | 16 | 17.23 | 1.19 | 0.5493 | not significant |
| Pure Error | 28.88 | 2 | 14.44 | |||
| Cor Total | 5256.03 | 26 |
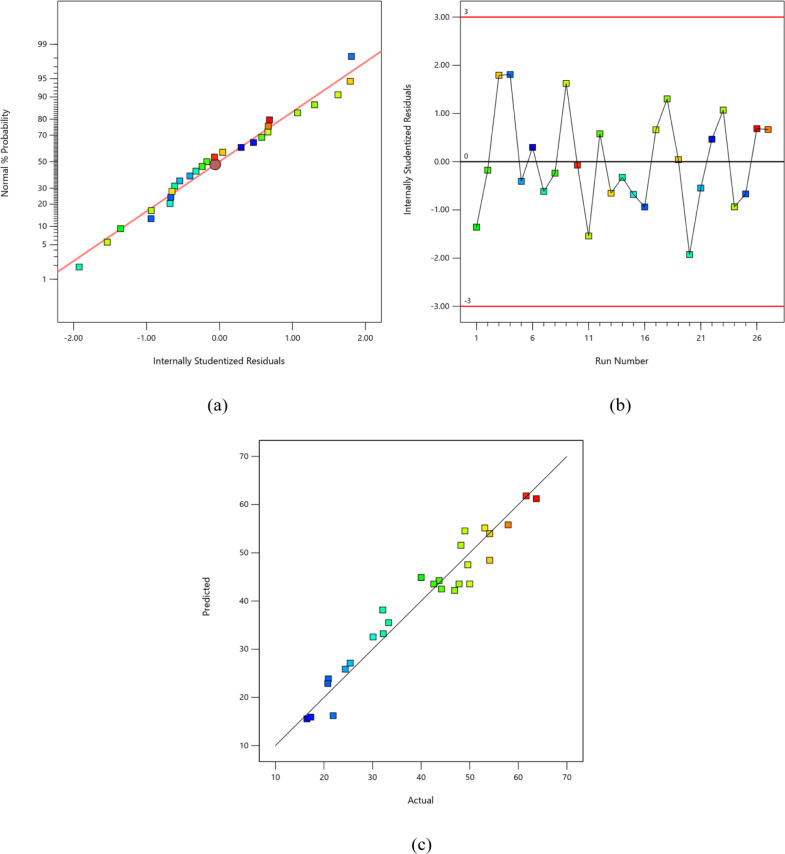
3.4. Diagnostic Plots for the Response Surface Model
The diagnostic plots illustrate the statistical performance of the MAOD system in relation to sulfur conversion in the BT model fuel using Fe (VI). The following diagnostic plots were generated by Design Expert v22 following model selection, as presented in Figure 4(a) normal probability, (b) residuals versus run number, and (c) predicted versus actual response for sulfur conversion. The normal probability plot in Figure 4a indicates that the internally studentized residuals follow a normal distribution, as specified by the data points lying close to the normal line in red. This highlights the suitability of the generated response surface quadratic model for the data. Moreover, there are no definitive patterns in the plot, such as an S-shaped curve, which may indicate that a response transformation is needed. Thus, the generated equation is adequate to model without requiring transformation.
Figure 4.
Diagnostic plots for the BT model: (a) internally studentized residuals, (b) residuals versus run number, and (c) predicted vs actual responses.
The residuals versus run number plot, as presented in Figure 4b, checks for a serial correlation among responses that may have been influenced by variables, such as time. The findings reveal a scattered distribution of data points with internally studentized residuals from the experimental runs falling within the range of ±3. Therefore, the chosen quadratic model demonstrates a favorable outcome, as there are no potential outliers in the experiment. Consequently, there is no requirement to repeat the experimental runs considering the errors and noise associated with the MAOD system.
Finally, the comparison between the predicted and actual response values is presented in Figure 4c. The predicted values correspond to the approximated sulfur conversion derived from the generated quadratic models, whereas the actual values represent the calculated sulfur conversions from the experimental runs. The BT model was observed to have responses along the diagonal line, indicating that the model can strongly predict the sulfur conversion response in the MAOD system with adequate accuracy.
3.5. Optimization
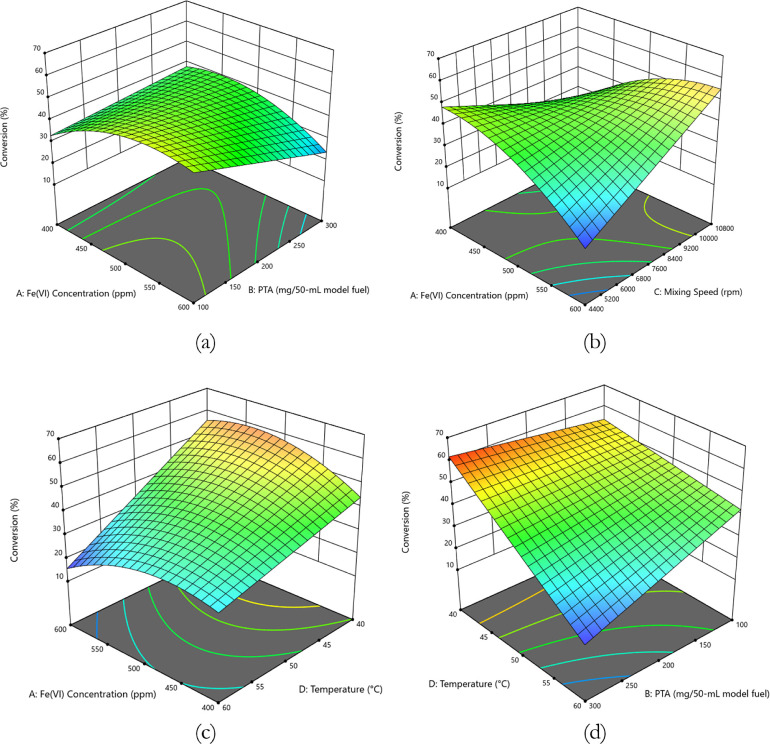
The 3D response surface graphs show significant interaction between two variables in the model, as indicated by the ANOVA results. Figure 5a shows the 3D response surfaces for the BT model with respect to the Fe (VI) concentration and PTA concentration. Meanwhile, Figure 5b presents the response with interacting variables of Fe (VI) concentration and agitation speed. Figure 5c illustrates the 3D response surface with respect to the Fe (VI) concentration and temperature. Finally, Figure 5d presents the interaction between temperature and PTA concentration.
Figure 5.
Model graphs of interacting variables for MAOD of BT model fuel: (a) Fe(VI) concentration and PTA 3D surface, (b) Fe(VI) concentration and agitation speed 3D surface, (c) Fe(VI) concentration and temperature 3D surface, and (d) temperature and PTA.
Figure 5a,b show an increasing trend in the BT response surfaces for Fe(VI) concentration, with the highest predicted responses ranging from 45% to 55% using 500 to 600 ppm Fe (VI). Meanwhile, Figure 5c projects the highest predicted sulfur conversion of 50% to 58% using 430 to 600 ppm Fe(VI). The trend suggests the significant effect of Fe(VI) concentration in the MAOD of a BT model fuel, wherein middle to high levels of Fe(VI) concentration are advantageous in generating Fe(VI) complexes to oxidize the model fuels.32
From Figure 5a, a low concentration of PTA is sufficient to produce a high sulfur conversion, implying that the oxidant was efficiently transferred to the organic phase. However, a high concentration of PTA is also predicted to decrease sulfur conversion. This is due to the creation of more turbid and thicker layers which add to the external mass transfer limitations present in the system.18
Utilizing a low temperature of 40 to 45 °C is also beneficial for sulfur conversion, as observed in Figure 5c. This indicates that effective molecular collisions between the oxidant and BT result in the formation of BTO. Higher temperatures may be detrimental to the system, as the reaction of Fe(VI) with water is promoted.33 Furthermore, the production of brominated byproducts may be promoted as temperature increases. These unwanted side reactions lower the availability of BT to be oxidized to BTO, resulting in a lowering of sulfur conversion. Finally, from Figure 5b, a high level of agitation speed (10 000 rpm to 10 800 rpm) is advantageous for BT conversion as it increases the formation of emulsion. This shows that external mass transfer limitations are reduced with high agitation speeds. High shear mixing enhances the oxidation rate by promoting the formation of uniformly shaped droplets and reducing mass transfer resistance.24
The optimal variables were obtained by setting the conversion to the maximum possible value in the model. The weights and importance for all variables were uniformly set. The optimal Fe(VI) concentration and PTA concentration were determined to be 600 ppm Fe(VI) and 101 mg PTA 50 mL–1 model fuel. Meanwhile, the optimal mixing speed and temperature were found to be 10 800 rpm and 40.0 °C. The predicted sulfur conversion for BT was 78.5% with a 95% confidence interval of 68.5 to 88.4%. In order to validate the developed quadratic model from the BBD-RSM, confirmatory runs were performed. The results indicated an average of 88.1% sulfur conversion, which falls within the determined confidence interval. This verified the precision of the response surface from the quadratic model equation under BBD-RSM.
3.6. Application to Pyrolysis Oil
The application of MOAD in a pyrolysis oil sample was performed using the conditions obtained from the optimization study using the model fuel. The original sulfur concentration of the pyrolysis oil sample was measured to be 8 804 ppm using a total sulfur analyzer. Following the MAOD process and solvent extraction, the sulfur concentration reached 3 990 ppm under the optimal BT conditions, resulting in a sulfur removal rate of 54.7%. The observed decline in desulfurization efficiency compared to that of the BT model fuel is attributed to the elevated presence of various sulfur compounds. In comparison, the optimized parameters from the preceding study using DBT resulted in a lower sulfur removal rate of 53.2%. Since BT is more difficult to oxidize compared to DBT, the optimized parameters using the BT model fuel were more effective in targeting more sulfur compounds in pyrolysis oil, resulting in a higher desulfurization rate than that with the DBT optimal parameters. Furthermore, pyrolysis oil is known to have a higher sulfur content, comprising of thiophene, BT, DBT, and their derivatives.30 With a significant amount of sulfur compounds and their derivatives competing for reaction, the optimal variables in the system may not be sufficient to achieve the predicted maximum sulfur oxidation.
4. Conclusions
K2FeO4 was prepared from a DWTS sample via the wet oxidation method and was successfully tested in the MAOD process of converting BT to BTO in a model fuel oil. BBD-RSM was employed to measure the effects of the Fe(VI) concentration, PTA concentration, agitation speed, and mixing temperature on sulfur conversion. The diagnostic plots and ANOVA validated the reliability of the generated quadratic model, indicating the goodness of fit and the reliable prediction of sulfur conversion. The 3D model graphs showed that sulfur conversion increased with the Fe(VI) concentration and PTA concentration. Meanwhile, low levels of agitation speed and temperature were favorable in the MAOD system. Actual sulfur conversions from experimental runs ranged from 16.5% to 63.7%. After MAOD and solvent extraction, the maximum desulfurization of 54.7% was reached under the BT optimal variables. The lower desulfurization results of the pyrolysis oil compared to those of the BT model fuel are attributed to the existence of more refractory sulfur compounds in the pyrolysis oil, such as thiophenes and their derivatives. Overall, the resulting S content confirms the effectiveness of MAOD in sulfur conversion using milder operating conditions compared to the conventional HDS method. Furthermore, the results support the promising applicability of MAOD in producing cleaner fuels via waste recovery for industrial applications. For future studies, it is recommended to test basic fuel properties before and after desulfurization for the future industrial applications of the MAOD system. In addition, the characterization of the oxidant, formation of the oxidant–catalyst complex, and kinetics of the reaction are proposed to be studied.
Acknowledgments
The authors acknowledge the Department of Science and Technology (DOST) of the Philippines through its Engineering Research and Development for Technology (ERDT) program and the Ministry of Science and Technology (MOST 111-2221-E-041-002-MY3) for providing financial support in this research undertaking.
Author Contributions
M.M.H. contributed to conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing – original draft, writing – review and editing, and visualization. A.E.S.C. contributed to conceptualization, supervision, and writing – review and editing. N.P.D. contributed to conceptualization, supervision, and writing – review and editing. M.W.W. contributed to conceptualization, resources, supervision, project administration, and funding acquisition.
The authors declare no competing financial interest.
References
- Serefentse R.; Ruwona W.; Danha G.; Muzenda E. A Review of the Desulphurization Methods Used for Pyrolysis Oil. Procedia Manuf. 2019, 35, 762–768. 10.1016/j.promfg.2019.07.013. [DOI] [Google Scholar]
- Han W.; Han D.; Chen H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 7. 10.3390/polym15071604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano F.; Abdul Jameel A. G.; Zhang W.; Emwas A.-H.; Agudelo A. F.; Martínez J. D.; Sarathy S. M. Fuel and Chemical Properties of Waste Tire Pyrolysis Oil Derived from a Continuous Twin-Auger Reactor. Energy Fuels 2020, 34 (10), 12688–12702. 10.1021/acs.energyfuels.0c02271. [DOI] [Google Scholar]
- Marshal S. J. J.; Samuel S. Y.; Sundari K. G.; Rajamohan S.. 4 - Diesel Engine Performance and Emissions with Fuels Derived from Waste Tyres; Khan A.; Pizzi A.; Jawaid M.; Azum N.; Asiri A.; Isa I. B. T.-A. T.; Woodhead Publishing, 2021; pp. 69–92.. DOI: 10.1016/B978-0-323-90150-5.00017-0. [DOI] [Google Scholar]
- Okoye C. O.; Jones I.; Zhu M.; Zhang Z.; Zhang D. Manufacturing of Carbon Black from Spent Tyre Pyrolysis Oil – A Literature Review. J. Cleaner Prod. 2021, 279, 123336. 10.1016/j.jclepro.2020.123336. [DOI] [Google Scholar]
- Zhang G.; Chen F.; Zhang Y.; Zhao L.; Chen J.; Cao L.; Gao J.; Xu C. Properties and Utilization of Waste Tire Pyrolysis Oil: A Mini Review. Fuel Process. Technol. 2021, 211, 106582. 10.1016/j.fuproc.2020.106582. [DOI] [Google Scholar]
- ">U.S. EPA. . Sulfur Dioxide (SO2) Pollution. United States Environmental Protection Agency. https://www.epa.gov/so2-pollution/sulfur-dioxide-basics#effects. (accessed 2020 December 23).
- ">US EPA . Air quality management; US EPA: Taiwan. https://air.epa.gov.tw/airepaEn/EnvTopics/MobilSource_11.aspx.
- Department of Environment and Natural Resources. Implementation Of Vehicle Emission Limits For Euro 4/Iv, And In-Use Vehicle Emission Standards; Department of Environment and Natural Resources, 2015. [Google Scholar]
- Pyshyev S.; Korchak B.; Miroshnichenko D.; Vytrykush N. Influence of Water on Noncatalytic Oxidative Desulfurization of High-Sulfur Straight-Run Oil Fractions. ACS Omega 2022, 7 (30), 26495–26503. 10.1021/acsomega.2c02527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcega A. A.; Futalan C. M.; Dalida M. L. P.; Wan M.-W.. Ultrasound-Assisted Oxidative Desulfurization of Organosulfur Compounds Using Ferrate(VI) Derived from Drinking Water Treatment Sludge. In 15th International Symposium on East Asian Resources Recycling Technology, EARTH 2019; 2020. [Google Scholar]
- Zhang X.; Shi Y.; Liu G. Direct Preparation of [(CH3)3NC16H33]4Mo8O26 and Its Catalytic Performance in Oxidative Desulfurization. Catal. Sci. Technol. 2016, 6 (4), 1016–1024. 10.1039/C5CY01439E. [DOI] [Google Scholar]
- Choi A. E. S.; Roces S.; Dugos N.; Wan M. W. Mixing-Assisted Oxidative Desulfurization of Model Sulfur Compounds Using Polyoxometalate/H2O2 Catalytic System. Sustain. Environ. Res. 2016, 26 (4), 184–190. 10.1016/j.serj.2015.11.005. [DOI] [Google Scholar]
- Sahraei S. Assessment of Reaction Parameters in the Oxidative Desulfurization Reaction. Energy Fuels 2023, 37 (20), 15373–15393. 10.1021/acs.energyfuels.3c02169. [DOI] [Google Scholar]
- Sun M.; Abazari R.; Chen J.; Hussain C. M.; Zhou Y.; Kirillov A. M. Encapsulation of H4SiW12O40 into an Amide-Functionalized MOF: A Highly Efficient Nanocomposite Catalyst for Oxidative Desulfurization of Diesel Fuel. ACS Appl. Mater. Interfaces 2023, 15 (45), 52581–52592. 10.1021/acsami.3c12374. [DOI] [PubMed] [Google Scholar]
- Choi A. E. S.; Roces S. A.; Dugos N. P.; Wan M.-W. Parametric Screening Analysis for the Oxidative Desulfurization of Diesel Oil. Chem. Eng. Trans. 2021, 88, 91–96. 10.3303/CET2188015. [DOI] [Google Scholar]
- Lu M.-C.; Biel L. C. C.; Wan M.-W.; de Leon R.; Arco S. The Oxidative Desulfurization of Fuels with a Transition Metal Catalyst: A Comparative Assessment of Different Mixing Techniques. Int. J. Green Energy 2014, 11 (8), 833–848. 10.1080/15435075.2013.830260. [DOI] [Google Scholar]
- Choi A. E. S.; Roces S.; Dugos N.; Futalan C. M.; Lin S. S.; Wan M. W. Optimization of Ultrasound-Assisted Oxidative Desulfurization of Model Sulfur Compounds Using Commercial Ferrate (VI). J. Taiwan Inst. Chem. Eng. 2014, 45 (6), 2935–2942. 10.1016/j.jtice.2014.08.003. [DOI] [Google Scholar]
- Barilla G. R. H.; Chen C. A. W.; Valencia M. Z. M.; Dugos N. P.; Choi A. E. S. Mixing Assisted Oxidative Desulfurization Using a Synthesized Catalyst of the Activated Carbon Supported Phosphotungstic Acid: A Process Optimization Study. South African J. Chem. Eng. 2022, 42, 61–71. 10.1016/j.sajce.2022.06.012. [DOI] [Google Scholar]
- Choi A. E. S.; Roces S.; Dugos N.; Futalan C. M.; Wan M.-W. Optimization Analysis of Mixing-Assisted Oxidative Desulfurization of Model Sulfur Compounds Using Commercial Ferrate(VI). Desalin. Water Treat. 2016, 57 (37), 17616–17623. 10.1080/19443994.2015.1088475. [DOI] [Google Scholar]
- Sikarwar P.; Gosu V.; Subbaramaiah V. An Overview of Conventional and Alternative Technologies for the Production of Ultra-Low-Sulfur Fuels:. Rev. Chem. Eng. 2019, 35 (6), 669–705. 10.1515/revce-2017-0082. [DOI] [Google Scholar]
- Sharma V. K. Ferrate(VI) and Ferrate(V) Oxidation of Organic Compounds: Kinetics and Mechanism. Coord. Chem. Rev. 2013, 257, 495–510. 10.1016/j.ccr.2012.04.014. [DOI] [Google Scholar]
- Hu J.; Li Z.; Zhang A.; Mao S.; Jenkinson I. R.; Tao W.. Using a Strong Chemical Oxidant, Potassium Ferrate (K2FeO4), in Waste Activated Sludge Treatment: A Review. In Environmental Research; Academic Press Inc, 2020. 10.1016/j.envres.2020.109764. [DOI] [PubMed] [Google Scholar]
- Huang D.; Wang Y. J.; Yang L. M.; Luo G. S. Chemical Oxidation of Dibenzothiophene with a Directly Combined Amphiphilic Catalyst for Deep Desulfurization. Ind. Eng. Chem. Res. 2006, 45 (6), 1880–1885. 10.1021/ie0513346. [DOI] [Google Scholar]
- Haboc M. M.; Dugos N. P.; Choi A. E. S.; Wan M.-W. Enhancing Oxidative Desulfurization Using Sludge-Derived Ferrate (VI) for Dibenzothiophene: An Optimization Study. J. Cleaner Prod. 2024, 470, 143307. 10.1016/j.jclepro.2024.143307. [DOI] [Google Scholar]
- Otsuki S.; Nonaka T.; Takashima N.; Qian W.; Ishihara A.; Imai T.; Kabe T. Oxidative Desulfurization of Light Gas Oil and Vacuum Gas Oil by Oxidation and Solvent Extraction. Energy Fuels 2000, 14 (6), 1232–1239. 10.1021/ef000096i. [DOI] [Google Scholar]
- Al-Abduly A.; Sharma V. K. Oxidation of Benzothiophene, Dibenzothiophene, and Methyl-Dibenzothiophene by Ferrate(VI). J. Hazard. Mater. 2014, 279, 296–301. 10.1016/j.jhazmat.2014.06.083. [DOI] [PubMed] [Google Scholar]
- Choi A. E. S.; Roces S.; Dugos N.; Wan M. W. Oxidation by H2O2 of Bezothiophene and Dibenzothiophene over Different Polyoxometalate Catalysts in the Frame of Ultrasound and Mixing Assisted Oxidative Desulfurization. Fuel 2016, 180, 127–136. 10.1016/j.fuel.2016.04.014. [DOI] [Google Scholar]
- Sharma V. K.; Luther G. W.; Millero F. J. Mechanisms of Oxidation of Organosulfur Compounds by Ferrate(VI). Chemosphere 2011, 82 (8), 1083–1089. 10.1016/j.chemosphere.2010.12.053. [DOI] [PubMed] [Google Scholar]
- Chen T.-C.; Shen Y.-H.; Lee W.-J.; Lin C.-C.; Wan M.-W. The Study of Ultrasound-Assisted Oxidative Desulfurization Process Applied to the Utilization of Pyrolysis Oil from Waste Tires. J. Cleaner Prod. 2010, 18 (18), 1850–1858. 10.1016/j.jclepro.2010.07.019. [DOI] [Google Scholar]
- Choi A. E. S.; Roces S. A.; Dugos N. P.; Wan M. W. A Comprehensive Process Optimization Study of the Mixing Assisted Oxidative Desulfurization of Diesel Oil. Environ. Technol. Innov. 2023, 31, 31. 10.1016/j.eti.2023.103144. [DOI] [Google Scholar]
- Liu S.; Wang B.; Cui B.; Sun L. Deep Desulfurization of Diesel Oil Oxidized by Fe (VI) Systems. Fuel 2008, 87 (3), 422–428. 10.1016/j.fuel.2007.05.029. [DOI] [Google Scholar]
- Talaiekhozani A.; Bagheri M.; Talaie Khozani M. R.; Jaafarzadeh N. An Overview on Production and Applications of Ferrate(VI). Jundishapur J. Heal. Sci. 2016, 8, e34904. 10.17795/jjhs-34904. [DOI] [Google Scholar]