Abstract
This review examines the convergence of silver nanoparticles (AgNPs), three-dimensional (3D) printing, and wound healing, focusing on significant advancements in these fields. We explore the unique properties of AgNPs, notably their strong antibacterial efficacy and their potential applications in enhancing wound recovery. Furthermore, the review delves into 3D printing technology, discussing its core principles, various materials employed, and recent innovations. The integration of AgNPs into 3D-printed structures for regenerative medicine is analyzed, emphasizing the benefits of this combined approach and identifying the challenges that must be addressed. This comprehensive overview aims to elucidate the current state of the field and to direct future research toward developing more effective solutions for wound healing.
1. Introduction
The process of 3D printing, also referred to as additive fabrication, involves the creation of three-dimensional objects through the gradual deposition of materials, enabling the construction of intricate structures with high precision.1,2 One of the most promising applications of 3D printing in healthcare is in the field of drug delivery.3−5 This technology allows for the precise fabrication of drug delivery systems tailored to the individual patient needs. For instance, 3D printing can produce oral drug delivery devices with customizable release profiles, enhancing the efficacy and safety of medications.3,6 The ability to design and manufacture dosage forms with specific geometries and release characteristics opens new possibilities for personalized medicine.7 Beyond drug delivery, 3D printing has made significant strides in the development of medical implants and prosthetics. Custom implants, such as cranial plates and hip joints, can be created to match a patient’s unique anatomy, improving the fit and function of these devices.8−10 Additionally, 3D printing has enabled the production of highly detailed anatomical models that assist surgeons in preoperative planning and intraoperative guidance.11−13 These models, derived from patient imaging data, provide a tangible reference that can enhance surgical precision and outcomes. Another exciting application of 3D printing in healthcare is the creation of bioprinted tissues and organs. While still in the experimental stages, researchers are exploring the potential of 3D printing to fabricate living tissues, such as skin, bone, and even entire organs.14−17 This technology holds the promise of addressing the shortage of donor organs and advancing regenerative medicine. Furthermore, 3D-printed scaffolds are being used in tissue engineering to support the growth and regeneration of damaged tissues.18 Hence, 3D printing is transforming healthcare by enabling the production of customized drug delivery systems, implants, prosthetics, and even bioprinted tissues. These advancements not only improve patient outcomes but also pave the way for more personalized and effective treatments. As the technology continues to evolve, its applications in healthcare are expected to expand, offering new solutions to some of the most pressing medical challenges.
Wound healing is a complex physiological process characterized by a series of cellular and mediator responses. Traditionally categorized into primary, secondary, and tertiary stages, each phase encompasses hemostasis, inflammation, proliferation, and remodeling.19−21 Despite advances in understanding this process, challenges persist in managing wounds, particularly in cases of chronic wounds that are resistant to conventional treatments. In the field of wound healing, 3D printing offers the potential to produce personalized wound coverings and supports precisely tailored to the shape and size of the wound, thereby enhancing therapeutic efficacy.22−29
Nanotechnology involves the precise manipulation of materials at the nanometer scale, typically ranging from 1 to 100 nm. At this scale, materials often demonstrate unique properties that are not apparent at larger scales.300,301 AgNPs exemplify this characteristic and are known for their potent antibacterial properties. When incorporated into wound dressings or scaffolds, these nanoparticles effectively prevent infection and promote the healing of wounds.302−36
The convergence of 3D printing and nanotechnology presents significant transformative opportunities in the realm of wound recovery. Incorporating AgNPs into 3D-printed constructs, researchers have achieved the development of wound dressings and scaffolds customized to individual wounds while also enhancing their antibacterial efficacy. This integration allows for precise control over the physical and chemical properties of the structures, thereby amplifying their effectiveness in promoting wound healing.37−44 For instance, tailored porosities in 3D-printed frameworks can influence the release rate of medications from the dressing or scaffold, while surface chemistry manipulation can promote crucial cell adhesion and proliferation processes essential for wound healing. Moreover, the inclusion of AgNPs further reinforces the antibacterial properties of these constructs.45−49 Consequently, the combination of 3D printing and nanotechnology offers innovative avenues for the development of advanced wound healing solutions. However, further research is imperative to optimize these technologies and fully exploit their potential in clinical applications.
Numerous studies have explored the integration of AgNPs onto 3D-printed scaffolds, leading to expanded biomedical applications. AgNPs are renowned for their distinct properties and broad utility in nanomedicine, including heightened antimicrobial efficacy, anticancer properties, and diverse therapeutic capabilities, making them a cost-effective and versatile solution for various applications.39,42,50−57 However, the full potential of AgNPs in the context of 3D printing and wound healing remains to be fully realized. While each domain, 3D printing, AgNPs, and wound healing, has been extensively investigated individually, there remains a significant gap in our understanding of their integrated potential. Specifically, how can we harness the antimicrobial and therapeutic properties of AgNPs in 3D printing techniques to enhance wound healing? This represents a frontier in wound healing research with promising therapeutic strategies. Scaffolds, often composed of materials such as poly(lactic acid) or poly(ether ether ketone) (PEEK), have shown potential in infection management and bone regeneration. Additionally, the development of multilayered scaffolds through a combination of 3D printing and electrospinning techniques has attracted attention. These scaffolds incorporate various substances, such as Mupirocin, Pluronic F127, and quaternized chitosan, enhancing their wound healing abilities. Research also extends to the fabrication of superporous hydrogels and functionalized membranes, characterized by high stretchability and directional water transport, making them suitable for applications such as joint wound therapy. Furthermore, the utilization of bioactive materials such as collagen, hyaluronic acid, and silk protein in 3D-printed constructs has been investigated for chronic diabetic wound rehabilitation and the repair of infective bone defects. When combined with AgNPs or nanoclusters, these materials give rise to composite hydrogels or biofunctionalized, hierarchically structured scaffolds.58−70
Despite the advancements made, understanding the long-term biocompatibility and in vivo performance of 3D-printed structures incorporating AgNPs requires thorough in vitro and in vivo analyses. It is crucial to assess the safety and efficacy of these innovative wound healing solutions extensively.71−76 Moreover, further investigation into the potential cytotoxicity of AgNPs within these structures is warranted. This review aims to provide a comprehensive overview of the current state of the field, including recent progress in the utilization of 3D printing technology and AgNPs for wound healing. It will discuss various methodologies employed to develop effective wound dressings and scaffolds, such as in situ synthesis of AgNPs on 3D-printed scaffolds, fabrication of multilayered scaffolds, and the creation of superporous hydrogels and functionalized membranes. Additionally, it will explore the use of bioactive materials like collagen, hyaluronic acid, and silk protein in 3D-printed constructs for chronic diabetic wound rehabilitation and the repair of infective bone defects. The discussion will also extend to how these materials, when combined with AgNPs or nanoclusters, can generate composite hydrogels or biofunctionalized hierarchically structured scaffolds. In addition to providing an overview of these areas, the review will highlight areas for future exploration, such as the prolonged biocompatibility and in vivo performance of these 3D-printed structures incorporating AgNPs. Despite promising results in laboratory settings, more extensive in vitro and in vivo analyses are essential to ensuring their safety and efficacy in real-world scenarios. Another area requiring further investigation is the potential cytotoxicity of AgNPs within these frameworks. Although AgNPs are valued for their antibacterial properties, thorough examination is necessary to guarantee the safety of these innovative wound healing solutions. By addressing these areas, the review aims to guide future research efforts toward the development of more effective wound healing treatments (Figure 1).
Figure 1.
An overview of the manuscript’s scope. The integration of silver nanoparticles and 3D printing for enhanced wound healing.
2. Silver Nanoparticles (AgNPs): An Overview
The synthesis of silver nanoparticles (AgNPs) can be achieved through various methods, each with its own advantages and disadvantages. Chemical reduction is the most common approach, involving the reduction of silver ions to silver atoms using a reducing agent. This method allows for precise control over nanoparticle characteristics by adjusting parameters such as silver ion concentration and reaction conditions.77−81 However, the use of hazardous chemicals poses environmental risks and limits the suitability of these nanoparticles for biological applications. Physical methods like evaporation–condensation and laser ablation eliminate the need for toxic chemicals but require significant energy and sophisticated equipment.82−88 Biological methods, using organisms like bacteria, fungi, or plant extracts, offer an environmentally friendly alternative, though they typically have lower and slower reaction rates.88−99
At the nanoscale, AgNPs exhibit unique properties, including potent antimicrobial activity due to their large surface area to volume ratio and silver ion release.100−111 These properties make AgNPs valuable in various fields such as wound care, water purification, and biosensing.107−111 Their antimicrobial efficacy is primarily due to the disruption of microbial membranes by silver ions, leading to cell death. Additionally, AgNPs’ optical properties, characterized by surface plasmon resonance, have applications in biosensing and bioimaging.112−124 Despite these benefits, the strong antimicrobial activity of AgNPs can also harm beneficial microorganisms, necessitating careful control and management in their applications.
3. 3D Printing Technology
Revolutionizing traditional manufacturing approaches, 3D printing, also known as additive manufacturing, revolutionizes fabrication by creating three-dimensional objects from digital blueprints. Unlike conventional methods that remove material to shape objects, this innovative technique builds objects layer by layer using various materials. The outcome? Highly detailed structures, particularly valued across diverse sectors, notably in the medical field.125−130 At its foundation, 3D printing begins with the creation of a digital model, typically generated using computer-aided design (CAD) software or acquired through advanced 3D scanning techniques.131,132 The essence of this process lies in its additive nature, with each layer incrementally contributing to the formation of the final product. This dynamic enables the production of complex geometries and internal intricacies, areas in which subtractive methods are limited.
The capabilities of 3D printing in the medical field have reached a peak, as illustrated in Scheme 1. Customized medical devices such as hearing aids and dental implants are now crafted using this technological advancement, ensuring a precise fit for each individual recipient. Additionally, prosthetic limbs produced by 3D printing offer not only structural integrity but also enhanced comfort and functionality, significantly improving the quality of life for users.133−137 Furthermore, 3D printing extends its reach into the realm of bioprinting, where living tissues are fabricated from bioinks containing living cells. While still in its early stages, bioprinting shows promise in regenerative medicine and organ reconstruction. By mimicking the intricate structure of natural tissues, bioprinted constructs represent a beacon of hope, signaling a new era in medical interventions.138−142 Therefore, 3D printing has emerged as a disruptive force, transforming industries with its ability to produce complex, personalized entities. Its impact is particularly profound in medicine, where it not only revolutionizes the manufacturing of medical devices but also paves the way toward a future where the repair of damaged tissues and organs becomes a feasible reality.
Scheme 1. Key Application of 3D Printing in Medical Sciences: A Schematic Representation.
The versatility of 3D printing encompasses a wide range of materials, including plastics, metals, ceramics, and biological substances. Material selection is tailored to the specific requirements of the objects being fabricated. For example, in the field of wound healing, materials like poly(lactic acid) (PLA) or polyetheretherketone (PEEK) are commonly used due to their combination of biocompatibility and mechanical strength.143−145 Plastics, such as PLA and acrylonitrile butadiene styrene (ABS), are prominent in 3D printing due to their ease of manipulation and cost-effectiveness. PLA, in particular, is favored in biomedical applications for its compatibility with living tissues and environmentally friendly biodegradability.132,146,147 Metals, including titanium, stainless steel, and gold, play a significant role in 3D printing, particularly in applications requiring high strength and durability, such as aerospace engineering and the production of medical implants.148−150 Ceramics, known for their heat resistance, hardness, and biocompatibility, are utilized in 3D printing for crafting dental prosthetics and bone implants, where precision and durability are essential considerations.151−154
In the emerging field of bioprinting, a combination of biological materials including cells, DNA, and bioinks is utilized to construct tissue-like structures, representing a significant advancement in regenerative medicine and wound management.145,155−157 A recent focus of research involves the incorporation of AgNPs into the 3D printing process. With their strong antibacterial properties, AgNPs offer a promising option for the production of wound dressings and scaffolds aimed at combating infections and promoting faster healing. The seamless integration of these nanoparticles into printing materials facilitates the creation of structures with inherent antibacterial capabilities.158−160 Material selection is intricately tied to the specific requirements of the object being fabricated, considering factors such as mechanical strength, intended application, and desired properties like biocompatibility and antibacterial activity, guiding the development and selection of materials for 3D printing.132,161−163 Recent advancements in 3D printing technology have enabled printing with multiple materials simultaneously, opening up possibilities for creating structures with diverse mechanical characteristics and incorporating active substances such as pharmaceuticals and AgNPs directly into the printed product. In wound healing, this advancement holds significant potential for fabricating customized coverings or frameworks that not only conform accurately to the wound’s contours but also provide tailored therapy.
The evolution of 3D printing technology into multimaterial printing is facilitated by advanced printer heads capable of transitioning between various materials during the printing process. This adaptability enables the creation of structures with a wide range of mechanical properties within a single printing session. For example, imagine a single object with both rigid and flexible segments, precisely tailored to meet design requirements.164−166 Another significant advancement is the incorporation of active substances, such as pharmaceuticals or AgNPs, directly into the printed object. In the medical field, this feature allows for the precise dispensing of medications to targeted areas using 3D-printed devices. Consider a 3D-printed wound dressing infused with a therapeutic drug, providing targeted therapy to accelerate healing.132,167,168 Furthermore, 3D printing technology offers unprecedented levels of customization that are unattainable with conventional manufacturing methods. This aspect is particularly advantageous in wound healing, where coverings or frameworks can be intricately designed to match the precise proportions and contours of the wound, ensuring optimal fit and enhancing therapeutic effectiveness.132,155,169 Bioprinting, an emerging area within 3D printing that utilizes biological components such as cells and growth factors, represents a significant advancement in the field. This technology has the potential to create tissue-like structures conducive to wound healing or tissue replacement. Bioprinting typically involves the use of bioink, a combination of cells and biomaterials, which is meticulously printed layer by layer to fabricate structures resembling natural tissue. Such technology holds great promise in regenerative medicine and wound management, envisioning bioprinted skin grafts that transform the treatment of burn victims or individuals with chronic wounds. Additionally, the prospect of bioprinting complex tissues and organs for transplantation is becoming increasingly feasible.143,170−172
In the dynamic field of 3D printing, persistent challenges endure despite significant progress. These obstacles encompass a range of issues, from the need to enhance both the accuracy and speed of 3D printers to ensuring the long-term durability and biocompatibility of 3D-printed medical devices. Additionally, understanding the complex interaction between printed materials and biological substrates presents another formidable barrier.132,173−175 However, a steadfast commitment to innovation and advancement continues to push the boundaries of what can be achieved with 3D printing technology. Particularly notable is the pursuit of improved resolution and speed in 3D printers. Higher resolution enables the fabrication of increasingly intricate and refined structures, while faster printing speeds hold the potential to enhance the overall manufacturing efficiency. Yet, striking the delicate balance between resolution and speed remains a key focus of exploration.176,177 Another significant challenge is ensuring the stability and safety of 3D-printed medical devices. This entails maintaining the mechanical integrity of printed components over extended periods and ensuring that the materials used demonstrate a high level of compatibility with biological systems to prevent adverse reactions in the human body. Furthermore, in the emerging field of bioprinting, preserving the viability and functionality of printed cells is of paramount importance for their intended biomedical applications.17,136,155,178−182
Despite the substantial challenges encountered, continuous advancement in research and development drives the field of 3D printing forward. Each achievement brings us closer to fully realizing the transformative capacity of this technology, particularly in areas such as regenerative medicine and beyond. The future holds great promise, positioning 3D printing as an intriguing frontier with a trajectory that remains dynamic and evolving.
4. AgNPs in 3D Printing
Incorporating AgNPs into the framework of 3D-printed structures represents a recent advancement in additive manufacturing. Typically, this integration involves mixing AgNPs with the base material, such as a polymer, used in the 3D printing process. Subsequently, the resulting composite material facilitates the creation of desired configurations and dimensions in the structures.68,183,184 Initially, a critical step involves blending AgNPs with the substrate material for the 3D-printed structures. This substrate material, often a polymer, serves as the foundation of the 3D-printed object. The dispersion of AgNPs within the substrate material can be achieved through various methods, including fusion blending, where the polymer undergoes fusion and incorporates the AgNPs, or solvent casting, where both the polymer and AgNPs are dissolved in a common solvent and then cast into a mold.184−188 Once the AgNPs are uniformly dispersed within the substrate material, the resulting blend is utilized in the 3D printing process. The specific method employed varies depending on the type of 3D printing technology that is utilized. This may involve heating the blend to produce a semiliquid filament that can be extruded through a nozzle, as seen in fused deposition modeling, or selectively solidifying a liquid resin containing the AgNPs, as observed in stereolithography.184−188 The primary benefit of incorporating AgNPs into 3D-printed structures lies in their antibacterial properties. AgNPs release silver ions, which possess broad-spectrum antibacterial efficacy. When AgNPs are infused into 3D-printed structures, they enable a continuous release of silver ions, providing persistent antibacterial protection. This feature is particularly advantageous in medical applications such as wound dressings or implants, where preventing bacterial contamination is crucial.159,189,190 Additionally, 3D printing technology offers the advantage of fabricating customized structures. By optimization of the design of the digital model used in the 3D printing process, structures can be precisely tailored to fit the specific contours and dimensions of a wound or implantation site. This level of customization significantly enhances the effectiveness of therapeutic interventions, providing tailored solutions for individual needs.68,184
The integration of AgNPs into 3D-printed structures combines the benefits of 3D printing technology, including customization and the ability to create complex designs, with the antimicrobial properties of AgNPs. This synergy presents novel opportunities for applications in wound healing and other areas. However, further research is necessary to optimize this approach and fully realize its potential.
5. Benefits and Challenges
The integration of AgNPs into 3D-printed constructs offers significant advancements, particularly due to their potent antimicrobial properties. These properties are crucial in medical applications such as wound dressings and implants, where infection prevention is essential. The versatility of 3D printing allows for the precise fabrication of structures tailored to the specific shapes and dimensions of wound or implant sites, thereby enhancing treatment efficacy.59,68 However, challenges exist, notably in achieving a uniform dispersion of AgNPs throughout the 3D-printed structure. This uniformity is critical, as uneven distribution can reduce the antimicrobial efficacy in certain areas of the construct.68,183 Additionally, potential cytotoxic effects of AgNPs pose a significant concern given their ability to harm human cells despite their antibacterial capabilities. Addressing this issue requires careful regulation of AgNP concentration and thorough safety assessments to ensure the safety of the 3D-printed devices.191−193 In summary, while the incorporation of AgNPs into 3D-printed constructs presents substantial benefits, it also necessitates addressing significant challenges through continuous research and development.
6. AgNPs in Wound Healing
6.1. Role of AgNPs in Wound Healing
In wound healing, AgNPs serve as effective antibacterial agents, preventing wound infections which can impede the healing process and lead to chronic wounds.194−196 Recent research suggests that AgNPs not only possess significant antibacterial properties but also exhibit potential anti-inflammatory effects and promote the proliferation and migration of skin cells, essential for wound repair. The antibacterial efficacy of AgNPs is attributed to the release of silver ions, which are effective against various bacterial strains, thereby protecting against infections. Furthermore, AgNPs may help regulate inflammation, which, while a natural part of the healing process, can be detrimental if excessive.195,197 By modulation of inflammation, AgNPs create an environment favorable for healing. Additionally, they support the proliferation and migration of skin cells, crucial steps in replacing damaged tissue and advancing wound closure. Thus, AgNPs hold promise in enhancing wound healing through multiple mechanisms, including antibacterial action, inflammation modulation, and cell proliferation and migration promotion.195,198
Explorations of 3D printing for wound healing applications have highlighted the potential of integrating AgNPs into 3D-printed constructs such as wound dressings or scaffolds. This innovative approach leverages the antibacterial, anti-inflammatory, and wound-healing properties of AgNPs, offering a promising new method for enhancing wound care.145,199,200 However, advancing this technology requires comprehensive research to fully understand the effects of AgNPs and to ensure their safe application. Key considerations include the potential cytotoxicity of AgNPs, necessitating a careful balance between their antibacterial efficacy and safety.192,193,201
6.2. Mechanism Unveiled
The effectiveness of AgNPs is largely attributed to their ability to release silver ions, which possess significant antibacterial properties. These ions disrupt bacterial cell walls and interfere with essential cellular functions by binding to proteins and DNA, thereby compromising cellular integrity.196,202−204 In the context of wound healing, AgNPs play a crucial role in preventing infections, which is essential for proper healing. Emerging research suggests that AgNPs may also have anti-inflammatory and wound-healing properties, further enhancing their therapeutic potential.205,206 However, the application of AgNPs requires careful consideration due to potential cytotoxic effects. Thorough research is necessary to fully understand their impact and to balance antibacterial efficacy with safety.192,193,196,201,207,208
6.3. Clinical Studies and Results
In wound care, AgNPs have been incorporated into various dressings, such as Acticoat and PolyMem Silver, to enhance healing by reducing infections, promoting re-epithelialization, and shortening healing times. These dressings are primarily tested in burn treatments but are also evaluated for other wound types, including diabetic ulcers.209−211 Despite their demonstrated efficacy, further research is essential to understanding the long-term safety and potential toxicity of AgNPs. Studies on human cells have shown that silver nanomaterials can cause varying degrees of toxicity including cell death and DNA damage, which are influenced by particle size. However, the overall risk posed by AgNPs to human health is considered relatively low.212−214 AgNPs offer a promising alternative to conventional antibiotics, particularly in the context of rising antimicrobial resistance (AMR). Their antimicrobial properties make them effective against drug-resistant infections through various mechanisms, although their efficacy can vary significantly based on the biomaterials used in their synthesis.215−218
A recent clinical trial (NCT05850819) evaluated the efficacy of Gelatamp, a colloidal silver gelatin sponge, in promoting the healing of intraoral wounds following mandibular teeth extraction. The hypothesis posited that the AgNPs in Gelatamp would provide antibacterial properties, thereby potentially accelerating the healing process. This randomized, double-masked intervention study included 60 participants, both healthy individuals and those with mild systemic disease, requiring mandibular teeth extraction. Participants were divided into two groups: the experimental group received Gelatamp in the extraction socket, while the control group received a gauze pack without Gelatamp. The primary outcomes were postoperative wound healing and pain, assessed using a 5-point early wound healing scale after 7 days and a 100 mm visual analogue scale on the first and second postoperative days, respectively.
Another clinical trial (NCT04213716) focused on wound healing by comparing the effectiveness of intracanal medications containing nanosilver combined with calcium hydroxide versus conventional calcium hydroxide in reducing postoperative pain in patients with symptomatic root canal treatment failure. This interventional trial assigned participants to two groups: the experimental group received 1 mL of a nanosilver particle solution (30 ppm) combined with 100 mg of calcium hydroxide as intracanal medication, while the control group received 100 mg of calcium hydroxide powder mixed with 1 mL of distilled water. The primary outcomes were evaluations of postoperative wound healing and pain.
Moreover, the clinical trial registered as NCT03401749 aimed to evaluate the effectiveness of preadmission Theraworx wipes in preventing surgical site infections in adult orthopedic surgery patients. This interventional study involved the division of participants into two groups: the first adhered to standard preadmission surgical instructions, while the second incorporated the use of Theraworx skin wipes the night before and 1 h prior to surgery. Primary objectives included safety monitoring, comparison of perioperative skin cultures between treatment groups, and assessment of surgical site infection rates. Secondary objectives included evaluating patient compliance, satisfaction levels among patients and nurses, and visual assessments of wound healing. While the trial did not explicitly outline the role of AgNPs, Theraworx likely incorporated silver, potentially in nanoparticle form, to utilize its antimicrobial properties for infection prevention in surgical site prophylaxis. However, the study faced premature termination due to funding constraints, recruitment challenges, and patient compliance issues.
7. 3D Printing and Wound Healing: A New Frontier
7.1. Synergy of AgNPs and 3D Printing in Wound Healing
The integration of AgNPs with 3D printing represents a significant advancement in wound healing. Known for their strong antibacterial properties, AgNPs play a crucial role in preventing wound infections, which is a common obstacle to healing. By incorporating AgNPs into 3D-printed structures such as wound dressings or scaffolds, these constructs not only conform closely to the unique contours of wounds but also provide targeted antibacterial reinforcement. The combination of AgNPs and 3D printing in wound healing offers several advantages. First, it allows for the fabrication of customized wound dressings or scaffolds tailored to enhance treatment efficacy. Second, the inherent antibacterial properties of AgNPs act as a barrier against wound infections, creating an environment conducive to optimal recovery. Recent advancements in this field include the development of multimaterial 3D printing techniques, enabling the integration of various materials, including AgNPs, into a single printed structure. This innovation enables the creation of structures with diverse mechanical properties and antibacterial capabilities, thereby enhancing their effectiveness in wound healing (Table 1).
Table 1. Examples of Silver Nanoparticles in 3D Printing for the Purpose of Wound Healing.
| Chemical information | Morphological structure | Mechanical properties | Functional | Antibacterial properties | Results | Ref |
|---|---|---|---|---|---|---|
| The presence of AgNPs in the hydrophobic layer | The hydrophilic layer had a nonwoven structure, while the hydrophobic layer had a 3D scaffold structure with different patterns | The knit-like pattern of the hydrophobic layer exhibited the best mechanical performance among the four patterns | The bilayer membrane had efficient directional-water-transport performance and excellent moisture management capability | The bilayer membrane had high antibacterial activity against E. coli and S. aureus due to the presence of AgNPs in the hydrophobic layer | These results demonstrate the potential of the bilayer membrane as a promising wound dressing for joint wounds | (65) |
| Made from Na-ALG, PVA, and Cu–Ag MBGNs | Porous structure with uniform elemental composition | Good strength and 45% ductility | Hydrophilic, biodegradable, and cytocompatible | Effective against S. aureus and E. coli | Promising for skin regeneration and wound healing | (66) |
| Scaffold made from polyurethane, Pluronic F127, quaternized chitosan, silver nitrate, mupirocin, pectin, and keratin | Three-layer structure with a top layer of polyurethane nanofibers, a middle layer of 3D-printed Pluronic F127-quaternized chitosan-silver nitrate, and a bottom layer of core–shell nanofibers of F127-mupirocin/pectin-keratin | Good mechanical properties with moderate tensile strength and elastic modulus | High swelling ratio and sustained release of Ag ions and mupirocin | Enhanced antibacterial activity against both Gram-positive and Gram-negative bacteria | Supported cell adhesion and viability, promoted angiogenesis, and accelerated wound healing in vitro and in vivo | (44) |
| RHCMA, HAMA, and AgNCs | Porous, interconnected network | Elastic modulus of 10R3H100Ag: 0.25 MPa | UV-responsive, shear-thinning, biocompatible | Effective against S. aureus and P. aeruginosa | Promoted wound healing in diabetic rats | (67) |
| PGSA prepolymer | 3D-printed scaffolds and conduits | Swelling ratio, degradation rate, mass loss | Electrical conductivity | Cell viability, proliferation, guidance | PGSA–PVP showed the best electrical conductivity, biodegradability, and biocompatibility among the PGSA composites. PGSA composites with microgrooves and electrical stimulation enhanced cell growth and alignment. PGSA–PVP conduits showed potential for nerve tissue regeneration | (49) |
| PCL modified with plasma polymer and AgNPs | 3D-printed scaffolds with interconnected pores and uniform distribution of AgNPs | Increased hardness and modulus compared to unmodified PCL | Improved hydrophilicity and biocompatibility | Effective against S. epidermidis and P. aeruginosa | Enhanced wound healing and angiogenesis in vivo | (59) |
| CNC/Chit-MA hydrogel with different ratios of CCNC to CChit-MA | Fibrillar structure with controlled pore size, dependent on ratio | Compression Young’s modulus decreased with increasing ratio | Shear-thinning and self-healing properties, water vapor transmission rate of 3210 ± 380 g/m2·24 h, high swelling ratio | Loaded with gentamicin or AgNPs for antibacterial activity against S. aureus and P. aeruginosa | Effective release of biologically active agents, biocompatible, improved wound healing in mice | (64) |
| AgNPs synthesized by UV irradiation method using silver nitrate as precursor and PLA as stabilizer | AgNPs have spherical shape and size ranging from 20 to 50 nm depending on UV exposure time | Similar thermal transitions in PLA and PLA/Ag | Increased hydrophilicity with AgNPs | Not investigated | Nontoxic, efficient, cost-effective method for biomedical applications | (68) |
| Silver–ethylene interaction: Ag ions chelate with MBAM monomers to form organometallic complexes, which are reduced to AgNPs in hydrogel matrix | Superporous hydrogels: prepared by using 3D-printed PLA templates and HPMC as pore-making materials | Mechanical strength: decreased with increasing AgNP content and porosity | Water uptake capacity: increased with increasing AgNP content and porosity | Increased with increasing AgNP content; effective against S. aureus and E. coli | Wound healing: AgNP cross-linked superporous hydrogel dressings promoted wound healing and reduced scar tissue formation in vivo | (69) |
| Ti6Al4V alloy, titanate nanowires, silk fibrin, AgNPs | Hierarchical porous structure, multilayered silk-on-silk assembly, Ag core/SF corona micelles | Adjustable elastic modulus and strength, sufficient space for bone and blood vessel ingrowth | Hydrophilic, protein-adsorbing, bioactive, osteoconductive, osteoinductive | Sustained Ag+ release, ROS production, surface nanostructure effects | Reduced bacterial adhesion and viability, enhanced cell proliferation and differentiation, improved bone regeneration | (70) |
| AgNPs coated on 3D PEEK scaffold via pDA nanolayer | AgNPs uniformly anchored on the surface with diameter of 100 nm; 33.59% porosity of 3D PEEK scaffold | No significant difference in elastic modulus among pure and modified 3D PEEK scaffolds | AgNPs endowed 3D PEEK scaffold with bioactivity and osteogenic differentiation | 3D PEEK/Ag (1 mM) scaffold showed significant antibacterial effect and antibiofilm formation against E. coli and S. aureus | 3D PEEK/Ag (1 mM) scaffold had good cytocompatibility and osteo-differentiation and could be a potential material for bone repair | (63) |
7.2. Case Studies or Examples
In the domain of biomedical applications, AgNPs are notable for their robust antimicrobial characteristics and extensive potential in tissue engineering. A noteworthy approach involves incorporating them into poly(lactic acid) (PLA) scaffolds, which can be fabricated by using diverse methods. Recently, a study focused on AgNP synthesis and characterization on 3D-printed PLA scaffolds, particularly highlighting the UV irradiation technique and its implications in biomedical engineering. Various synthesis methods for AgNPs have been investigated, such as photoreduction, in situ growth, and chemical reduction, each carrying distinct advantages and disadvantages. For instance, although chemical reduction offers efficiency, it also entails environmental and biological risks due to the use of chemical reducing agents. Conversely, the UV irradiation method, as demonstrated in this study, offers a straightforward, nontoxic, and cost-effective approach for AgNP synthesis on PLA scaffolds, devoid of chemical reducing agents, thus holding promise for biomedical applications due to its eco-friendliness and scalability. Incorporating AgNPs into PLA scaffolds modifies their mechanical, thermal, electrical, and biological properties, with parameters such as size, shape, distribution, and loading of AgNPs playing crucial roles. Research suggests that AgNP-functionalized PLA scaffolds exhibit increased surface hydrophilicity, enhancing cell adhesion and protein adsorption, making them suitable for various applications, such as cell culture, biosensing, and wound healing. Experimental validations have confirmed the effectiveness of the UV irradiation method in synthesizing AgNPs on 3D-printed PLA scaffolds. Utilizing a range of characterization techniques, including TEM, zeta sizer, UV–visible spectroscopy, ATR-FTIR, and DSC, researchers have successfully synthesized monodisperse AgNPs ranging in size from 20 ± 2.2 nm to 50 ± 4.8 nm. Furthermore, evaluations of surface wettability have supported the enhancement of surface hydrophilicity following AgNP integration, confirming its biomedical potential. In summary, the UV irradiation method has emerged as a promising approach for AgNP synthesis on PLA scaffolds, offering simplicity, safety, and cost-effectiveness. However, further research is necessary to fully determine its suitability across various biomedical contexts and optimize synthesis parameters for specific applications. By leveraging advancements in AgNP synthesis and characterization, researchers can pave the way for innovative solutions in biomedical engineering, contributing to advancements in tissue engineering, antimicrobial coatings, and beyond.68
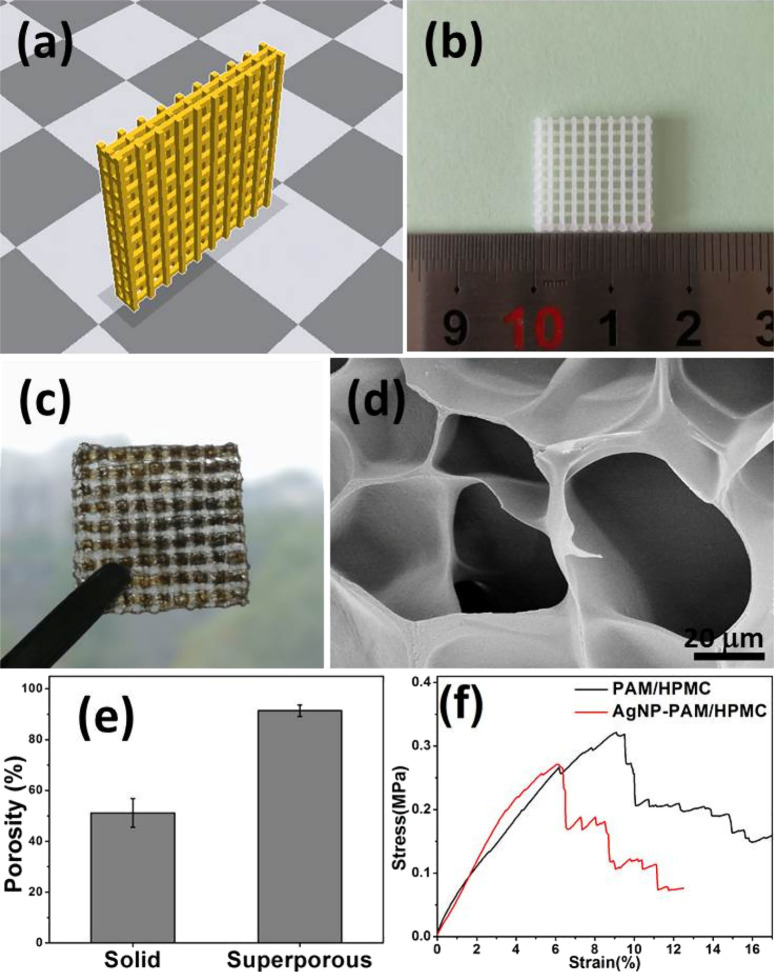
In a recent investigation, scholars delved into the advancement of antibacterial superporous hydrogel dressings for wound care. Employing a distinctive methodology that integrated the silver–ethylene interaction and 3D printing techniques, they engineered these dressings (as illustrated in Figure 2). Within this process, AgNPs were generated, dispersed, and cross-linked within the polyacrylamide (PAM)/hydroxypropyl methylcellulose (HPMC) hydrogel framework. This interaction facilitated the regulation of AgNP release, striking a balance between cytocompatibility and antibacterial efficacy. The application of 3D printing technology facilitated the fabrication of hydrogels characterized by high porosity and open pores, thereby enhancing water absorption and retention capabilities while mitigating the likelihood of swelling and detachment. Experimental findings corroborated the effectiveness of the AgNP-cross-linked superporous hydrogel dressings in fostering the healing of infected wounds and impeding scar tissue formation in vivo. These dressings demonstrated superior efficacy in reducing wound size compared to alternative hydrogel formulations, alongside noteworthy antibacterial properties against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), underscoring their potential utility in wound management.69
Figure 2.
Various stages and analyses of the development of a porous biomedical dressing. (a) The designed prototype is depicted as a 3D model of a yellow, grid-like structure intended for use as a template in creating biomedical dressings. (b) The printed PLA (poly(lactic acid)) template, shown alongside a ruler, has dimensions of 15 × 15 × 3 mm, with pore sizes of 1.2 × 1.2 × 0.8 mm and PLA wire diameters of 0.8 × 0.8 × 0.8 mm. This physical template is fabricated based on the initial 3D design. (c) Superporous AgNP-PAM/HPMC hydrogel dressings, derived from the PLA template, are illustrated being held by tweezers, emphasizing their high porosity aimed at enhancing biomedical application performance. (d) The SEM (scanning electron microscope) image offers a detailed view of the intricate porous structure of the superporous hydrogel dressing. (e) A bar graph compares the porosity levels of solid and superporous AgNP-PAM/HPMC hydrogels, revealing higher porosity in the superporous variants. (f) The stress–strain graph illustrates the mechanical properties of the superporous PAM/HPMC and AgNP-PAM/HPMC hydrogel dressings, showing that AgNP-PAM/HPMC demonstrates greater stress resistance at similar strains compared to PAM/HPMC. This figure is adapted with permission from the American Chemical Society.69,152
Embarking on innovative research, a recent investigation focused on the development and assessment of 3D-printed PGSA composites engineered to possess biodegradability and conductivity with a specific emphasis on nerve tissue regeneration. Utilizing poly(glycerol sebacate) acrylate (PGSA) as the base material, researchers augmented it with polyvinylpyrrolidone (PVP), AgNPs, and graphene to enhance its electrical conductivity and biocompatibility. This intricate synthesis was accompanied by a comprehensive evaluation encompassing electrical conductivity, biodegradability, 3D printability, and cell proliferation. The results revealed that these composites demonstrate notable conductivity upon swelling alongside rapid degradation and hydrolysis rates while simultaneously promoting cell growth and guidance. Moreover, employing PGSA composites in fabricating microgrooved scaffolds and conduits exhibited the ability to direct cell growth and enhance proliferation. The intricate microstructures within the printed materials, combined with electrical stimulation, particularly emphasized in PGSA–PVP compositions, underscore the significant potential of 3D-printed nerve conduits utilizing PGSA composites for nerve tissue regeneration, especially when complemented with electrical stimulation. These pioneering findings not only corroborate existing literature but also signify a substantial advancement in the field, highlighting the promising trajectory of these composites in nerve tissue regeneration applications.49
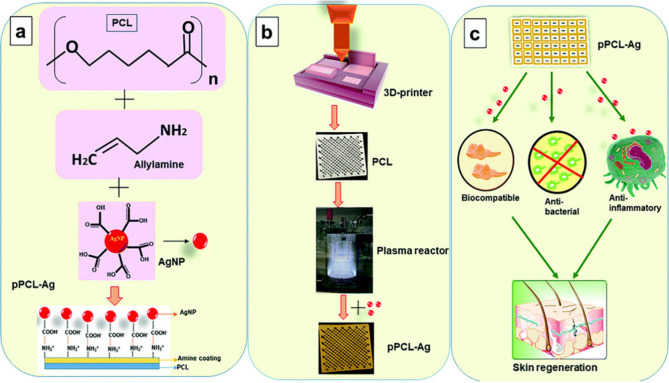
Concurrently, a separate investigation conducted an extensive inquiry into the development of biocompatible 3D-printed polycaprolactone (PCL) scaffolds integrated with potent antibacterial attributes (as depicted in Figure 3). This innovative approach combines plasma-assisted surface modification with the immobilization of AgNPs. Initially, PCL scaffolds were fabricated via fused deposition modeling, followed by a coating process with an allylamine plasma polymer. Subsequent steps involved the synthesis and immobilization of AgNPs onto the plasma-coated scaffolds for varying durations. A comprehensive array of characterization techniques was employed to evaluate the scaffolds for antibacterial efficacy, biocompatibility, immune response, and in vivo performance. Results demonstrated that the modified scaffolds maintained mechanical integrity while exhibiting improved hydrophilicity and surface energy, essential traits that enhance their functionality in biological environments. Incorporating immobilized AgNPs conferred robust antibacterial activity against common pathogens, such as S. epidermidis and P. aeruginosa, critical for combating wound infections. In vivo assessments on Sprague–Dawley rats revealed heightened angiogenesis and reduced foreign body reactions for scaffolds functionalized with AgNPs for 6 h, emphasizing their significant role in wound healing and tissue regeneration. The optimization potential of nanoparticle immobilization duration emerges as a noteworthy consideration crucial for achieving optimal outcomes.59
Figure 3.
Components, chemistry, and processes involved in the immobilization and creation of plasma-polymer-modified PCL scaffolds incorporating silver nanoparticles (AgNPs), designated as pPCL–Ag. (a) Elements and chemical reactions involved: Polycaprolactone (PCL) reacts with allylamine in the presence of AgNPs, resulting in pPCL-Ag, which is noted for its antibacterial properties. (b) Illustrates the equipment and processes used in the material’s fabrication, including a 3D printer that constructs the material’s layers and a plasma reactor that modifies the material to achieve a grid-like structure coated with pPCL-Ag. (c) Targeted outcomes of utilizing pPCL-Ag, emphasizing its biocompatibility, antibacterial, and anti-inflammatory properties, which make it suitable for skin regeneration applications. This figure is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported License.59
In conclusion, this groundbreaking research contributes substantially to the field of tissue engineering by highlighting the potential of 3D-printed PCL/silver nanoparticle scaffolds in advancing wound healing, consistent with prior studies emphasizing the crucial role of AgNPs in augmenting the antibacterial effectiveness of 3D-printed scaffolds.59,68 However, the inherent variability arising from methodologies and materials emphasizes the necessity of additional comparative studies to refine the scaffold design and fabrication processes.
Innovatively contributing to the spectrum of solutions, a pioneering investigation has revealed the emergence of an unprecedented 3D-printed poly(ether ether ketone) (PEEK) implant enhanced with AgNPs, meticulously designed to confer augmented antibacterial and osteogenic properties. Employing fused deposition modeling (FDM), researchers fabricated 3D PEEK scaffolds with varied pore dimensions and porosities. Subsequently, these structures underwent a comprehensive coating process involving polydopamine (PDA) and AgNPs via catechol-Ag+ chemistry. The antibacterial efficacy against E. coli and S. aureus, alongside biocompatibility and osteogenic differentiation potential, were meticulously evaluated through a series of in vitro analyses utilizing MG-63 cells. The findings revealed uniform and well-dispersed AgNPs adorning the surface of 3D PEEK/Ag constructs, highlighting notable antibacterial effectiveness against both Gram-negative E. coli and Gram-positive S. aureus. Furthermore, these constructs demonstrated commendable biocompatibility, fostering robust cellular adhesion, proliferation, and osteogenic differentiation, as validated by the increased expression of osteogenic genes and proteins. This underscores the significant potential of 3D PEEK/Ag constructs in clinical osseous tissue engineering, amalgamating the advantages of 3D printing, PEEK, and AgNPs into a cohesive solution poised to redefine bone regeneration.63
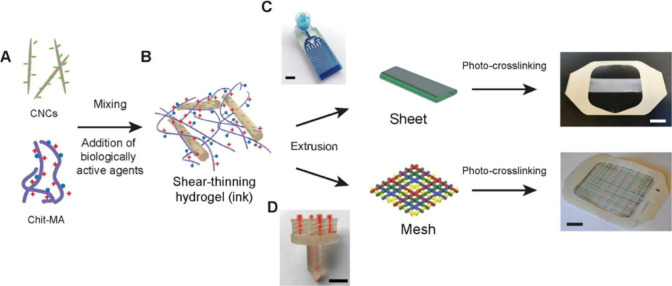
In a concurrent endeavor, Alizadehgiashi et al. introduced a groundbreaking approach in the development, characterization, and application of hydrogel dressings engineered to deliver a variety of therapeutic agents, including AgNPs, to wound sites with precisely controlled release profiles. The hydrogel ink, formulated with cellulose nanocrystals and chitosan methacrylamide, yielded a shear-thinning and self-healing bionanocomposite (as depicted in Figure 4). This ink, incorporating diverse biologically active agents, was adeptly 3D-printed into customizable structures using microfluidic printheads. Demonstrating adjustable mechanical and transport properties, these hydrogel dressings facilitated precise modulation of therapeutic agent release, including AgNPs. The effectiveness of these dressings was reinforced by comprehensive characterization of release profiles in phosphate-buffered saline along with thorough in vitro evaluations against 3T3 fibroblast cells and bacterial strains, revealing potent antibacterial properties associated with AgNPs. In vivo experiments on murine wound models unveiled the efficacy of these dressings in promoting granulation tissue formation and vascularization, contingent upon the release regimen of growth factors such as VEGF. Embarking on a multifaceted trajectory, these 3D-printed hydrogel wound dressings offer tailored solutions for wound management, promising expedited healing, and improved patient outcomes. However, further research and clinical validation are imperative to fully realize the therapeutic potential of this innovative technology in wound care practice.64
Figure 4.
Process of creating multifunctional wound dressings using cellulose nanocrystals (CNCs), chitosan methacrylamide (Chit-MA), and biologically active ingredients. Part (A) depicts the initial mixing of CNCs, Chit-MA, and biologically active substances such as proteins, small molecules, or nanoparticles. In the diagram, blue dots represent methacrylamide groups; red plus signs indicate positive charges; and green minus signs denote negative charges. Part (B) shows the formation of a shear-thinning hydrogel ink from this mixture, characterized by its solid-like behavior under low stress and liquidlike flow under high stress, suitable for extrusion-based 3D printing. Part (C) describes the extrusion of the hydrogel ink through a microfluidic printhead to fabricate sheets on a Tegaderm backing layer, followed by UV irradiation to initiate cross-linking and stabilize the hydrogel structure, with a scale bar indicating a thickness of 6 mm. Finally, part (D) details the extrusion process for creating multifunctional wound dressings on a Tegaderm backing layer, with subsequent UV exposure for cross-linking, and a scale bar indicating 1 cm. The use of food dyes demonstrates the capability to print filaments loaded with distinct active ingredients. This figure is adapted with permission from the American Chemical Society.64
Yang et al. described the creation of a double-layer membrane for joint wound treatment by using solution electrospinning and melt electrowriting techniques. They developed a hydrophilic layer of SBS nonwoven fabric through electrospinning and plasma treatment and a hydrophobic layer of PCL-AgNP scaffolds via melt electrowriting, each with distinct structures. By employing a hybrid manufacturing approach that integrates solution electrospinning (SE) and melt electrowriting (MEW), they produced a double-layer membrane with a hydrophilic electrospun SBS nonwoven fabric treated with oxygen plasma and a hydrophobic MEW scaffold containing PCL and AgNPs. The MEW scaffold was printed in four patterns: lattice, serpentine, sinusoidal, and knit-like. Morphological analysis confirmed the presence of AgNPs in the hydrophobic layer. Mechanical strength evaluation using a tensile tester indicated that the knit-like pattern exhibited superior mechanical performance among the four patterns. The directional water transport performance of the membrane was assessed by using a moisture management tester (MMT), which measured the water contact angle and breakthrough pressure. Results demonstrated efficient directional water transport and excellent moisture management capabilities. Additionally, the presence of AgNPs in the hydrophobic layer conferred high antibacterial activity against E. coli and S. aureus. Thus, the double-layer membrane, fabricated through a combination of solution electrospinning and melt electrowriting, shows promise as a wound dressing for joint wounds, offering stretchability, directional water transport, and antibacterial properties. Further research is needed to evaluate the biocompatibility and in vivo performance of this membrane.65
Mirhaj et al. recently advanced the field of infected wound treatment by developing a biomimetic three-layer scaffold using a combination of 3D printing and electrospinning techniques. The scaffold features a top layer of polyurethane nanofibers, a middle layer of Pluronic F127-quaternized chitosan-silver nitrate, and a bottom layer of core–shell nanofibers composed of F127-mupirocin/pectin-keratin. This innovative structure exhibits significant mechanical strength, a high swelling ratio, and the sustained release of silver ions and mupirocin. These properties result in enhanced antibacterial efficacy, improved cell adhesion and viability, stimulated angiogenesis, and accelerated wound healing. Due to its exceptional biological performance and wound healing capabilities, this scaffold represents a promising advancement in skin tissue engineering, with significant potential for treating skin injuries.44
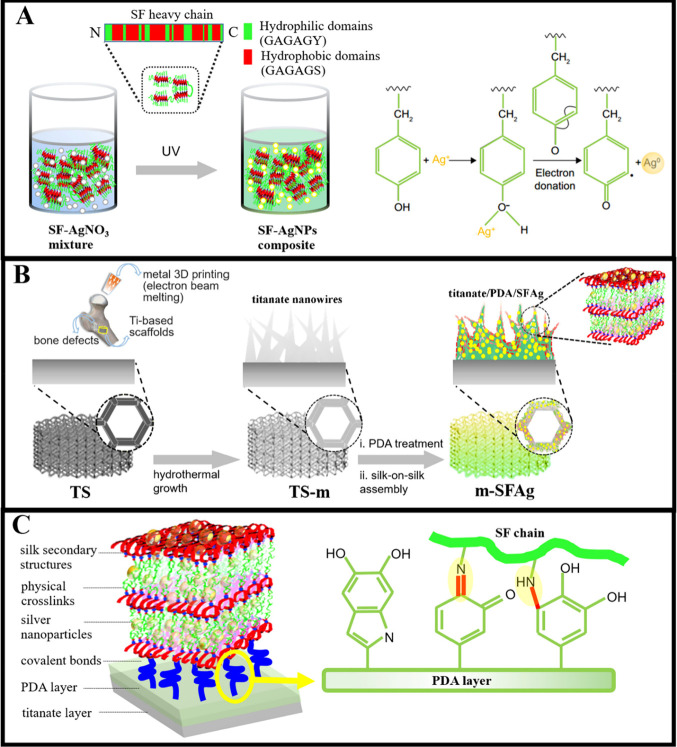
A recent study has introduced a pioneering approach for fabricating 3D-printed titanium scaffolds using biofunctionalized silk protein/nanosilver multilayers, as illustrated in Figure 5. This method aims to address infective bone defects by integrating 3D printing, surface nanomodification, and self-assembly techniques to produce structures with hierarchical organization, antibacterial properties, and osteogenic potential. The release kinetics of Ag+ from the m-SFAg scaffolds show an initial burst followed by sustained release over 6 weeks, with the total Ag+ release remaining below the cytotoxic threshold for MC3T3-E1 cells, ensuring safety. These scaffolds demonstrate significant antibacterial activity against Staphylococcus aureus, with high antibacterial rates observed after 12 h and sustained efficacy under continuous bacterial challenges for 6 weeks. Additionally, they exhibit effective antibiofilm activity, preventing visible biofilm formation on scaffold surfaces and inactivating preformed biofilms on titanium foils. The m-SFAg scaffolds also enhance osteogenic differentiation, indicated by increased alkaline phosphatase (ALP) activity and mineralization compared to control scaffolds while supporting cytoskeletal development and spatial growth of cells. This research suggests that 3D-printed titanium scaffolds biofunctionalized with silk protein/nanosilver multilayers present a promising solution for the tailored restoration of infective bone defects, combining robust antibacterial activity with osteogenic support.70
Figure 5.
Synthesis and structural design of the SFAg composite and its application in biomimetic scaffolds. (A) The SFAg composite is synthesized by mixing silk fibroin (SF) with silver nitrate (AgNO3) and exposing the mixture to UV light. The tyrosine residues in the silk chain play a key role by donating electrons to the Ag+ ions, reducing them to silver nanoparticles (AgNPs), thus forming the SF-AgNP composites. (B) This part details the combinatorial strategy for constructing hierarchically biomimetic and biofunctionalized scaffolds. Initially, a titanium-based scaffold is fabricated by using metal 3D printing with an electron beam melting process, followed by the hydrothermal growth of titania nanowires on this scaffold. The scaffold is then treated with polydopamine (PDA), leading to a silk-on-silk assembly process that results in the formation of m-SFAg. (C) The envisioned m-SFAg architecture comprises multiple layers and interactions among the different components. A titanate layer is hydrothermally grown in situ on the porous titanium scaffold, followed by a tightly adhered self-polymerized PDA layer. The PDA reacts with the silk precursor layer via Michael addition or Schiff base reactions, covalently grafting the silk onto it. Multiple SFAg layers are then constructed through a silk-on-silk assembly process, held together by the physical cross-links of silk secondary structures such as β-sheets. Nanosilver is encapsulated within the SF chains and fully embedded within the multilayered matrix. This figure is adapted with permission from the American Chemical Society.70
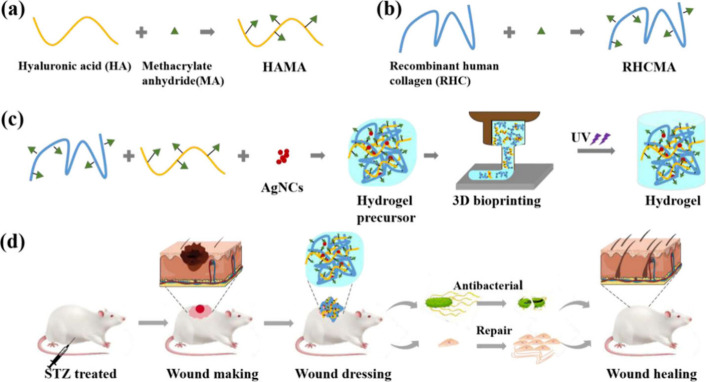
Exploring the capabilities of 3D-printed collagen–hyaluronic acid composite hydrogels fortified with silver nanoclusters, a recent investigation aimed to tackle the challenging issue of chronic diabetic wounds. Acknowledging the pressing need for effective treatments in this area, the study proposed hydrogels as adaptable tools suitable for various wound shapes and sizes through 3D bioprinting (as depicted in Figure 6). Researchers synthesized recombinant human collagen (RHC) and hyaluronic acid (HA), modified with methacrylate groups for UV cross-linking, and incorporated them with silver nanoclusters (AgNCs) for their antibacterial properties. Assessments covering morphology, mechanical properties, rheology, printability, biocompatibility, and in vitro and in vivo wound healing performance highlighted the favorable characteristics of the hydrogels. Notably, these hydrogels demonstrated notable UV responsiveness, porosity, mechanical strength, and biocompatibility. They effectively inhibited bacterial growth while promoting fibroblast proliferation, migration, and tissue regeneration both in vitro and in vivo, indicating a promising therapeutic effect on wound repair. Trials conducted on chronic diabetic wounds in rats demonstrated accelerated wound closure, reduced inflammation, and enhanced tissue regeneration and collagen deposition compared to controls. Histomorphological analysis further confirmed improvements in epidermal structure, gland and hair follicle formation, collagen fiber alignment, and reduction of the level of macrophage infiltration at wound sites. In conclusion, the study advocates for the 3D-printed RHAg hydrogels as promising candidates for addressing chronic diabetic wounds, owing to their UV responsiveness, biocompatibility, antibacterial efficacy, facilitation of cell migration, and potential for tissue regeneration.67
Figure 6.
Intricate overview of the synthesis of RHCMA and HAMA, the formation of RHAg composite hydrogels, and their implications in chronic wound healing, emphasizing their significance in medical and health sciences, particularly in wound healing and tissue engineering. (a) The synthesis of RHCMA involves the combination of recombinant human collagen (RHC) with methacrylate, yielding RHCMA, a crucial compound for hydrogel formation. (b) Similarly, the synthesis of HAMA entails the fusion of hyaluronic acid (HA) with methacrylate anhydride (MA), resulting in HAMA, another vital component of the hydrogel. (c) The formation of RHAg composite hydrogels initiated with a hydrogel precursor comprising AgNCs, which is subsequently transformed into a hydrogel through 3D bioprinting and UV light exposure, rendering it suitable for wound healing applications. (d) Demonstrating practical application, the figure showcases a STZ-treated mouse undergoing wound-making, dressing with antibacterial hydrogel, repair, and eventual healing stages, highlighting the RHAg composite hydrogels’ potential in promoting wound healing. This figure is adapted with permission from the American Chemical Society.67
In a comprehensive investigation, Ahmed et al. conducted an extensive study of the development of 3D-printed scaffolds tailored for skin tissue engineering applications. Utilizing a combination of sodium alginate (Na-ALG), poly(vinyl alcohol) (PVA), and copper–silver-doped mesoporous bioactive glass nanoparticles (Cu–Ag MBGNs), the researchers synthesized scaffolds employing an in-house-built 3D bioprinter and systematically characterized their properties. Investigation of various Na-ALG and PVA solution ratios revealed that the 3:1 ratio exhibited optimal printability and shear thinning behavior. The resulting scaffolds displayed a porous structure, homogeneous elemental distribution, and hydrophilic surfaces with confirmed chemical bonding between Na-ALG, PVA, and Cu–Ag MBGNs. Mechanical testing indicated satisfactory tensile strength and ductility attributed to cross-linking and porosity. Antibacterial evaluations against S. aureus and E. coli demonstrated robust antibacterial activity proportional to Cu–Ag MBGN concentration. Degradation and swelling studies revealed gradual degradation, facilitating water molecule diffusion and ion release. Assessment of angiogenic and cytocompatibility potential showcased the scaffolds’ proangiogenic nature and compatibility with human fibroblast cells. Overall, the study highlights the optimal printability and mechanical stability of the Na-ALG/PVA blend at a 3:1 ratio, the enhanced antibacterial and angiogenic potential conferred by Cu–Ag MBGNs, and the scaffolds’ hydrophilicity, biodegradability, and cytocompatibility.66
Bioactive glasses (BGs) serve as surface-reactive bioceramics capable of degrading within the body, thereby promoting tissue healing. However, their fabrication via 3D printing encounters challenges due to their inherent stiffness and brittleness. To overcome this, a study incorporated mesoporous bioactive glass nanoparticles (MBGNs), known for their increased surface area and porosity compared to traditional BGs, into a polymer matrix consisting of sodium alginate (Na-ALG) and poly(vinyl alcohol) (PVA) to enhance scaffold printability and mechanical properties. Sodium alginate (Na-ALG), a biocompatible and biodegradable polysaccharide, forms hydrogels when cross-linked with calcium ions but exhibits limited mechanical strength and stability. The addition of poly(vinyl alcohol) (PVA) aimed to improve the mechanical integrity and stability of Na-ALG hydrogels while enhancing hydrophilicity and water retention essential for wound healing. Incorporating copper and silver ions provides antibacterial and angiogenic benefits, although high concentrations may pose cytotoxic and inflammatory risks. Utilizing copper–silver doped MBGNs (Cu–Ag MBGNs) enabled controlled release of metal ions, with optimized MBGN composition balancing antibacterial, angiogenic, and cytocompatible properties. The study positions its fabrication approach and material selection as advancements in skin tissue engineering, acknowledging inherent limitations and challenges such as the need for further optimization of printing parameters, long-term scaffold performance evaluation, and the integration of stem cells and growth factors. Thus, the study refrains from claiming unrivaled success but presents a novel and promising avenue that warrants refinement and validation. The findings underscore the potential of 3D-printed Na-ALG/PVA/Cu–Ag MBGN scaffolds as promising candidates for skin regeneration and wound healing applications.66
7.3. Cytotoxicity Concerns of Excipients in 3D-Printed Wound Healing Applications
While the integration of AgNPs with 3D printing for wound healing presents numerous advantages, it is essential to consider the potential cytotoxic effects of the various excipients used in these applications. Excipients, which are inactive substances formulated alongside the active ingredients, can sometimes exhibit cytotoxic behavior, affecting the overall biocompatibility of wound healing devices. Here, we explore several examples of excipients commonly used in 3D-printed wound healing applications and their associated cytotoxic effects.
Poly(lactic acid) (PLA) is a widely used biopolymer in 3D printing due to its biodegradability and mechanical properties. However, its degradation products, primarily lactic acid, can lead to localized decreases in pH, which may induce cytotoxicity and inflammation.219−222 Research has shown that high concentrations of lactic acid can adversely affect cell viability and proliferation, potentially impeding wound healing processes. Polycaprolactone (PCL) is another commonly used polymer in 3D-printed scaffolds. Although it is generally considered biocompatible, the long-term degradation of PCL can result in the release of caproic acid, which can exhibit cytotoxic effects.223−226 Studies have reported that high concentrations of caproic acid can cause cellular damage and inflammation, highlighting the need for careful consideration of PCL’s degradation kinetics in wound healing applications.227,228
The synthesis of AgNPs often involves chemical reducing agents that can pose environmental and biological risks. For instance, sodium borohydride (NaBH4) is a common reducing agent used in AgNP synthesis but is known for its cytotoxicity. The presence of residual NaBH4 in 3D-printed wound dressings can lead to adverse cellular responses, including reduced cell viability and increased oxidative stress.229,230 UV-curable resins, used in some 3D printing processes, often contain photoinitiators that can generate reactive oxygen species (ROS) upon exposure to UV light. These ROS can induce cytotoxic effects, including DNA damage and apoptosis in the surrounding tissues. Common photoinitiators like benzoin methyl ether and camphorquinone have been associated with cytotoxicity in various cell lines.231−233
During the fabrication of 3D-printed scaffolds, solvents are sometimes used to dissolve or process the materials. Residual solvents, such as dichloromethane or dimethyl sulfoxide (DMSO), can remain trapped within the scaffold structure. These solvents are known for their cytotoxic properties, and their gradual release over time can negatively impact cell viability and tissue integration.234−236
The integration of AgNPs and 3D printing technology in wound healing presents a promising frontier, offering the potential for customized, effective, and antibacterial wound care solutions. However, the cytotoxicity associated with certain excipients used in these applications cannot be overlooked. Understanding the interactions between these excipients and biological tissues is crucial for developing safer and more effective wound healing devices. For instance, while PLA and PCL are favored for their biodegradability and mechanical properties, their degradation products can lead to localized cytotoxic effects, necessitating careful consideration of their use in wound healing applications.228,237,238 Similarly, the use of chemical reducing agents in AgNP synthesis and photoinitiators in UV-curable resins highlights the importance of optimizing synthesis and fabrication processes to minimize cytotoxic residues.239,240
To address these challenges, part of future research should focus on developing alternative, nontoxic reducing agents for AgNP synthesis, optimizing polymer compositions and degradation rates to minimize cytotoxic effects, investigating the long-term biocompatibility of residual solvents and other additives, and exploring advanced fabrication techniques that reduce the need for potentially harmful excipients. By addressing these cytotoxicity concerns, researchers can enhance the safety and efficacy of 3D-printed wound healing devices, paving the way for innovative solutions in tissue engineering and regenerative medicine. Moreover, ongoing advancements in material science and 3D printing technologies will likely yield new biocompatible materials and fabrication methods, further improving the landscape of wound healing applications.
7.4. Insights into Skin Dysbiosis
Skin dysbiosis, also termed skin dysbacteriosis, is an imbalance in the skin microbiome, which can lead to various dermatological conditions and impede wound healing. This condition arises when the delicate equilibrium between beneficial and pathogenic microorganisms on the skin is disrupted.241,242 Factors contributing to skin dysbiosis include environmental stressors, underlying health conditions, and the use of antibiotics or antimicrobial agents such as AgNPs.243−245 Silver nanoparticles are known for their potent antimicrobial properties, making them a common component in wound dressings and other medical applications. However, their impact on skin microbiota is a double-edged sword. While AgNPs are effective in eliminating pathogenic bacteria and preventing infections, they may also disrupt the balance of the skin microbiome, potentially leading to dysbiosis.246−249 The mechanisms of dysbiosis induced by AgNPs could include broad-spectrum antibacterial action, which does not differentiate between pathogenic and beneficial microorganisms, potentially reducing the population of commensal bacteria essential for skin health.250 Additionally, the antibacterial mechanism of AgNPs often involves the generation of reactive oxygen species (ROS), which can damage microbial cells. However, this oxidative stress can also harm skin cells and disrupt microbial communities.250−252 Long-term or high-dose exposure to AgNPs can lead to a decrease in microbial diversity, which is crucial for a healthy skin microbiome.196,253,254 The integration of AgNPs with 3D printing technology has revolutionized wound care by enabling the creation of customized wound dressings and scaffolds with enhanced antibacterial properties. These 3D-printed constructs can be tailored to the unique contours of wounds, providing targeted antibacterial reinforcement and promoting optimal healing environments (Table 1). The advantages of combining AgNPs and 3D printing include customization, antibacterial protection, and material integration. Studies have shown that incorporating AgNPs into poly(lactic acid) (PLA) scaffolds enhances their antibacterial properties, making them effective for wound healing applications. The UV irradiation method for synthesizing AgNPs on PLA scaffolds offers a nontoxic and cost-effective approach, ensuring biocompatibility and scalability. Researchers have developed superporous hydrogel dressings incorporating AgNPs, which exhibit high porosity, water absorption, and retention capabilities. These dressings have shown superior efficacy in reducing wound size and preventing scar tissue formation in vivo. Investigations into 3D-printed polycaprolactone (PCL) scaffolds with immobilized AgNPs have demonstrated robust antibacterial activity and biocompatibility. These scaffolds promote angiogenesis and reduce foreign body reactions, making them suitable for skin regeneration applications. The interplay among AgNPs, 3D printing, and skin dysbiosis highlights the need for balanced approaches in medical applications (Table 1). Future research should focus on optimizing the use of AgNPs to harness their antibacterial benefits while minimizing the risk of dysbiosis. Additionally, exploring alternative antimicrobial agents and developing strategies to restore and maintain a healthy skin microbiome will be crucial in advancing wound care and skin regeneration technologies.
7.5. AgNPs as Inducers of Cellular Stress and Proliferation
AgNPs have been shown to induce cellular stress, initiating a complex signaling cascade that can lead to changes in the cell’s structure and increased cell growth. The mechanisms underlying these effects are intricate, involving oxidative stress, modulation of growth factor signaling, and interactions with cellular proteins.255−257 AgNPs disrupt the redox balance within cells, which activates signaling pathways such as MAPK (Mitogen-Activated Protein Kinase)258,259 and PI3K/Akt (Phosphoinositide 3-Kinase/Protein Kinase B).260−264 These pathways are crucial for cell proliferation and survival. Furthermore, AgNPs have been found to promote the release of growth factors and cytokines, which further stimulate cell growth and tissue regeneration.265−270 AgNPs possess significant anti-inflammatory properties due to their ability to modulate immune responses and reduce the production of pro-inflammatory cytokines.271,272 They can inhibit the activation of NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells), a critical transcription factor in the inflammatory response.
Despite these promising findings, several aspects of AgNPs’ interactions with biological systems are still not fully understood. Key areas that require further investigation include the mechanisms of uptake, distribution within the body, excretion, toxicological effects, and detailed mechanisms of action. Gaining a deeper understanding of these parameters is essential for optimizing the use of AgNPs in biomedical applications and minimizing potential adverse effects.
8. Future Studies
Moving forward, the field of 3D printing in wound recovery demonstrates significant potential for further exploration. Subsequent investigations may focus on enhancing the integration of AgNPs into 3D-printed frameworks, exploring alternative antibacterial nanoparticles, and rigorously evaluating the long-term safety and efficacy of these frameworks in wound treatment.
Enduring biocompatibility and in vivo functionality: Despite the potential demonstrated by the integration of 3D printing and nanotechnology in wound recovery, understanding the long-term biocompatibility and in vivo functionality of these 3D-printed structures incorporating AgNPs presents a significant challenge. Biocompatibility refers to a material’s ability to perform its intended function in medical therapy without inducing undesirable local or systemic effects in the recipient. Long-term assessments are crucial to determine whether these structures elicit any adverse reactions upon interaction with bodily tissues over an extended period. Additionally, in vivo functionality concerns how these structures function within a living organism. More comprehensive in vivo evaluations are necessary to evaluate the safety and efficacy of these structures in real-world settings beyond the controlled environment of a laboratory.
Potential cytotoxicity of AgNPs: Despite their antibacterial properties, which make them attractive for wound recovery, AgNPs raise concerns regarding potential cytotoxicity. Cytotoxicity refers to the ability to cause harm to cells, suggesting that while AgNPs can eliminate bacteria they may also pose risks to healthy cells. This issue is especially relevant in wound recovery, where promoting the growth of healthy cells is crucial. Therefore, further investigation is necessary to fully understand the cytotoxic effects of AgNPs within these 3D-printed structures and to develop strategies for mitigating these effects.
These gaps represent significant areas of uncertainty in the current understanding of 3D-printed structures incorporating AgNPs for wound recovery. Addressing these gaps through future research efforts could lead to the development of safer and more effective wound recovery solutions. It is essential for upcoming studies to recognize these limitations and design experiments to specifically investigate these areas. This approach will not only enhance the knowledge base in this field but also ensure the development of solutions that are both effective and safe for patients.
Moreover, as bioprinting advances, there is the potential to create 3D-printed structures that incorporate not only antibacterial nanoparticles but also biological components, such as cells and growth factors. This has the potential to open up new avenues for regenerative medicine and wound healing, expanding the boundaries of the current feasibility.
Therefore, the integration of AgNPs and 3D printing represents a new frontier in wound healing, offering promising opportunities for the advancement of advanced wound care solutions. However, further research is necessary to exploit this potential and address the associated challenges.
9. Conclusion
The integration of silver nanoparticles (AgNPs) with three-dimensional (3D) printing presents a groundbreaking advancement in the field of wound healing. The antibacterial properties of AgNPs, when combined with the customization and precision of 3D printing, offer significant potential for developing advanced wound care solutions. Recent studies have demonstrated the efficacy of AgNP-infused 3D-printed scaffolds in promoting wound healing, reducing infection rates, and enhancing tissue regeneration. These constructs have shown promise in various applications from chronic wound management to bone regeneration, highlighting the versatility and effectiveness of this approach. Despite these promising advancements, several challenges and areas for further research remain. One of the primary challenges is ensuring the uniform dispersion of AgNPs within the 3D-printed structures. Achieving a consistent distribution is crucial for maintaining the antibacterial efficacy and overall functionality of the constructs. Additionally, the potential cytotoxic effects of AgNPs, particularly at higher concentrations, necessitate a thorough investigation to ensure the safety of these materials for clinical use. Understanding the long-term biocompatibility and in vivo performance of AgNP-infused 3D-printed scaffolds is essential to their successful application in wound healing. Future research should focus on optimizing the integration of AgNPs into 3D-printed frameworks, exploring alternative antibacterial nanoparticles, and rigorously evaluating the long-term safety and efficacy of these technologies in clinical settings. Advances in bioprinting techniques could also enable the incorporation of biological components such as cells and growth factors into 3D-printed scaffolds, opening new avenues for regenerative medicine and tissue engineering. Moreover, addressing the potential cytotoxicity and environmental impact of the materials and processes used in the synthesis of AgNPs is critical. Developing ecofriendly and biocompatible synthesis methods will enhance the sustainability and safety of these advanced wound care solutions. Hence, the convergence of AgNPs and 3D printing technology offers a promising frontier in wound healing with the potential to revolutionize the treatment of chronic wounds and other complex medical conditions. Ongoing research and innovation in this field are essential to fully harness the benefits of these technologies and overcome the associated challenges, ultimately leading to more effective and personalized wound healing therapies.
Data Availability Statement
The data sets generated and/or analyzed during the current study are publicly available.
Author Contributions
Mohammad Ebrahim Astaneh: Investigation, Writing—Review & Editing. Narges Fereydouni: Conceptualization, Writing—Original Draft.
The authors declare no competing financial interest.
References
- Gardin C.; Ferroni L.; Latremouille C.; Chachques J. C.; Mitrecic D.; Zavan B. Recent Applications of Three Dimensional Printing in Cardiovascular Medicine. Cells 2020, 9 (3), 742. 10.3390/cells9030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W.; Mei H.; Zhao S. Applications of 3D Bio-Printing in Tissue Engineering and Biomedicine. Journal of biomedical nanotechnology 2021, 17 (6), 989–1006. 10.1166/jbn.2021.3078. [DOI] [PubMed] [Google Scholar]
- Beg S.; Almalki W. H.; Malik A.; Farhan M.; Aatif M.; Rahman Z.; Alruwaili N. K.; Alrobaian M.; Tarique M.; Rahman M. 3D printing for drug delivery and biomedical applications. Drug Discovery Today 2020, 25 (9), 1668–1681. 10.1016/j.drudis.2020.07.007. [DOI] [PubMed] [Google Scholar]
- Hurst E. J. 3D Printing in Healthcare: Emerging Applications. Journal of Hospital Librarianship 2016, 16 (3), 255–267. 10.1080/15323269.2016.1188042. [DOI] [Google Scholar]
- Ma W. C.; Goh G. L.; Priyadarshini B. M.; Yeong W. Y. 3D printing and 3D-printed electronics: Applications and future trends in smart drug delivery devices. Int. J. Bioprint 2023, 9 (4), 725. 10.18063/ijb.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M.; Choudhury H.; Fern J. L. C.; Kee A. T. K.; Kou J.; Jing J. L. J.; Her H. C.; Yong H. S.; Ming H. C.; Bhattamisra S. K.; Gorain B. 3D printing for oral drug delivery: a new tool to customize drug delivery. Drug Delivery and Translational Research 2020, 10 (4), 986–1001. 10.1007/s13346-020-00737-0. [DOI] [PubMed] [Google Scholar]
- Mahmood M. A. 3D Printing in Drug Delivery and Biomedical Applications: A State-of-the-Art Review. Compounds 2021, 1 (3), 94–115. 10.3390/compounds1030009. [DOI] [Google Scholar]
- Kadakia R. J.; Wixted C. M.; Allen N. B.; Hanselman A. E.; Adams S. B. Clinical applications of custom 3D printed implants in complex lower extremity reconstruction. 3D Printing in Medicine 2020, 6, 1–6. 10.1186/s41205-020-00083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreiros F. M. M.; Heuzé Y.; Verius M.; Unterhofer C.; Freysinger W.; Recheis W. Custom implant design for large cranial defects. International Journal of Computer Assisted Radiology and Surgery 2016, 11 (12), 2217–2230. 10.1007/s11548-016-1454-8. [DOI] [PubMed] [Google Scholar]
- Dias J. M.; da Silva F. S. C. P.; Gasik M.; Miranda M. G. M.; Bartolomeu F. J. F. Unveiling additively manufactured cellular structures in hip implants: a comprehensive review. International Journal of Advanced Manufacturing Technology 2024, 130 (9), 4073–4122. 10.1007/s00170-023-12769-0. [DOI] [Google Scholar]
- Brumpt E.; Bertin E.; Tatu L.; Louvrier A. 3D printing as a pedagogical tool for teaching normal human anatomy: a systematic review. BMC Medical Education 2023, 23 (1), 783. 10.1186/s12909-023-04744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartikian M.; Ferreira A.; Gonçalves-Ferreira A.; Neto L. L. 3D printing anatomical models of head bones. Surgical and Radiologic Anatomy 2019, 41 (10), 1205–1209. 10.1007/s00276-018-2148-4. [DOI] [PubMed] [Google Scholar]
- Jones J. F. X.; McCaul C.; Gorman L.; Campbell T.; Pickering M.. 3D Printing in Anatomy. In Teaching Anatomy: A Practical Guide; Chan L. K., Pawlina W., Eds.; Springer International Publishing, 2020; pp 349–357. [Google Scholar]
- Walters-Shumka J. P.; Sorrentino S.; Nygaard H. B.; Willerth S. M. Recent advances in personalized 3D bioprinted tissue models. MRS Bull. 2023, 48 (6), 632–642. 10.1557/s43577-023-00551-2. [DOI] [Google Scholar]
- Zhang Y. S.; Yue K.; Aleman J.; Mollazadeh-Moghaddam K.; Bakht S. M.; Yang J.; Jia W.; Dell’Erba V.; Assawes P.; Shin S. R.; Dokmeci M. R.; Oklu R.; Khademhosseini A. 3D Bioprinting for Tissue and Organ Fabrication. Annals of Biomedical Engineering 2017, 45 (1), 148–163. 10.1007/s10439-016-1612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. H.; Jang J.; Lee J.-S.; Cho D.-W. Current advances in three-dimensional tissue/organ printing. Tissue Engineering and Regenerative Medicine 2016, 13 (6), 612–621. 10.1007/s13770-016-8111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaci A.; Camci-Unal G.; Guvendiren M.; Guest E. Three-dimensional bioprinting for medical applications. MRS Bull. 2023, 48 (6), 624–631. 10.1557/s43577-023-00546-z. [DOI] [Google Scholar]
- Bhatti S. S.; Singh J. 3D printing of biomaterials for biomedical applications: a review. International Journal on Interactive Design and Manufacturing (IJIDeM) 2023, 10.1007/s12008-023-01525-z. [DOI] [Google Scholar]
- Suh D. Y.; Hunt T. K. Time line of wound healing. Clinics in Podiatric Medicine and Surgery 1998, 15 (1), 1–9. 10.1016/S0891-8422(23)01024-8. [DOI] [PubMed] [Google Scholar]
- Fu X. Translation medicine in wound healing: Successful cases and personal deliberation. Chinese Journal of Burns 2014, 30 (1), 3–5. 10.3760/cma.j.issn.1009-2587.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Bodeker G. C.; Ryan T. J.; Ong C. K. Traditional approaches to wound healing. Clinics in Dermatology 1999, 17 (1), 93–98. 10.1016/S0738-081X(98)00056-X. [DOI] [PubMed] [Google Scholar]
- Wu H.; Chen J.; Zhao P.; Liu M.; Xie F.; Ma X. Development and Prospective Applications of 3D Membranes as a Sensor for Monitoring and Inducing Tissue Regeneration. Membranes 2023, 13 (9), 802. 10.3390/membranes13090802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Wang Y.; Teng Y.; Shi J.; Yang X.; Ding Z.; Guo X.; Hou S.; Lv Q. 3D bioprinting: opportunities for wound dressing development. Biomedical Materials (Bristol) 2023, 18 (5), 052001. 10.1088/1748-605X/ace228. [DOI] [PubMed] [Google Scholar]
- Norahan M. H.; Pedroza-González S. C.; Sánchez-Salazar M. G.; Álvarez M. M.; Trujillo de Santiago G. Structural and biological engineering of 3D hydrogels for wound healing. Bioactive Materials 2023, 24, 197–235. 10.1016/j.bioactmat.2022.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseri E.; Ahmadi A. A review on wound dressings: Antimicrobial agents, biomaterials, fabrication techniques, and stimuli-responsive drug release. Eur. Polym. J. 2022, 173, 111293 10.1016/j.eurpolymj.2022.111293. [DOI] [Google Scholar]
- Liu C.; Wang X.; Shu D.; Li S.; Zhnag L.; Cui X.; Li H.; Pan H. Progress on Promoting Wound Healing with Boric Acid/Borosilicate Bioactive Glass. Kuei Suan Jen Hsueh Pao/Journal of the Chinese Ceramic Society 2024, 52 (2), 681–693. 10.14062/j.issn.0454-5648.20230592. [DOI] [Google Scholar]
- Lin Z.; Zhang X.; Chen Y.; Tian Y.; Yang X.; Zhao Z. Negative pressure wound therapy for flap closed-incisions after 3D-printed prosthesis implantation in patients with chronic osteomyelitis with soft tissue defects. BMC Musculoskeletal Disorders 2023, 24 (1), 827. 10.1186/s12891-023-06970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgar S.; Ulag S.; Sahin A.; Gunduz O.; Ustundag C. B. Controlled Delivery of Amoxicillin and Rifampicin by Three-Dimensional Polyvinyl alcohol/Bismuth Ferrite Scaffolds. ChemistrySelect 2023, 10.1002/slct.202204798. [DOI] [Google Scholar]
- Hu Y.; Yang K.; Liu H.; Wang L.; Wang S.; Zhang X.; Qu B.; Yang H. 3D-printed custom implant for the management of “locked” posterior dislocation of the shoulder joint with reverse Hill-Sachs lesion: a case report. Frontiers in Bioengineering and Biotechnology 2023, 11, 1259255 10.3389/fbioe.2023.1259255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereydouni N.; Movaffagh J.; Amiri N.; Darroudi S.; Gholoobi A.; Goodarzi A.; Hashemzadeh A.; Darroudi M. Synthesis of nano-fibers containing nano-curcumin in zein corn protein and its physicochemical and biological characteristics. Scientific Reports 2021, 11 (1), 1902. 10.1038/s41598-020-73678-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereydouni N.; Sadeghnia H. R.; Ghayour Mobarhan M.; Movaffagh J.; Baradaran Rahimi V.; Hashemzadeh A.; Mardani Z.; Darroudi M. Nanoceria: Polyphenol-based green synthesis, mechanism of formation, and evaluation of their cytotoxicity on L929 and HFFF2 cells. Journal of Molecular Structure 2019, 1186, 23–30. 10.1016/j.molstruc.2019.03.014. [DOI] [Google Scholar]
- Fereydouni N.; Zangouei M.; Darroudi M.; Hosseinpour M.; Gholoobi A. Antibacterial activity of chitosan-polyethylene oxide nanofibers containing silver nanoparticles against aerobic and anaerobic bacteria. Journal of Molecular Structure 2023, 1274, 134304. 10.1016/j.molstruc.2022.134304. [DOI] [Google Scholar]
- Al-Harmooshee A. H. W.; Tabrizi M. H.; Roodbari N. H. In vitro pro-apoptotic and anti-metastatic potentials of harmaline-silver containing folate-linked chitosan nano-drug delivery system. Iranian Journal of Basic Medical Sciences 2024, 27 (2), 180–187. 10.22038/IJBMS.2023.71731.15602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nadaf A. H.; Awadallah A.; Thiab S. Superior rat wound-healing activity of green synthesized silver nanoparticles from acetonitrile extract of Juglans regia L: Pellicle and leaves. Heliyon 2024, 10 (2), e24473 10.1016/j.heliyon.2024.e24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asefian S.; Ghavam M. Green and environmentally friendly synthesis of silver nanoparticles with antibacterial properties from some medicinal plants. BMC Biotechnology 2024, 24 (1), 5. 10.1186/s12896-023-00828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaikwad D.; Sutar R.; Patil D. Polysaccharide mediated nanodrug delivery: A review. Int. J. Biol. Macromol. 2024, 261, 129547 10.1016/j.ijbiomac.2024.129547. [DOI] [PubMed] [Google Scholar]
- Meher M. K.; Naidu G.; Mishra A.; Poluri K. M. A review on multifaceted biomedical applications of heparin nanocomposites: Progress and prospects. Int. J. Biol. Macromol. 2024, 260, 129379 10.1016/j.ijbiomac.2024.129379. [DOI] [PubMed] [Google Scholar]
- Niculescu A. G.; Georgescu M.; Marinas I. C.; Ustundag C. B.; Bertesteanu G.; Pinteală M.; Maier S. S.; Al-Matarneh C. M.; Angheloiu M.; Chifiriuc M. C. Therapeutic Management of Malignant Wounds: An Update. Current Treatment Options in Oncology 2024, 25 (1), 97–126. 10.1007/s11864-023-01172-2. [DOI] [PubMed] [Google Scholar]
- Prabhu N.; Nivetha S.; Pooja G.; Nivedhitha B.; Baskamary M.; Pooja S. Mycosynthesis of Nanoparticles and Their Application in Medicine. In. Environmental Science and Engineering 2024, 207–226. 10.1007/978-3-031-45956-6_8. [DOI] [Google Scholar]
- Yayehrad A. T.; Siraj E. A.; Matsabisa M.; Birhanu G. 3D printed drug loaded nanomaterials for wound healing applications. Regenerative Therapy 2023, 24, 361–376. 10.1016/j.reth.2023.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashak Golroudbari H.; Banikarimi S. P.; Ayati A.; Hadizadeh A.; Khorasani Zavareh Z.; Hajikhani K.; Heirani-Tabasi A.; Ahmadi Tafti M.; Davoodi S.; Ahmadi Tafti H. Advanced micro-/nanotechnologies for exosome encapsulation and targeting in regenerative medicine. Clinical and Experimental Medicine 2023, 23 (6), 1845–1866. 10.1007/s10238-023-00993-7. [DOI] [PubMed] [Google Scholar]
- Prete S.; Dattilo M.; Patitucci F.; Pezzi G.; Parisi O. I.; Puoci F. Natural and Synthetic Polymeric Biomaterials for Application in Wound Management. Journal of Functional Biomaterials 2023, 14 (9), 455. 10.3390/jfb14090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini F.; Mirzaei Chegeni M.; Bidaki A.; Zaer M.; Abolhassani H.; Seyedi S. A.; Nabipoorashrafi S. A.; Ashrafnia Menarbazari A.; Moeinzadeh A.; Farmani A. R.; Tavakkoli Yaraki M. 3D-printing-assisted synthesis of paclitaxel-loaded niosomes functionalized by cross-linked gelatin/alginate composite: Large-scale synthesis and in-vitro anti-cancer evaluation. Int. J. Biol. Macromol. 2023, 242, 124697 10.1016/j.ijbiomac.2023.124697. [DOI] [PubMed] [Google Scholar]
- Choi J.; Lee E. J.; Jang W. B.; Kwon S. M. Development of Biocompatible 3D-Printed Artificial Blood Vessels through Multidimensional Approaches. Journal of Functional Biomaterials 2023, 14 (10), 497. 10.3390/jfb14100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L.; Zhou Y.; Zhang J.; Liang H.; Chen X.; Tan H. Natural Polymer-Based Hydrogels: From Polymer to Biomedical Applications. Pharmaceutics 2023, 15 (10), 2514. 10.3390/pharmaceutics15102514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Dutta S. D.; Acharya R.; Park H.; Kim H.; Randhawa A.; Patil T. V.; Ganguly K.; Luthfikasari R.; Lim K. T. Stimuli-Responsive 3D Printable Conductive Hydrogel: A Step toward Regulating Macrophage Polarization and Wound Healing. Adv. Healthcare Mater. 2024, 13 (4), e2302394 10.1002/adhm.202302394. [DOI] [PubMed] [Google Scholar]
- Mirhaj M.; Varshosaz J.; Labbaf S.; Emadi R.; Marcus Seifalian A.; Sharifianjazi F. An antibacterial Multi-Layered scaffold fabricated by 3D printing and electrospinning methodologies for skin tissue regeneration. Int. J. Pharm. 2023, 645, 123357 10.1016/j.ijpharm.2023.123357. [DOI] [PubMed] [Google Scholar]
- Wu X.; Liu S.; Chen K.; Wang F.; Feng C.; Xu L.; Zhang D. 3D printed chitosan-gelatine hydrogel coating on titanium alloy surface as biological fixation interface of artificial joint prosthesis. Int. J. Biol. Macromol. 2021, 182, 669–679. 10.1016/j.ijbiomac.2021.04.046. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Contreras A.; Torres D.; Piñera-Avellaneda D.; Pérez-Palou L.; Ortiz-Hernández M.; Ginebra M. P.; Calero J. A.; Manero J. M.; Rupérez E. Dual-Action Effect of Gallium and Silver Providing Osseointegration and Antibacterial Properties to Calcium Titanate Coatings on Porous Titanium Implants. International Journal of Molecular Sciences 2023, 24 (10), 8762. 10.3390/ijms24108762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Yao X.; Ye J.; Zhang C.; Lin H.; Fu J. A printing-spray-transfer process for attaching biocompatible and antibacterial coatings to the surfaces of patient-specific silicone stents. Biomedical Materials (Bristol) 2020, 10.1088/1748-605X/ab99d6. [DOI] [PubMed] [Google Scholar]
- Jia Z.; Xiu P.; Xiong P.; Zhou W.; Cheng Y.; Wei S.; Zheng Y.; Xi T.; Cai H.; Liu Z.; Wang C.; Zhang W.; Li Z. Additively Manufactured Macroporous Titanium with Silver-Releasing Micro-/Nanoporous Surface for Multipurpose Infection Control and Bone Repair - A Proof of Concept. ACS Appl. Mater. Interfaces 2016, 8 (42), 28495–28510. 10.1021/acsami.6b10473. [DOI] [PubMed] [Google Scholar]
- Huang W.-J.; Wang J. Development of 3D-Printed, Biodegradable, Conductive PGSA Composites for Nerve Tissue Regeneration. Macromol. Biosci. 2023, 23 (3), 2200470. 10.1002/mabi.202200470. [DOI] [PubMed] [Google Scholar]
- Εkonomou S. Ι.; Soe S.; Stratakos A. C. An explorative study on the antimicrobial effects and mechanical properties of 3D printed PLA and TPU surfaces loaded with Ag and Cu against nosocomial and foodborne pathogens. Journal of the Mechanical Behavior of Biomedical Materials 2023, 137, 105536. 10.1016/j.jmbbm.2022.105536. [DOI] [PubMed] [Google Scholar]
- Yurttas E.; Tetik N.; Ayrilmis N. Antimicrobial properties of 3D printed biocomposites with heat-treated wood flour using silver nanoparticles with leaf extract. Wood Material Science and Engineering 2023, 18 (2), 663–671. 10.1080/17480272.2022.2061869. [DOI] [Google Scholar]
- Venkatesan K.; Tchekep A. G. K.; Anadebe V. C.; Mathew A. M.; Sreya P. V.; Rajendran A.; Barik R. C.; Pattanayak D. K. Development of bioactive and antimicrobial nano-topography over selective laser melted Ti6Al4V implant and its in-vitro corrosion behavior. Journal of the Mechanical Behavior of Biomedical Materials 2024, 149, 106210. 10.1016/j.jmbbm.2023.106210. [DOI] [PubMed] [Google Scholar]
- Valencia L. M.; Herrera M.; de la Mata M.; Hernández-Saz J.; Romero-Ocaña I.; Delgado F. J.; Benito J.; Molina S. I. Stereolithography of Semiconductor Silver and Acrylic-Based Nanocomposites. Polymers 2022, 14 (23), 5238. 10.3390/polym14235238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh J. M.; Lim Y. J. L.; Tay J. T.; Cheng H. M.; Tey H. L.; Liang K. Design and fabrication of customizable microneedles enabled by 3D printing for biomedical applications. Bioactive Materials 2024, 32, 222–241. 10.1016/j.bioactmat.2023.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akshaya S.; Rowlo P. K.; Dukle A.; Nathanael A. J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11 (12), 1719. 10.3390/antibiotics11121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S.; Rahman K.; Cheema T. A.; Shakeel M.; Khan A.; Bermak A. Fabrication of Low-Cost Resistance Temperature Detectors and Micro-Heaters by Electrohydrodynamic Printing. Micromachines 2022, 13 (9), 1419. 10.3390/mi13091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah T.; İlyasoğlu G.; Memić A. Designing Lignin-Based Biomaterials as Carriers of Bioactive Molecules. Pharmaceutics 2023, 15 (4), 1114. 10.3390/pharmaceutics15041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaggi G.; Bergamonti L.; Graiff C.; Ossiprandi M. C.; Elviri L. Rapid Prototyping of 3D-Printed AgNPs- and Nano-TiO2-Embedded Hydrogels as Novel Devices with Multiresponsive Antimicrobial Capability in Wound Healing. Antibiotics 2023, 12 (7), 1104. 10.3390/antibiotics12071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninan N.; Joseph B.; Visalakshan R. M.; Bright R.; Denoual C.; Zilm P.; Dalvi Y. B.; Priya P. V.; Mathew A.; Grohens Y.; Kalarikkal N.; Vasilev K.; Thomas S. Plasma assisted design of biocompatible 3D printed PCL/silver nanoparticle scaffolds: in vitro and in vivo analyses. Materials Advances 2021, 2 (20), 6620–6630. 10.1039/D1MA00444A. [DOI] [Google Scholar]
- Mojena-Medina D.; Hubl M.; Bäuscher M.; Jorcano J. L.; Ngo H. D.; Acedo P. Real-time impedance monitoring of epithelial cultures with inkjet-printed interdigitated-electrode sensors. Sensors (Switzerland) 2020, 20 (19), 5711. 10.3390/s20195711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. J.; Wang J. Development of 3D-Printed, Biodegradable, Conductive PGSA Composites for Nerve Tissue Regeneration. Macromol. Biosci. 2023, 10.1002/mabi.202200470. [DOI] [PubMed] [Google Scholar]
- Gao-Qiang S.; Gao-Xing L.; Xi-Wei C.; Jiang-Lin T.; Meng-Long L.; Teng-Fei L.; Xiao-Rong X.; Jun W. A novel 3D printed biomimicry, made from oil infused layer on poly (dimethylsiloxane), promotes infected wound healing through antifouling and antimicrobial activity. Medical Journal of Chinese People’s Liberation Army 2018, 43 (10), 840–848. 10.11855/j.issn.0577-7402.2018.10.06. [DOI] [Google Scholar]
- Deng L.; Deng Y.; Xie K. AgNPs-decorated 3D printed PEEK implant for infection control and bone repair. Colloids Surf., B 2017, 160, 483–492. 10.1016/j.colsurfb.2017.09.061. [DOI] [PubMed] [Google Scholar]
- Alizadehgiashi M.; Nemr C. R.; Chekini M.; Pinto Ramos D.; Mittal N.; Ahmed S. U.; Khuu N.; Kelley S. O.; Kumacheva E. Multifunctional 3D-Printed Wound Dressings. ACS Nano 2021, 15 (7), 12375–12387. 10.1021/acsnano.1c04499. [DOI] [PubMed] [Google Scholar]
- Yang L.; Lou Y.; Zhang G.; Sun Y.; Yang Y.; Wu J.; Ye Y.; Chu X.; Du L.; Jiang Z.; Xu H. Hybrid manufacturing of highly stretchable functionalized membrane for joint wound treatment. Colloids Surf., A 2024, 680, 132655 10.1016/j.colsurfa.2023.132655. [DOI] [Google Scholar]
- Ahmed S.; Hussain R.; Khan A.; Batool S. A.; Mughal A.; Nawaz M. H.; Irfan M.; Wadood A.; Avcu E.; Rehman M. A. U. 3D Printing Assisted Fabrication of Copper–Silver Mesoporous Bioactive Glass Nanoparticles Reinforced Sodium Alginate/Poly(vinyl alcohol) Based Composite Scaffolds: Designed for Skin Tissue Engineering. ACS Appl. Bio Mater. 2023, 6 (11), 5052–5066. 10.1021/acsabm.3c00726. [DOI] [PubMed] [Google Scholar]
- Liang M.; Dong L.; Guo Z.; Liu L.; Fan Z.; Wei C.; Mi S.; Sun W. Collagen–Hyaluronic Acid Composite Hydrogels with Applications for Chronic Diabetic Wound Repair. ACS Biomater. Sci. Eng. 2023, 9 (9), 5376–5388. 10.1021/acsbiomaterials.3c00695. [DOI] [PubMed] [Google Scholar]
- Calamak S.; Ermis M. In situ silver nanoparticle synthesis on 3D-printed polylactic acid scaffolds for biomedical applications. J. Mater. Res. 2021, 36 (1), 166–175. 10.1557/s43578-020-00064-7. [DOI] [Google Scholar]
- Wu Z.; Hong Y. Combination of the Silver–Ethylene Interaction and 3D Printing To Develop Antibacterial Superporous Hydrogels for Wound Management. ACS Appl. Mater. Interfaces 2019, 11 (37), 33734–33747. 10.1021/acsami.9b14090. [DOI] [PubMed] [Google Scholar]
- Jia Z.; Zhou W.; Yan J.; Xiong P.; Guo H.; Cheng Y.; Zheng Y. Constructing Multilayer Silk Protein/Nanosilver Biofunctionalized Hierarchically Structured 3D Printed Ti6Al4 V Scaffold for Repair of Infective Bone Defects. ACS Biomater. Sci. Eng. 2019, 5 (1), 244–261. 10.1021/acsbiomaterials.8b00857. [DOI] [PubMed] [Google Scholar]
- Ghahtan N.; Dehghan N.; Ullah M.; Khoradmehr A.; Habibi H.; Nabipour I.; Baghban N. From seaweed to healing: the potential of fucoidan in wound therapy. Nat. Prod Res. 2024, 1–14. 10.1080/14786419.2024.2358387. [DOI] [PubMed] [Google Scholar]
- Bhatia A.; Prakash S. Topical phenytoin for wound healing. Dermatol Online J. 2004, 10 (1), 5. 10.5070/D30Z3612W1. [DOI] [PubMed] [Google Scholar]
- Totoraitis K.; Cohen J. L.; Friedman A. Topical approaches to improve surgical outcomes and wound healing: A review of efficacy and safety. Journal of Drugs in Dermatology 2017, 16 (3), 209–212. [PubMed] [Google Scholar]
- Khouri C.; Kotzki S.; Roustit M.; Blaise S.; Gueyffier F.; Cracowski J. L. Hierarchical evaluation of electrical stimulation protocols for chronic wound healing: An effect size meta-analysis. Wound Repair and Regeneration 2017, 25 (5), 883–891. 10.1111/wrr.12594. [DOI] [PubMed] [Google Scholar]
- Toleubayev M.; Dmitriyeva M.; Kozhakhmetov S.; Sabitova A. Efficacy of erythropoietin for wound healing: A systematic review of the literature. Ann. Med. Surg (Lond) 2021, 65, 102287 10.1016/j.amsu.2021.102287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Mei Z.; Shi L.; Wan Y.; Zhou X.; Zeng T.; Liu Y.; Yang J. Y.; Shi Z. Evaluation of bevacizumab biosimilar on wound healing complications in patients with colorectal cancer undergoing endoscopic mucosal resection: A systematic review and meta-analysis in anorectal medicine. Int. Wound J. 2024, 21 (1), e14638 10.1111/iwj.14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte M.; Metha G.; Zevenhoven R. Life cycle indicator comparison of copper, silver, zinc and aluminum nanoparticle production through electric arc evaporation or chemical reduction. International Journal of Energy and Environmental Engineering 2015, 6 (3), 233–243. 10.1007/s40095-015-0171-3. [DOI] [Google Scholar]
- Pourzahedi L.; Vance M.; Eckelman M. J. Life Cycle Assessment and Release Studies for 15 Nanosilver-Enabled Consumer Products: Investigating Hotspots and Patterns of Contribution. Environ. Sci. Technol. 2017, 51 (12), 7148–7158. 10.1021/acs.est.6b05923. [DOI] [PubMed] [Google Scholar]
- Jassim A. Y.; Wang J.; Chung K. W.; Loosli F.; Chanda A.; Scott G. I.; Baalousha M. Comparative assessment of the fate and toxicity of chemically and biologically synthesized silver nanoparticles to juvenile clams. Colloids Surf., B 2022, 209, 112173. 10.1016/j.colsurfb.2021.112173. [DOI] [PubMed] [Google Scholar]
- Kaur R.; Singh K.; Agarwal S.; Masih M.; Chauhan A.; Gautam P. K. Silver nanoparticles induces apoptosis of cancer stem cells in head and neck cancer. Toxicology Reports 2024, 12, 10–17. 10.1016/j.toxrep.2023.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheybari S.; Samadi N.; Hosseini S. V.; Fazeli A.; Fazeli M. R. Synthesis and antimicrobial effects of silver nanoparticles produced by chemical reduction method. DARU, Journal of Pharmaceutical Sciences 2010, 18 (3), 168–172. [PMC free article] [PubMed] [Google Scholar]
- Saadh M. J. Synthesis, characterization, and applications of silver nanoparticles. Pharmacologyonline 2021, 3, 2040–2045. [Google Scholar]
- Lytvyn S. Y.; Kurapov Y. A.; Ruban N. M.; Churkina L. N.; Andrusyshyna I. M.; Didikin G. G.; Boretskyi V. V. Influence of temperature on the physical properties and bio-activity of pure (ligand-free) EB PVD silver nanoparticles. Applied Nanoscience (Switzerland) 2023, 13 (7), 5171–5183. 10.1007/s13204-022-02730-0. [DOI] [Google Scholar]
- Kurapov Y.; Litvin S.; Romanenko S. M.; Didikin G.; Belyavina N. Synthesis of copper and silver nanoparticles by molecular beam method. Micro and Nanosystems 2018, 10 (2), 148–157. 10.2174/1876402911666181128101907. [DOI] [Google Scholar]
- Gundo S.; Parauha Y. R.; Singh N.; Dhoble S. J. Eco-friendly synthesis route of silver nanoparticle: A review. In. Journal of Physics: Conference Series 2021, 1913, 012052. 10.1088/1742-6596/1913/1/012052. [DOI] [Google Scholar]
- Grzesiakowska A.; Kasprowicz M. J.; Kuchta-Gładysz M.; Rymuza K.; Szeleszczuk O. Genotoxicity of physical silver nanoparticles, produced by the HVAD method, for Chinchilla lanigera genome. Sci. Rep. 2021, 10.1038/s41598-021-97926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorashi S. A. A.; Kamali M. Synthesis of Silver Nanoparticles Using Complexing Agent Method: Comparing the Effect of Ammonium Hydroxide and Nitric Acid on Some Physical Properties of Nanoparticles. Journal of Cluster Science 2011, 22 (4), 667–672. 10.1007/s10876-011-0413-3. [DOI] [Google Scholar]
- Farheen A. Methodologies for synthesizing silver nanoparticles. In Green Synthesis of Silver Nanomaterials 2022, 589–606. 10.1016/B978-0-12-824508-8.00024-1. [DOI] [Google Scholar]
- Vivekanandhan S.; Misra M.; Mohanty A. K. Biological synthesis of silver nanoparticles using Glycine max (Soybean) leaf extract: An investigatioion different soybean varieties. J. Nanosci. Nanotechnol. 2009, 9 (12), 6828–6833. 10.1166/jnn.2009.2201. [DOI] [PubMed] [Google Scholar]
- Iqbal M. J.; Ali S.; Rashid U.; Kamran M.; Malik M. F.; Sughra K.; Zeeshan N.; Afroz A.; Saleem J.; Saghir M. Biosynthesis of silver nanoparticles from leaf extract of Litchi chinensis and its dynamic biological impact on microbial cells and human cancer cell lines. Cellular and Molecular Biology 2018, 64 (13), 42–47. 10.14715/cmb/2018.64.13.9. [DOI] [PubMed] [Google Scholar]
- Barbhuiya R. I.; Singha P.; Asaithambi N.; Singh S. K. Ultrasound-assisted rapid biological synthesis and characterization of silver nanoparticles using pomelo peel waste. Food Chem. 2022, 385, 132602. 10.1016/j.foodchem.2022.132602. [DOI] [PubMed] [Google Scholar]
- Srivastava A.; Chauhan P. S.; Singh R. Characterization of Stress-Tolerant Bacteria for the Biosynthesis of Silver Nanoparticles and their Applications. Journal of Nano Research 2021, 68, 70–80. 10.4028/www.scientific.net/JNanoR.68.70. [DOI] [Google Scholar]
- Johnson D. L.; Wang Y.; Stealey S. T.; Alexander A. K.; Kaltchev M. G.; Chen J.; Zhang W. Biosynthesis of silver nanoparticles using upland cress: Purification, characterisation, and antimicrobial activity. Micro and Nano Letters 2020, 15 (2), 110–113. 10.1049/mnl.2019.0528. [DOI] [Google Scholar]
- Farrag H. M. M.; Mostafa F. A. A. M.; Mohamed M. E.; Huseein E. A. M. Green biosynthesis of silver nanoparticles by Aspergillus niger and its antiamoebic effect against Allovahlkampfia spelaea trophozoite and cyst. Experimental Parasitology 2020, 219, 108031. 10.1016/j.exppara.2020.108031. [DOI] [PubMed] [Google Scholar]
- Ezzat A. O.; Aigbodion V. S.; Ohiemi I. E. Multifaceted incorporation of epoxy/functionalized maize cob nanoparticle with biosynthesis silver nanoparticles for composite coating of mild steel. International Journal of Advanced Manufacturing Technology 2024, 130 (3–4), 1465–1476. 10.1007/s00170-023-12793-0. [DOI] [Google Scholar]
- Taghavizadeh Yazdi M. E.; Hamidi A.; Amiri M. S.; Kazemi Oskuee R.; Hosseini H. A.; Hashemzadeh A.; Darroudi M. Eco-friendly and plant-based synthesis of silver nanoparticles using Allium giganteum and investigation of its bactericidal, cytotoxicity, and photocatalytic effects. Materials Technology 2019, 34 (8), 490–497. 10.1080/10667857.2019.1583408. [DOI] [Google Scholar]
- Maleki P.; Nemati F.; Gholoobi A.; Hashemzadeh A.; Sabouri Z.; Darroudi M. Green facile synthesis of silver-doped cerium oxide nanoparticles and investigation of their cytotoxicity and antibacterial activity. Inorg. Chem. Commun. 2021, 131, 108762. 10.1016/j.inoche.2021.108762. [DOI] [Google Scholar]
- Elahi N. J.; Salehmoghadam M.; Taherzadeh D.; Hashemzadeh A.; Darroudi M. Ammonia sensing and cytotoxicity of the biosynthesized silver nanoparticle by arabic gum (AG). Recent patents on biotechnology 2019, 13 (3), 228–238. 10.2174/1872208313666190118123141. [DOI] [PubMed] [Google Scholar]
- Asgharzadeh F.; Hashemzadeh A.; Yaghoubi A.; Avan A.; Nazari S. E.; Soleimanpour S.; Hassanian S. M.; Ferns G. A.; Rahmani F.; Khazaei M. Therapeutic effects of silver nanoparticle containing sulfasalazine on DSS-induced colitis model. Journal of Drug Delivery Science and Technology 2021, 61, 102133. 10.1016/j.jddst.2020.102133. [DOI] [Google Scholar]
- Sivalingam A. M.; Pandian A.; Rengarajan S.; Ramasubbu R. Polyphenol-compounds From Green Synthesis of Antimicrobial property of Silver Nanoparticles using Eichhornia crassipes: Characterization and Applications. Silicon 2023, 15 (17), 7415–7429. 10.1007/s12633-023-02593-2. [DOI] [Google Scholar]
- Shinde M.; Patil R.; Karmakar S.; Bhoraskar S.; Rane S.; Gade W.; Amalnerkar D. Antimicrobial properties of uncapped silver nanoparticles synthesized by DC arc thermal plasma technique. J. Nanosci. Nanotechnol. 2012, 12 (2), 887–893. 10.1166/jnn.2012.5152. [DOI] [PubMed] [Google Scholar]
- Safari S.; Barani M.; Sadrmohammadi R. Antimicrobial properties of tissue conditioner modified with chitosan and green-synthesized silver nanoparticles: a promising approach for preventing denture stomatitis. BMC Oral Health 2024, 10.1186/s12903-024-03880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A.; Akpobolokemi T.; Chung E.; Ren G.; Raimi-Abraham B. T. pH Alteration in Plant-Mediated Green Synthesis and Its Resultant Impact on Antimicrobial Properties of Silver Nanoparticles (AgNPs). Antibiotics 2022, 11 (11), 1592. 10.3390/antibiotics11111592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. S.; Madhu G.; John E.; Kuttinarayanan S. V.; Nair S. K. Optical and antimicrobial properties of silver nanoparticles synthesized via green route using honey. Green Processing and Synthesis 2020, 9 (1), 268–274. 10.1515/gps-2020-0029. [DOI] [Google Scholar]
- Kalam A.; Al-Sehemi A. G.; Alrumman S.; Assiri M. A.; Alfaify A. M.; Moustafa M. F. Antimicrobial properties of silver nanoparticles prepared by Acacia etbaica (Schweinf.) valve extract. Mater. Lett. 2021, 300, 130233. 10.1016/j.matlet.2021.130233. [DOI] [Google Scholar]
- Gumber S.; Kanwar S.; Mazumder K. Properties and antimicrobial activity of wheat-straw nanocellulose-arabinoxylan acetate composite films incorporated with silver nanoparticles. Int. J. Biol. Macromol. 2023, 246, 125480. 10.1016/j.ijbiomac.2023.125480. [DOI] [PubMed] [Google Scholar]
- Rahman H.; Kumar V.; Singh P.; Kumar A. Catalytic effect of silver nanoparticles on ZnO surface for CO gas-sensing applications. Applied Nanoscience (Switzerland) 2022, 12 (11), 3517–3527. 10.1007/s13204-022-02423-8. [DOI] [Google Scholar]
- Mahajan P. G.; Dige N. C.; Vanjare B. D.; Phull A. R.; Kim S. J.; Lee K. H. Gallotannin mediated silver colloidal nanoparticles as multifunctional nano platform: Rapid colorimetric and turn-on fluorescent sensor for Hg2+, catalytic and In vitro anticancer activities. J. Lumin. 2019, 206, 624–633. 10.1016/j.jlumin.2018.10.095. [DOI] [Google Scholar]
- Lee S. J.; Begildayeva T.; Yeon S.; Naik S. S.; Ryu H.; Kim T. H.; Choi M. Y. Eco-friendly synthesis of lignin mediated silver nanoparticles as a selective sensor and their catalytic removal of aromatic toxic nitro compounds. Environ. Pollut. 2021, 269, 116174. 10.1016/j.envpol.2020.116174. [DOI] [PubMed] [Google Scholar]
- Jothi Ramalingam R.; Arunachalam P.; Amer M. S.; AlOthman Z. A.; Alanazi A. G.; Al-Anazy M. M.; A Al-Lohedan H.; Mohammed Dahan W. Facile sonochemical synthesis of silver nanoparticle and graphene oxide deposition on bismuth doped manganese oxide nanotube composites for electro-catalytic sensor and oxygen reduction reaction (ORR) applications. Intermetallics 2021, 131, 107101. 10.1016/j.intermet.2021.107101. [DOI] [Google Scholar]
- Al-Onazi W. A.; Abdel-Lateef M. A. Catalytic oxidation of O-phenylenediamine by silver nanoparticles for resonance Rayleigh scattering detection of mercury (II) in water samples. Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy 2022, 264, 120258. 10.1016/j.saa.2021.120258. [DOI] [PubMed] [Google Scholar]
- Izak-Nau E.; Huk A.; Reidy B.; Uggerud H.; Vadset M.; Eiden S.; Voetz M.; Himly M.; Duschl A.; Dusinska M.; Lynch I. Impact of storage conditions and storage time on silver nanoparticles’ physicochemical properties and implications for their biological effects. RSC Adv. 2015, 5 (102), 84172–84185. 10.1039/C5RA10187E. [DOI] [Google Scholar]
- Calderón-Jiménez B.; Johnson M. E.; Montoro Bustos A. R.; Murphy K. E.; Winchester M. R.; Vega Baudrit J. R. Silver Nanoparticles: Technological Advances, Societal Impacts, and Metrological Challenges. Frontiers in Chemistry 2017, 10.3389/fchem.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zou’by J. Y.; Alsamarraie L. a. A.; Al-Zboon K. K. A study of the physicochemical properties of silver nanoparticles dispersed in various water chemistry settings. J. Nanopart. Res. 2023, 25 (12), 239. 10.1007/s11051-023-05895-z. [DOI] [Google Scholar]
- Ritu; Verma K. K.; Das A.; Chandra P. Phytochemical-Based Synthesis of Silver Nanoparticle: Mechanism and Potential Applications. BioNanoScience 2023, 13 (3), 1359–1380. 10.1007/s12668-023-01125-x. [DOI] [Google Scholar]
- Xu Y.; Yan J.; Zhu Y.; Chen H.; Wu C.; Zhu X.; Zhang Y.; Li H.; Liu M.; Yao S. Self-Cascade Nanoenzyme of Cupric Oxide Nanoparticles (CuO NPs) Induced in Situ Catalysis Formation of Polyelectrolyte as Template for the Synthesis of Near-Infrared Fluorescent Silver Nanoclusters and the Application in Glutathione Detection and Bioimaging. Anal. Chem. 2022, 94 (42), 14642–14651. 10.1021/acs.analchem.2c02832. [DOI] [PubMed] [Google Scholar]
- Wang R.; Liu H.; Meng X.; Qian Y.; Wang X.; Zhu F.; Nie R.; Wang H. In situ immobilization of silver nanocrystals in carbon nanoparticles for intracellular fluorescence imaging and hydroxyl radicals detection. J. Colloid Interface Sci. 2022, 608, 2672–2680. 10.1016/j.jcis.2021.10.195. [DOI] [PubMed] [Google Scholar]
- Wang J.; Moore J.; Laulhe S.; Nantz M.; Achilefu S.; Kang K. A. Fluorophoregold nanoparticle complex for sensitive optical biosensing and imaging. Nanotechnology 2012, 23 (9), 095501. 10.1088/0957-4484/23/9/095501. [DOI] [PubMed] [Google Scholar]
- Teixeira M. I.; Lopes C. M.; Amaral M. H.; Costa P. C. Silver nanoparticles for the management of neurological diseases. Silver Nanoparticles for Drug Delivery 2024, 209–239. 10.1016/B978-0-443-15343-3.00002-4. [DOI] [Google Scholar]
- Rhee K.; Tukova A.; Tavakkoli Yaraki M.; Wang Y. Nanosupernova: a new anisotropic nanostructure for SERS. Nanoscale 2023, 15 (5), 2087–2095. 10.1039/D2NR05287C. [DOI] [PubMed] [Google Scholar]
- Paul G.; Gupta U.; Shah H.; Mazahir F.; Yadav A. K. Inorganic and metal-based nanoparticles. Molecular Pharmaceutics and Nano Drug Delivery: Fundamentals and Challenges 2024, 203–235. 10.1016/B978-0-323-91924-1.00006-X. [DOI] [Google Scholar]
- Pan M.; Liang M.; Sun J.; Liu X.; Wang F. Lighting Up Fluorescent Silver Clusters via Target-Catalyzed Hairpin Assembly for Amplified Biosensing. Langmuir 2018, 34 (49), 14851–14857. 10.1021/acs.langmuir.8b01576. [DOI] [PubMed] [Google Scholar]
- Li S.; Xu L.; Sun M.; Wu X.; Liu L.; Kuang H.; Xu C. Hybrid Nanoparticle Pyramids for Intracellular Dual MicroRNAs Biosensing and Bioimaging. Adv. Mater. 2017, 10.1002/adma.201606086. [DOI] [PubMed] [Google Scholar]
- Kotcherlakota R.; Das S.; Patra C. R. Therapeutic applications of green-synthesized silver nanoparticles. Green Synthesis, Characterization and Applications of Nanoparticles 2018, 389–428. 10.1016/B978-0-08-102579-6.00017-4. [DOI] [Google Scholar]
- Yang L.; Feng H. Y.; Xu Y. J. Current status of 3D printing medical applications and center construction. Chinese Journal of Tissue Engineering Research 2023, 27 (13), 2110–2115. 10.12307/2023.294. [DOI] [Google Scholar]
- Stolz B.; Mönkemeyer F.; Mader M.; Schmidt S.; Volk L.; Steinberg T.; Bruchmann B.; Mülhaupt R. Polyhydroxymethylenes as Multifunctional High Molecular Weight Sugar Alcohols Tailored for 3D Printing and Medical Applications. Macromol. Chem. Phys. 2020, 10.1002/macp.202000132. [DOI] [Google Scholar]
- Shannon A.; O’Connell A.; O’Sullivan A.; Byrne M.; Clifford S.; O’Sullivan K. J.; O’Sullivan L. A Radiopaque Nanoparticle-Based Ink Using PolyJet 3D Printing for Medical Applications. 3D Printing and Additive Manufacturing 2020, 7 (6), 259–268. 10.1089/3dp.2019.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B.; Wang H.; Zhang T. Application Research and Prospect of 3D Modeling, Printing Medical Course Teaching Aids. Recent Innovations in Chemical Engineering 2023, 16 (3), 158–166. 10.2174/2405520416666230809124219. [DOI] [Google Scholar]
- Mirtaheri P.; Güler E.; Gjoovaag T.. 3D printing in medical application: An educational design perspective. Proceedings of the 19th International Conference on Engineering and Product Design Education: Building Community: Design Education for a Sustainable Future, E and PDE; 2017; Vol. 2017, pp 176–181.
- Arefin A. M. E.; Khatri N. R.; Kulkarni N.; Egan P. F. Polymer 3D printing review: Materials, process, and design strategies for medical applications. Polymers 2021, 13 (9), 1499. 10.3390/polym13091499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson H. A.; Dubé A. K. 3D printing as an educational technology: theoretical perspectives, learning outcomes, and recommendations for practice. Education and Information Technologies 2022, 27 (3), 3037–3064. 10.1007/s10639-021-10733-7. [DOI] [Google Scholar]
- Bai W.; Fang H.; Wang Y.; Zeng Q.; Hu G.; Bao G.; Wan Y. Academic Insights and Perspectives in 3D Printing: A Bibliometric Review. Applied Sciences 2021, 11 (18), 8298. 10.3390/app11188298. [DOI] [Google Scholar]
- Tetsuka H.; Shin S. R. Materials and technical innovations in 3D printing in biomedical applications. J. Mater. Chem. B 2020, 8 (15), 2930–2950. 10.1039/D0TB00034E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack P.; Victor J.; Gemmel P.; Annemans L. 3D-printing techniques in a medical setting: a systematic literature review. BioMedical Engineering OnLine 2016, 15 (1), 115. 10.1186/s12938-016-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N. R.; Subburaj K.; Sandhu K.; Sharma V.. Applications of 3D printing in Biomedical Engineering; Springer, 2021. [Google Scholar]
- Alzoubi L.; Aljabali A. A. A.; Tambuwala M. M. Empowering Precision Medicine: The Impact of 3D Printing on Personalized Therapeutic. AAPS PharmSciTech 2023, 24 (8), 228. 10.1208/s12249-023-02682-w. [DOI] [PubMed] [Google Scholar]
- Jamróz W.; Szafraniec J.; Kurek M.; Jachowicz R. 3D Printing in Pharmaceutical and Medical Applications – Recent Achievements and Challenges. Pharm. Res. 2018, 35 (9), 176. 10.1007/s11095-018-2454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.; Bit A. 3D bioprinting in tissue engineering and regenerative medicine. Cell and Tissue Banking 2022, 23 (2), 199–212. 10.1007/s10561-021-09936-6. [DOI] [PubMed] [Google Scholar]
- Jovic T. H.; Combellack E. J.; Jessop Z. M.; Whitaker I. S. 3D Bioprinting and the Future of Surgery. Frontiers in surgery 2020, 7, 609836. 10.3389/fsurg.2020.609836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. G.Bioprinting: Techniques and Risks for Regenerative Medicine; Elsevier Science, 2017. [Google Scholar]
- Edgar L.; Pu T.; Porter B.; Aziz J. M.; La Pointe C.; Asthana A.; Orlando G. Regenerative medicine, organ bioengineering and transplantation. British Journal of Surgery 2020, 107 (7), 793–800. 10.1002/bjs.11686. [DOI] [PubMed] [Google Scholar]
- Agarwal S.; Saha S.; Balla V. K.; Pal A.; Barui A.; Bodhak S. Current Developments in 3D Bioprinting for Tissue and Organ Regeneration–A Review. Frontiers in Mechanical Engineering 2020, 10.3389/fmech.2020.589171. [DOI] [Google Scholar]
- Zhang M.; Zhang C.; Li Z.; Fu X.; Huang S. Advances in 3D skin bioprinting for wound healing and disease modeling. Regenerative Biomaterials 2023, 10.1093/rb/rbac105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadhif M. H.; Assyarify H.; Irsyad M.; Pramesti A. R.; Suhaeri M. Recent advances in 3D printed wound dressings. AIP Conf. Proc. 2021, 10.1063/5.0047183. [DOI] [Google Scholar]
- Choudary R.; Saini N.; Chopra D. S.; Singh D.; Singh N. A comprehensive review of 3D bioprinting biomaterials: Properties, strategies and wound healing application. J. Mater. Res. 2023, 38 (13), 3264–3300. 10.1557/s43578-023-01078-7. [DOI] [Google Scholar]
- Lodha S.; Song B.; Park S.-I.; Choi H.-J.; Lee S. W.; Park H. W.; Choi S.-K. Sustainable 3D printing with recycled materials: a review. Journal of Mechanical Science and Technology 2023, 37 (11), 5481–5507. 10.1007/s12206-023-1001-9. [DOI] [Google Scholar]
- Mikula K.; Skrzypczak D.; Izydorczyk G.; Warchoł J.; Moustakas K.; Chojnacka K.; Witek-Krowiak A. 3D printing filament as a second life of waste plastics—a review. Environmental Science and Pollution Research 2021, 28 (10), 12321–12333. 10.1007/s11356-020-10657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velásquez-García L. F.; Kornbluth Y. Biomedical applications of metal 3D printing. Annu. Rev. Biomed. Eng. 2021, 23, 307–338. 10.1146/annurev-bioeng-082020-032402. [DOI] [PubMed] [Google Scholar]
- Gupta M. 3D Printing of Metals. Metals 2017, 7 (10), 403. 10.3390/met7100403. [DOI] [Google Scholar]
- Babu S. S.; Bourell D. L.; Das S. Metallic materials for 3D printing. MRS Bull. 2016, 41 (10), 729–741. 10.1557/mrs.2016.217. [DOI] [Google Scholar]
- Hussain M. I.; Xia M.; Ren X.; Ge C.; Jamil M.; Gupta M. K. Digital light processing 3D printing of ceramic materials: a review on basic concept, challenges, and applications. International Journal of Advanced Manufacturing Technology 2024, 130 (5), 2241–2267. 10.1007/s00170-023-12847-3. [DOI] [Google Scholar]
- Abdelkader M.; Petrik S.; Nestler D.; Fijalkowski M. Ceramics 3D Printing: A Comprehensive Overview and Applications, with Brief Insights into Industry and Market. Ceramics 2024, 7 (1), 68–85. 10.3390/ceramics7010006. [DOI] [Google Scholar]
- Chen Z.; Sun X.; Shang Y.; Xiong K.; Xu Z.; Guo R.; Cai S.; Zheng C. Dense ceramics with complex shape fabricated by 3D printing: A review. Journal of Advanced Ceramics 2021, 10 (2), 195–218. 10.1007/s40145-020-0444-z. [DOI] [Google Scholar]
- Sohl J.; Dias J.. 3D Printing of Ceramics for Modern Medical Engineering. In Additive Manufacturing in Multidisciplinary Cooperation and Production; Drstvensek I., Pal S., Ihan Hren N., Eds.; Springer International Publishing, 2024; pp 235–243. [Google Scholar]
- Santoni S.; Gugliandolo S. G.; Sponchioni M.; Moscatelli D.; Colosimo B. M. 3D bioprinting: current status and trends—a guide to the literature and industrial practice. Bio-Design and Manufacturing 2022, 5 (1), 14–42. 10.1007/s42242-021-00165-0. [DOI] [Google Scholar]
- Suntornnond R.; An J.; Chua C. K. Roles of support materials in 3D bioprinting – Present and future. IJB 2017, 3 (1), 83. 10.18063/IJB.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovic T. H.; Kungwengwe G.; Mills A. C.; Whitaker I. S. Plant-Derived Biomaterials: A Review of 3D Bioprinting and Biomedical Applications. Frontiers in Mechanical Engineering 2019, 10.3389/fmech.2019.00019. [DOI] [Google Scholar]
- de Faria J. V. G.; Pontes L. M.; Bomfim C. C.; Ciuffi K. J.; Rocha L. A.; Nassar E. J.; da Silva J. V. L.; de Oliveira M. F.; Maia I. A. Incorporation of silver nanoparticles into polyamide powder for application in 3D printing. Progress in Additive Manufacturing 2023, 8 (6), 1423–1431. 10.1007/s40964-023-00410-1. [DOI] [Google Scholar]
- Larder R. R.; Krumins E.; Jacob P. L.; Kortsen K.; Cavanagh R.; Jiang L.; Vuotto C.; Francolini I.; Tuck C.; Taresco V.; Howdle S. M. Antimicrobial ‘inks’ for 3D printing: block copolymer-silver nanoparticle composites synthesised using supercritical CO2. Polym. Chem. 2022, 13 (25), 3768–3779. 10.1039/D2PY00398H. [DOI] [Google Scholar]
- Ruiz S.; Wang F.; Liu L.; Lu Y.; Duan B.; Korshoj L. E.; Kielian T.; Cui B. Antibacterial properties of silver nanoparticles synthesized via nanosecond pulsed laser ablation in water. Journal of Laser Applications 2022, 10.2351/7.0000603. [DOI] [Google Scholar]
- Ronca A.; Abbate V.; Redaelli D. F.; Storm F. A.; Cesaro G.; De Capitani C.; Sorrentino A.; Colombo G.; Fraschini P.; Ambrosio L. A Comparative Study for Material Selection in 3D Printing of Scoliosis Back Brace. Materials 2022, 15 (16), 5724. 10.3390/ma15165724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. L.Multi-material 3D printing guided by machine vision; Nature Publishing Group UK: London, 2023. [DOI] [PubMed] [Google Scholar]
- Nguyen T. T.; Tran V. T.; Pham T. H. N.; Nguyen V.-T.; Thanh N. C.; Thi H. M. N.; Duy N. V. A.; Thanh D. N.; Nguyen V. T. T. Influences of Material Selection, Infill Ratio, and Layer Height in the 3D Printing Cavity Process on the Surface Roughness of Printed Patterns and Casted Products in Investment Casting. Micromachines 2023, 14 (2), 395. 10.3390/mi14020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain N.; Chowdhury M. A.; Shuvho M. B. A.; Kashem M. A.; Kchaou M. 3D-Printed Objects for Multipurpose Applications. Journal of Materials Engineering and Performance 2021, 30 (7), 4756–4767. 10.1007/s11665-021-05664-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J.; Leong K. F. Multi-material and Multi-dimensional 3D Printing for Biomedical Materials and Devices. Biomedical Materials & Devices 2023, 1 (1), 38–48. 10.1007/s44174-022-00038-9. [DOI] [Google Scholar]
- Espalin D.; Muse D. W.; MacDonald E.; Wicker R. B. 3D Printing multifunctionality: structures with electronics. International Journal of Advanced Manufacturing Technology 2014, 72 (5), 963–978. 10.1007/s00170-014-5717-7. [DOI] [Google Scholar]
- Jagadeesh P.; Puttegowda M.; Rangappa S. M.; Alexey K.; Gorbatyuk S.; Khan A.; Doddamani M.; Siengchin S. A comprehensive review on 3D printing advancements in polymer composites: technologies, materials, and applications. International Journal of Advanced Manufacturing Technology 2022, 121 (1), 127–169. 10.1007/s00170-022-09406-7. [DOI] [Google Scholar]
- Jacob S.; Nair A. B.; Patel V.; Shah J. 3D Printing Technologies: Recent Development and Emerging Applications in Various Drug Delivery Systems. AAPS PharmSciTech 2020, 21 (6), 220. 10.1208/s12249-020-01771-4. [DOI] [PubMed] [Google Scholar]
- Ishutov S.; Hodder K.; Chalaturnyk R.; Zambrano-Narvaez G. A 3D printing Short Course: A Case Study for Applications in the Geoscience Teaching and Communication for Specialists and Non-experts. Frontiers in Earth Science 2021, 10.3389/feart.2021.601530. [DOI] [Google Scholar]
- Mao S.; Man J.; Wang J.; Fu L.; Yin C.; Karimi-Maleh H. Research progress and challenges of bioprinting in wound dressing and healing: Bibliometrics-based analysis and perspectives. IJB 2023, 9 (2), 653. 10.18063/ijb.v9i2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian Q.; Jiao T.; Zhao T.; Wang H.; Yang S.; Li D. 3D Bioprinted Skin Substitutes for Accelerated Wound Healing and Reduced Scar. Journal of Bionic Engineering 2021, 18 (4), 900–914. 10.1007/s42235-021-0053-8. [DOI] [Google Scholar]
- Luo Y.; Xu X.; Ye Z.; Xu Q.; Li J.; Liu N.; Du Y. 3D bioprinted mesenchymal stromal cells in skin wound repair. Frontiers in Surgery 2022, 10.3389/fsurg.2022.988843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning X.; Liu T.; Wu C.; Wang C. 3D Printing in Construction: Current Status, Implementation Hindrances, and Development Agenda. Advances in Civil Engineering 2021, 2021, 6665333 10.1155/2021/6665333. [DOI] [Google Scholar]
- El-Sayegh S.; Romdhane L.; Manjikian S. A critical review of 3D printing in construction: Benefits, challenges, and risks. Archives of Civil and Mechanical Engineering 2020, 20, 1–25. 10.1007/s43452-020-00038-w. [DOI] [Google Scholar]
- Ferrero Guillén R. From enemies to allies: 3D printing, IP and sustainability. Journal of Intellectual Property Law & Practice 2023, 18 (5), 375–381. 10.1093/jiplp/jpad040. [DOI] [Google Scholar]
- Chadha U.; Abrol A.; Vora N. P.; Tiwari A.; Shanker S. K.; Selvaraj S. K. Performance evaluation of 3D printing technologies: a review, recent advances, current challenges, and future directions. Progress in Additive Manufacturing 2022, 7 (5), 853–886. 10.1007/s40964-021-00257-4. [DOI] [Google Scholar]
- Nulty A. A comparison of trueness and precision of 12 3D printers used in dentistry. BDJ. Open 2022, 8 (1), 14. 10.1038/s41405-022-00108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricles L. M.; Coburn J. C.; Di Prima M.; Oh S. S. Regulating 3D-printed medical products. Science Translational Medicine 2018, 10 (461), eaan6521 10.1126/scitranslmed.aan6521. [DOI] [PubMed] [Google Scholar]
- Christensen A.; Rybicki F. J. Maintaining safety and efficacy for 3D printing in medicine. 3D printing in medicine 2017, 3 (1), 1–10. 10.1186/s41205-016-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn J. C.; Grant G. T.. FDA Regulatory Pathways and Technical Considerations for the 3D Printing of Medical Models and Devices. In 3D Printing in Medicine: A Practical Guide for Medical Professionals; Rybicki F. J., Grant G. T., Eds.; Springer International Publishing, 2017; pp 97–111. [Google Scholar]
- Diment L. E.; Thompson M. S.; Bergmann J. H. M. Clinical efficacy and effectiveness of 3D printing: a systematic review. BMJ. Open 2017, 7 (12), e016891 10.1136/bmjopen-2017-016891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C.; Yan J.; Fu Y.; Guo J.; Shi Y.; Guo J. Organoid bioprinting strategy and application in biomedicine: A review. IJB 2023, 9 (6), 0112. 10.36922/ijb.0112. [DOI] [Google Scholar]
- Bergonzi C.; Remaggi G.; Graiff C.; Bergamonti L.; Potenza M.; Ossiprandi M. C.; Zanotti I.; Bernini F.; Bettini R.; Elviri L. Three-Dimensional (3D) Printed Silver Nanoparticles/Alginate/Nanocrystalline Cellulose Hydrogels: Study of the Antimicrobial and Cytotoxicity Efficacy. Nanomaterials 2020, 10 (5), 844. 10.3390/nano10050844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantino E.; Chiappone A.; Calignano F.; Fontana M.; Pirri F.; Roppolo I. In Situ Thermal Generation of Silver Nanoparticles in 3D Printed Polymeric Structures. Materials 2016, 9 (7), 589. 10.3390/ma9070589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Jiang S.; Huo Y.; Ning T.; Liu A.; Zhang C.; He Y.; Wang M.; Li C.; Man B. 3D silver nanoparticles with multilayer graphene oxide as a spacer for surface enhanced Raman spectroscopy analysis. Nanoscale 2018, 10 (13), 5897–5905. 10.1039/C7NR09276H. [DOI] [PubMed] [Google Scholar]
- Abd-Elaziem W.; Khedr M.; Abd-Elaziem A.-E.; Allah M. M. A.; Mousa A. A.; Yehia H. M.; Daoush W. M.; El-Baky M. A. A. Particle-Reinforced Polymer Matrix Composites (PMC) Fabricated by 3D Printing. Journal of Inorganic and Organometallic Polymers and Materials 2023, 33 (12), 3732–3749. 10.1007/s10904-023-02819-1. [DOI] [Google Scholar]
- Park S.; Shou W.; Makatura L.; Matusik W.; Fu K. K. 3D printing of polymer composites: Materials, processes, and applications. Matter 2022, 5 (1), 43–76. 10.1016/j.matt.2021.10.018. [DOI] [Google Scholar]
- Ang X.; Owi C. K.; Tey J. Y.; Yeo W. H.; Yee P.; Shak K. 3D printing of composite material through blending of PLA and PETG using fused deposition modelling. AIP Conf. Proc. 2023, 10.1063/5.0165316. [DOI] [Google Scholar]
- Marambio-Jones C.; Hoek E. M. V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12 (5), 1531–1551. 10.1007/s11051-010-9900-y. [DOI] [Google Scholar]
- Yan X.; He B.; Liu L.; Qu G.; Shi J.; Hu L.; Jiang G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: proteomics approach†. Metallomics 2018, 10 (4), 557–564. 10.1039/C7MT00328E. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Wang L.; Chen Q.; Chen C. Cytotoxic Potential of Silver Nanoparticles. Yonsei medical journal 2014, 55, 283–291. 10.3349/ymj.2014.55.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzadi I.; Aziz Shah S. M.; Shah M. M.; Ismail T.; Fatima N.; Siddique M.; Waheed U.; Baig A.; Ayaz A. Antioxidant, Cytotoxic, and Antimicrobial Potential of Silver Nanoparticles Synthesized using Tradescantia pallida Extract. Frontiers in Bioengineering and Biotechnology 2022, 10.3389/fbioe.2022.907551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman D. B.; Ostrovskaya L. A.; Bluhterova N. V.; Rikova V. A.; Fomina M. M. Silver Nanoparticles: Cytotoxic Activity and Mechanisms of Action. Biophysics 2022, 67 (4), 571–581. 10.1134/S000635092204011X. [DOI] [Google Scholar]
- Pangli H.; Vatanpour S.; Hortamani S.; Jalili R.; Ghahary A. Incorporation of Silver Nanoparticles in Hydrogel Matrices for Controlling Wound Infection. Journal of Burn Care & Research 2021, 42 (4), 785–793. 10.1093/jbcr/iraa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berihu H. T.; Welderfael T.; Tekluu B.; Gopalakrishnan V. K.; Rao M. R.; Kumar P. P. N. V.; Shameem U.; Dogulas P. J.; Chaithanya K. K. Anti-inflammatory and Cytotoxicity activities of Green Synthesized Silver Nanoparticles from Stem Bark of Terminalia brownii. BioNanoScience 2021, 11 (4), 998–1016. 10.1007/s12668-021-00885-8. [DOI] [Google Scholar]
- Bruna T.; Maldonado-Bravo F.; Jara P.; Caro N. Silver Nanoparticles and Their Antibacterial Applications. International Journal of Molecular Sciences 2021, 22 (13), 7202. 10.3390/ijms22137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold B.-E.; Urnukhsaikhan E.; Mishig-Ochir T. Biosynthesis of silver nanoparticles with antibacterial, antioxidant, anti-inflammatory properties and their burn wound healing efficacy. Frontiers in Chemistry 2022, 10.3389/fchem.2022.972534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Fan J.; Lv M.; She K.; Sun J.; Lu Q.; Han C.; Ding S.; Zhao S.; Wang G.; Zhang Y.; Zang G. Photocrosslinking silver nanoparticles–aloe vera–silk fibroin composite hydrogel for treatment of full-thickness cutaneous wounds. Regenerative Biomaterials 2021, 10.1093/rb/rbab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida D. T.; Bruschi M. L. 3D Printing as a Technological Strategy for the Personalized Treatment of Wound Healing. AAPS PharmSciTech 2023, 24 (1), 41. 10.1208/s12249-023-02503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh S.; Shabangiz S.; Koupaei N.; Hassanzadeh-Tabrizi S. A. 3D printed bio polymeric materials as a new perspective for wound dressing and skin tissue engineering applications: a review. Journal of Polymer Research 2022, 29 (2), 50. 10.1007/s10965-022-02899-6. [DOI] [Google Scholar]
- Tao L.; Chen X.; Sun J.; Wu C. Silver nanoparticles achieve cytotoxicity against breast cancer by regulating long-chain noncoding RNA XLOC_006390-mediated pathway. Toxicology Research 2021, 10 (1), 123–133. 10.1093/toxres/tfaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W.-Y.; Ran S.-Y. Two-stage DNA compaction induced by silver ions suggests a cooperative binding mechanism. J. Chem. Phys. 2018, 10.1063/1.5025348. [DOI] [PubMed] [Google Scholar]
- Hachicho N.; Hoffmann P.; Ahlert K.; Heipieper H. J. Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2. FEMS Microbiology Letters 2014, 355 (1), 71–77. 10.1111/1574-6968.12460. [DOI] [PubMed] [Google Scholar]
- Terzioğlu E.; Arslan M.; Balaban B. G.; Çakar Z. P. Microbial silver resistance mechanisms: recent developments. World J. Microbiol. Biotechnol. 2022, 38 (9), 158. 10.1007/s11274-022-03341-1. [DOI] [PubMed] [Google Scholar]
- Ashij M. A.; Al-Shmgani H. S.; Sulaiman G. M.; Mohammed H. A.; Abdalrazaq E. A.; Albukhaty S. Investigation of Antibacterial Activity and Wound Healing Promotion Properties Induced by Bromelain-Loaded Silver Nanoparticles. Plasmonics 2024, 19, 1903. 10.1007/s11468-023-02127-x. [DOI] [Google Scholar]
- Alavi M.; Varma R. S. Antibacterial and wound healing activities of silver nanoparticles embedded in cellulose compared to other polysaccharides and protein polymers. Cellulose 2021, 28 (13), 8295–8311. 10.1007/s10570-021-04067-3. [DOI] [Google Scholar]
- Dong Y.; Zhu H.; Shen Y.; Zhang W.; Zhang L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio Natriegens. PLoS One 2019, 14 (9), e0222322 10.1371/journal.pone.0222322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; Mao J.; Zheng P.; Wang R.; Yang Z.; Qian S. Improving the biocompatibility and antibacterial efficacy of silver nanoparticles functionalized with (LLRR)3 antimicrobial peptide. World J. Microbiol. Biotechnol. 2024, 40 (1), 1. 10.1007/s11274-023-03792-0. [DOI] [PubMed] [Google Scholar]
- Lafontaine N.; Jolley J.; Kyi M.; King S.; Iacobaccio L.; Staunton E.; Wilson B.; Seymour C.; Rogasch S.; Wraight P. Prospective randomised placebo-controlled trial assessing the efficacy of silver dressings to enhance healing of acute diabetes-related foot ulcers. Diabetologia 2023, 66 (4), 768–776. 10.1007/s00125-022-05855-7. [DOI] [PubMed] [Google Scholar]
- Rybka M.; Mazurek Ł.; Konop M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice. Life 2023, 13 (1), 69. 10.3390/life13010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantari K.; Mostafavi E.; Afifi A. M.; Izadiyan Z.; Jahangirian H.; Rafiee-Moghaddam R.; Webster T. J. Wound dressings functionalized with silver nanoparticles: promises and pitfalls. Nanoscale 2020, 12 (4), 2268–2291. 10.1039/C9NR08234D. [DOI] [PubMed] [Google Scholar]
- Noga M.; Milan J.; Frydrych A.; Jurowski K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. International Journal of Molecular Sciences 2023, 24 (6), 5133. 10.3390/ijms24065133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu A. N.; Kudesia N.; Raut A. M.; Pakrudheen I.; Wahengbam J. Toxicity, bioaccumulation, and transformation of silver nanoparticles in aqua biota: a review. Environmental Chemistry Letters 2021, 19 (6), 4275–4296. 10.1007/s10311-021-01304-w. [DOI] [Google Scholar]
- Kuempel E. D.; Roberts J. R.; Roth G.; Zumwalde R. D.; Nathan D.; Hubbs A. F.; Trout D.; Holdsworth G.. Current Intelligence Bulletin 70: health effects of occupational exposure to silver nanomaterials; Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, 2021. 10.26616/NIOSHPUB2021112. [DOI]
- Zhang L.; Wei Y.; Wang H.; Wu F.; Zhao Y.; Liu X.; Wu H.; Wang L.; Su H. Green Synthesis of Silver Nanoparticles Using Mushroom Flammulina velutipes Extract and Their Antibacterial Activity Against Aquatic Pathogens. Food and Bioprocess Technology 2020, 13 (11), 1908–1917. 10.1007/s11947-020-02533-7. [DOI] [Google Scholar]
- Dove A. S.; Dzurny D. I.; Dees W. R.; Qin N.; Nunez Rodriguez C. C.; Alt L. A.; Ellward G. L.; Best J. A.; Rudawski N. G.; Fujii K.; et al. Silver nanoparticles enhance the efficacy of aminoglycosides against antibiotic-resistant bacteria. Frontiers in Microbiology 2023, 13, 1064095. 10.3389/fmicb.2022.1064095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher P.; Singh M.; Mudila H. Silver nanoparticles as antimicrobial therapeutics: current perspectives and future challenges. 3 Biotech 2018, 8 (10), 411. 10.1007/s13205-018-1436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franci G.; Falanga A.; Galdiero S.; Palomba L.; Rai M.; Morelli G.; Galdiero M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20 (5), 8856–8874. 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone L. P.; Best S. M.; Cameron R. E. Accelerated degradation testing impacts the degradation processes in 3D printed amorphous PLLA. Frontiers in Bioengineering and Biotechnology 2024, 10.3389/fbioe.2024.1419654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C. P. Use of recycled poly lactic acid (PLA) polymer in 3D printing: a review. Tc (C) 2019, 123 (104.34), 98.32. [Google Scholar]
- de Souza Medeiros C. B.; Silva B. L.; Medeiros A. M.; Melo J. D. D.; Barbosa A. P. C. Degradation of 3D-printed poly(lactic acid) for biomedical applications. Polym. Bull. 2024, 81 (7), 6271–6281. 10.1007/s00289-023-04992-2. [DOI] [Google Scholar]
- Taib N.-A. A. B.; Rahman M. R.; Huda D.; Kuok K. K.; Hamdan S.; Bakri M. K. B.; Julaihi M. R. M. B.; Khan A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80 (2), 1179–1213. 10.1007/s00289-022-04160-y. [DOI] [Google Scholar]
- Duan J.; Lei D.; Ling C.; Wang Y.; Cao Z.; Zhang M.; Zhang H.; You Z.; Yao Q. Three-dimensional-printed polycaprolactone scaffolds with interconnected hollow-pipe structures for enhanced bone regeneration. Regenerative Biomaterials 2022, 10.1093/rb/rbac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncal K. K.; Heo D. N.; Godzik K. P.; Sosnoski D. M.; Mrowczynski O. D.; Rizk E.; Ozbolat V.; Tucker S. M.; Gerhard E. M.; Dey M.; Lewis G. S.; Yang J.; Ozbolat I. T. 3D printing of poly(ε-caprolactone)/poly(D,L-lactide-co-glycolide)/hydroxyapatite composite constructs for bone tissue engineering. J. Mater. Res. 2018, 33 (14), 1972–1986. 10.1557/jmr.2018.111. [DOI] [Google Scholar]
- Rezania N.; Asadi-Eydivand M.; Abolfathi N.; Bonakdar S.; Mehrjoo M.; Solati-Hashjin M. Three-dimensional printing of polycaprolactone/hydroxyapatite bone tissue engineering scaffolds mechanical properties and biological behavior. J. Mater. Sci.: Mater. Med. 2022, 33 (3), 31. 10.1007/s10856-022-06653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Wang Y.; Zhou Y.; Chen J.; Wan Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13 (16), 2754. 10.3390/polym13162754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heris M. A.; Jahandideh A.; Akbarzadeh A.; Mortazavi P. Dual-Loaded Poly(ε-caprolactone) Nanofibers for Wound Healing Applications: Co-Delivery of Clindamycin and Propolis Extract for Synergistic Therapeutic Effects. BioNanoScience 2024, 10.1007/s12668-024-01337-9. [DOI] [Google Scholar]
- Raina N.; Pahwa R.; Khosla J. K.; Gupta P. N.; Gupta M. Polycaprolactone-based materials in wound healing applications. Polym. Bull. 2022, 79 (9), 7041–7063. 10.1007/s00289-021-03865-w. [DOI] [Google Scholar]
- Kaur M.; Singh K.; Kumar V. Green Synthesis of Silver Nanoparticles Using Penicillium camemberti and its Biological Applications. BioNanoScience 2024, 10.1007/s12668-024-01507-9. [DOI] [Google Scholar]
- León-Silva S.; Fernández-Luqueño F.; López-Valdez F. Silver Nanoparticles (AgNP) in the Environment: a Review of Potential Risks on Human and Environmental Health. Water, Air, & Soil Pollution 2016, 227 (9), 306. 10.1007/s11270-016-3022-9. [DOI] [Google Scholar]
- Sakai N.; Hirasawa T. Photo-curable Resins for UV Nanoinprint and their Evaluation Methods. KOBUNSHI RONBUNSHU 2009, 66 (3), 88–96. 10.1295/koron.66.88. [DOI] [Google Scholar]
- Gatechair L. R.; Wostratzky D.. Photoinitiators: A Review of Mechanisims and Applications. In Adhesive Chemistry: Developments and Trends; Lee L.-H., Ed.; Springer: US, 1984; pp 409–438. [Google Scholar]
- Sakai N. Photo-curable Resin for UV-Nanoimprint Technology. J. Photopolym. Sci. Technol. 2009, 22 (2), 133–145. 10.2494/photopolymer.22.133. [DOI] [Google Scholar]
- dos Santos Jorge Sousa K.; de Souza A.; de Almeida Cruz M.; de Lima L. E.; do Espirito Santo G.; Amaral G. O.; Granito R. N.; Renno A. C. 3D printed scaffolds of biosilica and spongin from marine sponges: analysis of genotoxicity and cytotoxicity for bone tissue repair. Bioprocess Biosyst. Eng. 2024, 47, 1483. 10.1007/s00449-024-03042-z. [DOI] [PubMed] [Google Scholar]
- Koumentakou I.; Michopoulou A.; Noordam M. J.; Terzopoulou Z.; Bikiaris D. N. 3D-printed scaffolds for tissue engineering applications using thermosensitive hydrogels based on biopolymer blends. J. Mater. Sci. 2024, 59 (20), 9021–9041. 10.1007/s10853-024-09707-0. [DOI] [Google Scholar]
- Hatt L. P.; Wirth S.; Ristaniemi A.; Ciric D. J.; Thompson K.; Eglin D.; Stoddart M. J.; Armiento A. R. Micro-porous PLGA/β-TCP/TPU scaffolds prepared by solvent-based 3D printing for bone tissue engineering purposes. Regenerative Biomaterials 2023, 10.1093/rb/rbad084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar N.; Mondal A. Synthesis, properties, environmental degradation, processing, and applications of Polylactic Acid (PLA): an overview. Polym. Bull. 2024, 81, 11421. 10.1007/s00289-024-05252-7. [DOI] [Google Scholar]
- Vieira A.; Vieira J.; Ferra J.; Magalhães F.; Guedes R.; Marques A. Mechanical study of PLA–PCL fibers during in vitro degradation. Journal of the mechanical behavior of biomedical materials 2011, 4 (3), 451–460. 10.1016/j.jmbbm.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Quintero-Quiroz C.; Acevedo N.; Zapata-Giraldo J.; Botero L. E.; Quintero J.; Zárate-Triviño D.; Saldarriaga J.; Pérez V. Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomaterials Research 2019, 23 (1), 27. 10.1186/s40824-019-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh W. A.; Chakraborty S.; Owens G.; Islam R. U. A review of the phytochemical mediated synthesis of AgNP (silver nanoparticle): the wonder particle of the past decade. Applied Nanoscience 2021, 11 (11), 2625–2660. 10.1007/s13204-021-02135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canchy L.; Kerob D.; Demessant A. L.; Amici J. M. Wound healing and microbiome, an unexpected relationship. Journal of the European Academy of Dermatology and Venereology 2023, 37, 7–15. 10.1111/jdv.18854. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Huang J.; Zeng A.; Long X.; Yu N.; Wang X. The role of the skin microbiome in wound healing. Burns & Trauma 2024, 10.1093/burnst/tkad059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott S. L.; Larcombe D.-L.; Logan A. C.; West C.; Burks W.; Caraballo L.; Levin M.; Etten E. V.; Horwitz P.; Kozyrskyj A.; Campbell D. E. The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. World Allergy Organization Journal 2017, 10 (1), 29. 10.1186/s40413-017-0160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; Ou C.; Zhuang J.; Li J.; Zhang F.; Zhong Y.; Chen Y. Interplay Between Skin Microbiota Dysbiosis and the Host Immune System in Psoriasis: Potential Pathogenesis. Frontiers in Immunology 2021, 10.3389/fimmu.2021.764384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting C.; Abdel Azim S.; Friedman A. The Skin Microbiome and its Significance for Dermatologists. American Journal of Clinical Dermatology 2024, 25 (2), 169–177. 10.1007/s40257-023-00842-z. [DOI] [PubMed] [Google Scholar]
- Bjerre R. D.; Holm J. B.; Palleja A.; Solberg J.; Skov L.; Johansen J. D. Skin dysbiosis in the microbiome in atopic dermatitis is site-specific and involves bacteria, fungus and virus. BMC Microbiology 2021, 21 (1), 256. 10.1186/s12866-021-02302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norreslet L. B.; Agner T.; Clausen M.-L. The Skin Microbiome in Inflammatory Skin Diseases. Current Dermatology Reports 2020, 9 (2), 141–151. 10.1007/s13671-020-00297-z. [DOI] [Google Scholar]
- Yang Y.; Qu L.; Mijakovic I.; Wei Y. Advances in the human skin microbiota and its roles in cutaneous diseases. Microbial Cell Factories 2022, 21 (1), 176. 10.1186/s12934-022-01901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. R.; Powell E. J.; Tomasovic L. M.; Marcheskie R. L.; Girish V.; Warman A.; Sivaloganathan D. Changes in the Skin Microbiome Following Dermatological Procedures: A Scoping Review. Appl. Microbiol. 2024, 4 (2), 972–985. 10.3390/applmicrobiol4020066. [DOI] [Google Scholar]
- Dakal T. C.; Kumar A.; Majumdar R. S.; Yadav V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front Microbiol 2016, 7, 1831. 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Sun X. Antibacterial Mechanism of Nanosilvers. Current Pharmacology Reports 2019, 5 (6), 401–409. 10.1007/s40495-019-00204-6. [DOI] [Google Scholar]
- Zhang L.; Wu L.; Mi Y.; Si Y. Silver Nanoparticles Induced Cell Apoptosis, Membrane Damage of Azotobacter vinelandii and Nitrosomonas europaea via Generation of Reactive Oxygen Species. Bull. Environ. Contam. Toxicol. 2019, 103 (1), 181–186. 10.1007/s00128-019-02622-0. [DOI] [PubMed] [Google Scholar]
- Gherasim O.; Puiu R. A.; Bîrcă A. C.; Burduşel A.-C.; Grumezescu A. M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10 (11), 2318. 10.3390/nano10112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández S.; Lozano-Iturbe V.; García B.; Andrés L. J.; Menéndez M. F.; Rodríguez D.; Vazquez F.; Martín C.; Quirós L. M. Antibacterial effect of silver nanorings. BMC Microbiology 2020, 20 (1), 172. 10.1186/s12866-020-01854-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M. J.; Kabeer A.; Abbas Z.; Siddiqui H. A.; Calina D.; Sharifi-Rad J.; Cho W. C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Communication and Signaling 2024, 22 (1), 7. 10.1186/s12964-023-01398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde M. M.; Snyder C. M.; Sloop J.; Solst S. R.; Donati G. L.; Spitz D. R.; Furdui C. M.; Singh R. The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Particle and Fibre Toxicology 2021, 18 (1), 37. 10.1186/s12989-021-00430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-F.; Shen W.; Gurunathan S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. International Journal of Molecular Sciences 2016, 17 (10), 1603. 10.3390/ijms17101603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saafane A.; Durocher I.; Vanharen M.; Girard D. Impact of ultra-small silver nanoparticles of 2 nm (AgNP2) on neutrophil biology: AgNP2 alter the actin cytoskeleton and induce karyorrhexis by a mitogen-activated protein kinase-dependent mechanism in vitro and transitorily attract neutrophils in vivo. Chemico-Biological Interactions 2022, 365, 110096. 10.1016/j.cbi.2022.110096. [DOI] [PubMed] [Google Scholar]
- Ma J.; Zhao D.; Lu H.; Huang W.; Yu D. Apoptosis signal-regulating kinase 1 (ASK1) activation is involved in silver nanoparticles induced apoptosis of A549 lung cancer cell line. Journal of Biomedical Nanotechnology 2017, 13 (3), 349–354. 10.1166/jbn.2017.2359. [DOI] [PubMed] [Google Scholar]
- Yin Q.; Zhou Q.; Hu J.; Weng J.; Liu S.; Yin L.; Long L.; Tong Y.; Tang K.; Bai S.; Ou L. Fabrication of bimetallic Ag@ZnO nanocomposite and its anti-cancer activity on cervical cancer via impeding PI3K/AKT/mTOR pathway. Journal of Trace Elements in Medicine and Biology 2024, 84, 127437. 10.1016/j.jtemb.2024.127437. [DOI] [PubMed] [Google Scholar]
- Vahabirad M.; Daei S.; Abbasalipourkabir R.; Ziamajidi N. Anticancer Action of Silver Nanoparticles in SKBR3 Breast Cancer Cells through Promotion of Oxidative Stress and Apoptosis. BioMed. Research International 2024, 2024, 1. 10.1155/2024/7145339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaneethan R. D.; Packia Lekshmi N. C. J.; Ramaiah M.; Ravindran R.; Ananth Kumar T.; Chinnathambi A.; Ali Alharbi S.; Sivagnanam A.; Mohemedibrahim P. K. M. Caralluma pauciflora based Ag-NPs activate ROS - induced apoptosis through down-regulation of AKT, mTOR and pI3K signaling in human gastric cancer (AGS) cells. Nanotechnology 2024, 35 (19), 195102. 10.1088/1361-6528/ad26d9. [DOI] [PubMed] [Google Scholar]
- Khorrami S.; Dogani M.; Mahani S. E.; Moghaddam M. M.; Taheri R. A. Neuroprotective activity of green synthesized silver nanoparticles against methamphetamine-induced cell death in human neuroblastoma SH-SY5Y cells. Sci. Rep. 2023, 10.1038/s41598-023-37917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X.; Wang X.; Li J.; Shang M.; Niu S.; Zhang W.; Li Y.; Sun Z.; Gan J.; Li W.; Tang M.; Xue Y. Silver nanoparticles induced cytotoxicity in HT22 cells through autophagy and apoptosis via PI3K/AKT/mTOR signaling pathway. Ecotoxicology and Environmental Safety 2021, 208, 111696. 10.1016/j.ecoenv.2020.111696. [DOI] [PubMed] [Google Scholar]
- Pang S.; Gao Y.; Wang F.; Wang Y.; Cao M.; Zhang W.; Liang Y.; Song M.; Jiang G. Toxicity of silver nanoparticles on wound healing: A case study of zebrafish fin regeneration model. Sci. Total Environ. 2020, 717, 137178. 10.1016/j.scitotenv.2020.137178. [DOI] [PubMed] [Google Scholar]
- He Y.; Zhang Y.; Hu F.; Chen M.; Wang B.; Li Y.; Xu H.; Dong N.; Zhang C.; Hu Y.; Lin Z.; Peng Y.; Ye Q.; Luo L. Photosensitive Hydrogels Encapsulating DPSCs and AgNPs for Dental Pulp Regeneration. International Dental Journal 2024, 74 (4), 836–846. 10.1016/j.identj.2024.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales Matushita L. M.; Palomino L.; Rodriguez-Reyes J. C. F. Silver Nanoparticles Coated with Recombinant Human Epidermal Growth Factor: Synthesis, Characterization, Liberation and Anti-Escherichia coli Activity. Reactions 2023, 4 (4), 713–724. 10.3390/reactions4040041. [DOI] [Google Scholar]
- Li R.; Xu Z.; Jiang Q.; Zheng Y.; Chen Z.; Chen X. Characterization and biological evaluation of a novel silver nanoparticle-loaded collagen-chitosan dressing. Regenerative Biomaterials 2020, 7 (4), 371–380. 10.1093/rb/rbaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.; Chen-wen L.; Qing W.; Min H.; San-jun S.; Zi-wei L.; Guo-lin W.; Huan-huan C.; Yuan-yuan L.; Qian Z.; Xiu-heng Y.; Li J. Silver nanoparticles/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber promotes wound healing by activating TGFβ1/Smad signaling pathway. Int. J. Nanomed. 2016, 11, 373–387. 10.2147/IJN.S91975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franková J.; Pivodová V.; Vágnerová H.; Juráňová J.; Ulrichová J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. Journal of Applied Biomaterials and Functional Materials 2016, 14 (2), e137–e142. 10.5301/jabfm.5000268. [DOI] [PubMed] [Google Scholar]
- Shi J.; Sun X.; Lin Y.; Zou X.; Li Z.; Liao Y.; Du M.; Zhang H. Endothelial cell injury and dysfunction induced by silver nanoparticles through oxidative stress via IKK/NF-κB pathways. Biomaterials 2014, 35 (24), 6657–6666. 10.1016/j.biomaterials.2014.04.093. [DOI] [PubMed] [Google Scholar]
- Abdellatif A. A. H.; Rasheed Z.; Alhowail A. H.; Alqasoumi A.; Alsharidah M.; Khan R. A.; Aljohani A. S. M.; Aldubayan M. A.; Faisal W. Silver citrate nanoparticles inhibit pma-induced tnfα expression via deactivation of nf-κb activity in human cancer cell-lines, mcf-7. Int. J. Nanomed. 2020, 15, 8479–8493. 10.2147/IJN.S274098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and/or analyzed during the current study are publicly available.