Abstract
Background
Lumbar disc surgery is among the more common spinal procedures. In this paper, we report the current treatment recommendations for patients with symptomatic disc herniation.
Methods
This review is based on pertinent publications retrieved by a selective literature search in PubMed using the terms [timing] AND [lumbar disc herniation], supplemented by other relevant articles and guidelines.
Results
Symptoms resolve in 60% to 80% of patients with herniated discs in 6–12 weeks, and in 80% to 90% over the long term (≥ 1 year). According to the guidelines, 6–12 weeks of conservative treatment are recommended in the absence of significant neurologic deficits. Early surgery is indicated in case of worsening pain or new onset of neurologic deficits. Lumbar disc herniation associated bladder or bowel dysfunction (cauda equina syndrome) is considered an absolute surgical emergency that requires immediate decompression (within 24 to 48 hours). Patients with severe motor deficits (MRC ≤ 3/5) benefit from early intervention and should be offered surgery within three days, if possible, for the best chance of recovery. The degree of weakness and the duration of symptoms have been identified as risk factors for incomplete recovery. Early surgery can be considered in patients with mild paresis (MRC 4/5) in case of functional impairment (e.g., quadriceps paresis).
Conclusion
Longer symptom duration and lower motor scores are associated with worse outcome and a lower chance of neurologic recovery. The recovery rate for motor deficits ranges from 33% to 75%, depending on the timing and modality of treatment as well as the motor score.
Degenerative changes of the spine and the associated symptoms are among the more common reasons for medical consultation in western industrialized countries (1). Disc herniation can cause mechanical and secondary inflammatory irritation of the nerve roots, triggering sciatica (alternative term: lumboischialgia) in the lumbosacral segments (2).
In the general population, the annual incidence of sciatica is 1–5%, and the overall lifetime prevalence is as high as 43% (e1, e2). The pain usually radiates from the low back along the distribution of the sciatic nerve into the leg, but affection of the upper lumbar segments can also cause pain along the distribution of the femoral nerve (ventral thigh).
Incidence and prevalence.
In the general population, the annual incidence of sciatica is 1–5%, and the overall lifetime prevalence is as high as 43%.
Pain is generally the leading symptom; it can be accompanied by a sensory and/or motor deficit of the corresponding dermatome or myotome (2). Intervertebral disc surgery is one of the more common spinal operations and has become more common all over the world in the past 20 years (e3, e4).
Low back pain and sciatica due to lumbar disc herniation carry a high socioeconomic cost because of prolonged absences from work, expensive diagnostic evaluations, costly conservative treatments, and, above all, expensive interventional procedures and operations (1).
Lumbar disc surgery.
Intervertebral disc surgery is one of the more common spinal operations and has become more common all over the world in the past 20 years (e3, e4). Low back pain and sciatica due to lumbar disc herniation, and its surgical treatment, carry a high socioeconomic cost.
This disease is, however, generally benign and self-limiting, with symptom resolution in 6–12 weeks in 60–80% of cases and long-term improvement in 80–90% (3, e5, e6).
The important exception to this rule is lumbar disc herniation with manifestations of cauda equina compression, i.e., bladder and/or rectal dysfunction. This situation is an absolute surgical emergency in which immediate surgery (i.e., within 24–48 hours) is needed to enable functional recovery; the earlier the intervention, the higher the chance that bladder and rectal dysfunction will recover (4, 5). In general, surgery is recommended for lumbar disc herniation with persistent radiculopathy, worsening pain, or neurologic deficits that cause functional impairment (6). Despite the commonness of this type of surgery, there is no consensus on the optimal timing of treatment for patients suffering “only” from persistent pain, or for those who have a motor deficit. We address this issue in the present review on the basis of up-to-date evidence retrieved by an an extensive literature search, in order to provide recommendations for treatment based on the particular clinical manifestations in the individual case (7).
We carried out a selective search in PubMed for articles including the search terms (“timing”) AND (“lumbar disc herniation”), supplemented by other suitable or associated articles. The 124 retrieved articles were screened independently by two of the authors of this review independently for clinical relevance with the PRISMA criteria on the basis of the title, abstract, and full text (if available); in case the two screeners arrived at different conclusions, these were harmonized in a further discussion (e7). Randomized controlled trials (RCTs), reviews, meta-analyses, and both prospective and retrospective studies were selected for further analysis. The breadth of types of publication that were selected for analysis here is a result of the nature of the topic itself, the lack of RCTs concerning early treatment for patients with paresis, and the known methodological limitations of meta-analyses. The main publications on the timing of treatment of radiculopathy due to lumbar disc herniation are listed in the Table. Publications on paresis and bladder and/or rectal dysfunction are listed in the eTable. Only limited and low-level evidence for timing of treatment is available, derived mainly from retrospective analyses or small-scale prospective trials. On the other hand, there is high-level evidence from a large number of RCTs, reviews, and meta-analyses on the question of surgical versus conservative treatment for persistent sciatica. The interpretability of the results is impaired by high cross-over rates and intention-to-treat analyses.
Table. Studies with the highest level of evidence* evaluating the benefits of surgery for disc herniation and the importance of the timing of surgery.
|
Author (year) Study design |
Number of patients Parameters |
Comparison groups | Outcome [95% CI] | Limitations |
| Liu (9) Meta-analysis |
24 studies, 1711 patients; primary outcome: leg pain secondary outcome: back pain, disability, safety of treatment |
OP vs. conservative therapy vs. ESI | benefit of disc surgery vs. conservative/ESI: immediate pain reduction (-12.1), short-term (-11.7) and medium/long-term relief (-6.5) of leg pain with negligible long-term effect (-2.3) similar effect size for ESI and for short-term improvement of functional impairment by surgery adverse events, 1.34 % [0.91; 1.98] | invalid for heterogeneous LDH population focus on sciatica symptom duration: 49 days – 5 years |
| Sabnis (10) Meta-analysis |
21 studies on sciatica; effect of symptom duration on outcome |
OP vs. conservative therapy | 2 RCTs show no difference 10/12 studies with medium and 5/7 with low evidence showed better outcome after surgery for patients with shorter symptom duration risk factors for negative outcome: 6 months of sciatica, > 2 months of inability to work, compensation payments | no recommendation |
| Östermann (31) Randomized controlled trial |
56 (20–50 years); sciatica (± deficit) primary outcome: leg and back pain |
OP vs. conservative therapy | 6 –12 weeks of initial conservative therapy; no significant difference after 2 years in primary outcome; better short-term data because of faster recovery after surgery; 11/28 cross-over; 12/29 with residual paresis at 1 year | exclusion of severe pain, CES, progressive deficits, longer duration of symptoms before surgery; unknown degree of paresis |
| Weber (32) Randomized controlled trial |
126: 67 primary surgery, 87 conservative; sciatica | OP vs. conservative therapy | 1, 4, 10 years FU: better outcome for surgically treated patients at 1 year, statistically insignificant thereafter | only sciatica, 30% cross-over rate, exclusion of patients with severe pain |
| Hofstee (19) Randomized controlled trial |
250 (younger than 60 years); sciatica (< 1 month) | bed rest vs. physiotherapy vs. ADL | no significant differences between groups (at 1, 2, and 6 months) with respect to the pain scale or functional impairment in everyday life (Quebec Disability Scale) | 6-month FU: cross-over rate: 17–25% |
| Peul (8) Randomized controlled trial |
283; 141 early surgery vs. 142 conservative treatment | early surgery vs. conservative therapy | initially: in patients with sciatica of 6 –12 weeks‘ duration, earlier and faster recovery and pain relief in those who underwent early surgery (HR 1.97; [1.72; 2.22]; p < 0.001)at 1 year: convergence of outcomes | cross-over rate of 39% after only 19 weeks |
| Buttermann (20) Randomized controlled trial |
100; radiculopathy ≥ 6 weeks (LDH > 25% of the spinal canal) | ESI vs. surgery | rapid clinical improvement after surgery (92–98%) relief from ESI in 42–56% outcome no worse with longer duration of symptoms | high cross-over rate, no data on motor deficits |
| Weinstein (21) Randomized controlled trial |
RCT: 245 surgery vs. 256 conservative; observational study with 743 patients; radiculopathy ≥ 6 weeks | conservative therapy vs. surgery | surgery led to significantly better short- and long-term outcomes (as-treated analysis) for the primary parameters pain (45.6 vs. 30.7), physical performance level (44.6 vs. 29.7), and ODI (-38.1 vs. –24.9) | high cross-over rate (30% of the conservative cohort underwent surgery within three months, while only 50% of the “surgical” cohort did) initial intention-to-treat analysis (2006) |
| Rihn (14) Randomized controlled trial |
1192; 927 vs. 265; LDH associated radiculopathy | duration of symptoms (≤ 6 months vs. > 6 months) | better outcome at 4 years for patients with shorter symptom duration: significant improvement in the pain and function domain of the SF-36 (48.3 vs. 41.9) and reduction in the ODI (41.1 vs. 34.6) after surgery (p < 0.001) in both cohorts | differences at baseline: in particular, type of LDH, distribution of neurologic deficits, depression rate |
| Bailey (22) Randomized controlled trial |
128; 64 vs. 64; L4 – S1 LDH with sciatica for 4 – 12 months | microdiscectomy vs. conservative therapy | one-year follow-up: significantly less leg pain after surgery (2.8 vs. 5.2; [1.4; 3.4]; p < 0.001] 9/64 adverse events, 1 recurrence; two-year-follow-up: mcid not achieved | 12.5% / 34% cross-over rate within 11 months, low FU rate |
* meta-analysis, systematic review and randomized controlled trials
ADL, activities of daily living; CES, caude equina syndrome; CI, confidence interval; ESI, epidural steroid injection;
FU, follow-up; LDH, lumbar disc herniation; MCID minimal clinically important change (i.e., of at least some importance to the patient);
ODI, Oswestry Disability Index; OP, operation; pat., patient(s)
eTable. Studies of the effect of symptom duration and degree of paresis on the outcome of patients with motor deficits and bladder/rectum dysfunction related disc herniations*.
|
Author / year / study design |
Number of patients; intended analysis |
Outcome | Limitations |
| (Ahn 2000) Meta-analysis |
322 with cauda equina syndrome; symptom duration: < 24 hr, 24–48 rh, 2–10 days, 11 days –1 month, > 1 month | OP ≤ 48 hr vs. > 48 h; odds ratios (OR)2.5 for urinary incontinence; 9.1 for motor deficits; 9.1 for rectal dysfunction, 3.5 for sensory deficits | statistical design (binomial/categorical), data pooling with partly unknown information on timing; no MRC scale; range of incontinence |
| (Kumar 2022) Meta-analysis |
22 studies, 852 with CES, symptom duration < 48 h vs. ≥ 48 h; minimum FU: 1 year | outcome: 43% residual urinary incontinence, 31% residual rectal dysfunction, 40% sexual dysfunction; 53% sensory and 38% motor deficits; OP ≤ 48 h vs. > 48 h: 24.6 % vs. 50.3 % residual urinary incontinence | diverse pathologies included |
| (Schoenfeld 2015) Systematic review |
11 studies, effect of symptom duration before disc surgery on functional outcome | 9 of 11 studies: negative correlation between symptom duration and outcome majority of studies: 6 months cut-off | study selection, no details on symptom severity, heterogeneous symptom duration 2 –12 months |
| (Sharma 2012) Systematic review |
review on the effect of the timing of treatment and the degree of paresis on the outcome | 30 – 50 % LDH-associated motor deficits, 45% improve spontaneously, 25 – 76% recovery rate, OP: better short-term outcome. Earlier tends to be better. High grade paresis (MRC ≤ 2/5) is associated with poor functional outcome. | SCS and LDH associated paresis, study selection (recovery: MRC ≥ 4/5), baseline characteristics (conservative vs. surgery) different. Wide range of symptom duration or degree of paresis, cross-over rate, no definition of “early” |
| (Overdevest 2014) Subgroup analysis, randomized controlled trial |
150 patients with motor deficits (6–12 weeks); early surgery (1.8 weeks) vs. delayed surgery (15 weeks); 126 moderate (MRC 4/5), 24 severe deficits (MRC 3/5) | 53% had motor deficits; faster recovery in early operated cohort; recovery rate 87% vs. 84 % with initial MRC 4/5 and 58% vs. 50% with initial MRC 3/5); RF: > 25% of the spinal canal | cross-over rate 40%, no precise symptom duration, no definition of early surgery, „severe“ = MRC 3/5, where patients with MRC ≤ 2/5, worsening symptoms, and CES were excluded; 6–12 weeks of sciatica before surgery |
| (Thomé 2022) Prospective registry study |
390; prospective registry study of acute LDH with associated motor deficits: 118 (30.3%) mild, 191 (49%) moderate (3/5), 81 (20.8%) severe (≤ 2/5); unbiased recursive partitioning, conditional inference tree (URP-CTREE) | MRC ≤ 2 predicts worse outcome (p < 0.001), surgery within 3 days: superior outcome for mrc 3/5 and mrc ≤ 2/5, 61 % vs. 0 % (severe ≤ 2/5), nnt 1.4; 97.4 % recovery rate for early treated moderate paresis (surgery < 3 days) early surgery predicted better result for patients with mild paresis ≤ 8 days (98%) | no conservatively treated control group; strength: precise strength grading and symptom duration, objective statistical method, large cohort, 1–5 years FU |
| (Dubourg 2002) Multicenter, prospective cohort study |
LDH with associated MRC ≤ 3/5 foot drop (< 1 month); outcome of motor deficits (conservative vs. operative) | 53% improved (incl. 30% recovery rate); surgery vs. conservative: 53% vs. 56% improvement rate | baseline characteristics (type of prolapse, more muscle groups affected, symptom duration); FU up to 6 months |
| (Sangondimath 2020) Retrospective study |
43 CES, (27 LDH) ≥ 1 year FU; effect of symptom duration; urodynamic analysis including sexual dysfunction analysis | age and duration of urinary incontinence with postoperative sexual dysfunction (SD) 70% in men, 60% in women. CES recovery associated with shorter symptom duration | retrospective analysis, missing data (especially preoperative details), mixed clinical pictures, average duration of saddle hypesthesia was 24 days, number of patients |
| (Beculic 2016) Retrospective study |
25 with CES; outcome after surgery (within 24 hr after hospitalization) | Overall: recovery of 36% of urinary and 44% of fecal incontinence, 48% of motor and 64% of sensory deficits. 89% recovery (< 48 h) vs. 16.7% (2–5 days) | few patients, no precise information on symptom duration |
| (Nielsen 1980) Retrospective study |
22 with CES, outcome after surgery, including urodynamic analysis; symptom duration: < 48 hr, 9 patients 2–8 days, 4 patients with ces ≥ 10 days | 50% were able to urinate by detrusor contraction (better outcome if surgery < 48 h), 6/7 who were able to urinate with effort had symptoms for > 2 days | small cohort, no details on sensorimotor deficits (13/22 postoperatively), strength: urodynamic analysis supports clinical outcome |
| (Krishnan 2017) Retrospective study |
140 (70 motor deficits vs. 70 neurologically intact); motor deficit defined as MRC ≤ 3/5; descriptive, RF for neurological deficits | RF for neurological deficits: acute onset of symptoms, free sequestrum, cranial luxation, median herniation, DM, L3/4 segment, pre-existing SCS | retrospective, no information on outcome |
| (Aono 2007) Retrospective study |
46 patients with foot drop (24 LDH), defined as MRC < 3/5 (3-/5 = incomplete rom in sitting), surgical outcome after 1 year | 30% recovery rate, 70% of which within 1 year; better recovery rate MRC 2–3-/5 vs. MRC 0–1 (0.047); better outcome if shorter symptom duration (undefined) and in younger patients (p = 0.027; OR 1.48) | mixed cohort: LDH, LSS, spondylolisthesis, multilevel in > 33%, symptom duration 4–720 days |
| (Macki 2016) Retrospective study |
71 (45 LDH) with paresis; outcome after 1and 6 weeks, 3 and 6 months, 1 year | 73% improve, association with timing (HR 0.67, p = 0.004), inverse correlation between grade and recovery rate (p = 0.010), 95% improve within 3 months | partly only by telephone. FU, LSS and LDH patients, foot drop in 28% (MRC 4-/4/4 +); foot drop for 6 weeks on average; < 20% treated within 1 week |
| (Takenaka 2017) Retrospective study |
102; surgical outcome after foot drop (MRC ≤ 3-/5); good ≥ 3/5, excellent ≥ 4/5 | longer symptom duration (> 30 days), worse paresis grade (MRC ≤ 1/5) associated with poor outcome (< 0.001); age, soft disc herniation and leg pain are negative predictors; 84% of mrc 2–3/5 (< 30 days) improve to mrc ≥ 4/5 | retrospective, 72% FU rate, diverse pathologies, statistical model, average symptom duration 106 days |
| (Ghahreman 2008) Retrospective study |
56 (88 % LDH); surgical outcome for foot drop ≤ MRC 3-/5 (66%):27% with MRC 3/5, 7% with MRC 4/5 | 41% recovery rate (27% in ≤ 2/5), RF: degree of paresis (p < 0.001), and older age (p = 0.03) are negative predictors | retrospective, diverse pathologies |
| (Postacchini 2002) Retrospective study |
116 patients with LDH-associated paresis (= 27% of the overall cohort); outcome of motor deficits depending on duration and degree of paresis; 67% mild (MRC 4/5), 21% severe (MRC 3/5), 12% very severe (MRC ≤ 2/5) | 76% recovery rate (84% of mild and 61% of severe deficits), incomplete especially in L5 and S1 nerve root involvement, 83% recovery achieved after 2–4 months; shorter symptom duration associated with higher recovery rate, inverse correlation of the degree of paresis with recovery (p = 0.0046) | retrospective, definition of plantar flexion paresis (MRC 2/5 = tiptoe standing < 2 cm, mrc 3/5 = 2 cm); duration of paresis 7–730 days |
| (Lonne 2012) Retrospective study |
91 with 1 year FU, outcome of surgically treated motor deficits | recovery rate: 55% (MRC (0–3) vs. 84% (MRC 4/5) (p = 0.003); no effect of symptom duration; HRQL, pain and functional status improved in all (p < 0.001), recovery led to better outcome and fewer compensation payments | retrospective, multiple examiners, 18 symptomatic recurrences excluded, no conservative control group, limited data on riming |
| (Masuda 2020) Retrospective study |
87 patients with foot drop; outcome of motor deficits (purely surgical cohort) | 32% complete recovery, age, degree of paresis, and symptom duration (> 2 months) were RF for incomplete recovery | diverse pathologies (46 LDH-associated paresis) and heterogeneous surgical treatments; symptom duration 0 – 60 months; recovery = MRC ≥ 4/5 |
| (Girardi 2002) Retrospective study |
55 patients with foot drop (MRC 2–4/5 [average: 3]); outcome of motor deficits (purely surgical cohort) | 71% recovery ratefor foot drop and 64 % for big-toe drop | diverse pathologies (LDH and LSS), few cases, foot drop defined as MRC 4/5, missing subanalysis |
* with high-level evidence (meta-analysis, systematic review and RCTs), medium-level evidence (prospective studies with large numbers of cases, small RCTs), and low evidence (small cohort studies, retrospective analyses)
CES, cauda equina syndrome; FU, follow-up; HR, hazard tatio; LDH, lumbar disc herniation; LSS, lumbar spinal stenosis; MRC, Medical Resaerch Council; OR, odds ratio; pat., patient(s); RCT, randomized controlled trial; RF, risk factor
Lack of consensus on the timing of surgery.
Despite the commonness of this type of surgery, there is no consensus on the optimal timing of treatment for patients suffering “only” from persistent pain, or for those who have a motor deficit.
Learning objectives
The purpose of this review is to provide an understanding of the main predictors of outcome in the treatment of lumbar disc herniation so that these can be integrated into routine clinical practice. After reading the article, readers should know:
which patients with symptomatic disc herniation should be referred immediately for surgery;
how soon a cauda equina syndrome due to lumbar disc herniation should be treated in order to enable recovery, or at least, improvement of bladder and rectal function;
and what the risk factors are for incomplete recovery of paresis due to lumbar disc herniation
Symptom duration and outcome of sciatica
The duration of sciatica and the outcome of treatment.
Sciatica is usually self-limiting: most patients with acute symptoms report marked improvement within 10 days, and 75% within one month.
Sciatica is usually self-limiting: most patients with acute symptoms report marked improvement within 10 days, and 75% within one month. Nevertheless, around 30% of patients who do not undergo surgery still complain of intermittent pain one year after symptom onset (8). Conservative measures include physical rest including short-term bed rest, physiotherapy, and analgesic drugs according to the WHO scheme. Locally acting medications and periradicular infiltrations are available as well (9, e8). The AWMF guideline currently recommends sustained analgesic therapy in the acute stage through to the chronic phase, as well as short-term rest in the acute phase with the introduction of appropriate exercise therapy in the subacute stage. Back training, manipulation or traction if the affected segment, electrotherapy, ultrasound and massages should be avoided in the acute phase. Orthoses (corsets) should be considered on a case-by-case basis and should not be given to patients at risk of chronification.
Consideration of the potential indication for surgery is recommended at 6–12 weeks (3). Most of the studies that were examined in a systematic review published in 2014 found that longer symptom duration before surgery is associated with poorer outcomes, and that surgery within 6 months of symptom onset yields better results (10). The reported durations of conservative treatment are distributed over a wide range (2–12 months) (10). Accordingly, some studies have shown that surgery yields better outcomes when the symptoms have been present for less than 8 months (11, 12), while others have shown the same with a cutoff of 6 months (13, e9).
The well-known Spine Patient Outcomes Research Trial (SPORT) also identified six months as the critical duration of symptoms. In both the conservative and the surgical arm, patients with a shorter duration of symptoms (< 6 months) had a better long-term outcome, in particular a higher level of activity and less residual pain. Those who were treated surgically had better patient-reported outcome measures (PROMs) at all time points, independently of the timing of treatment (14).
Fisher et al., in their prospective study, stressed the importance of PROMs in modern spinal surgery and documented a significant clinical improvement in the first 6 months after surgery. The beneficial effect of the operation leveled off by 1 year (15). This can be explained by the generally self-limiting and benign course of sciatica due to lumbar disc herniation, but possibly also by late adverse effects of surgery.
The evidence for surgical treatment.
The evidence supporting the conclusion that surgery yields better outcomes than conservative treatment or epidural steroid injection (ESI) is on a low level. The benefit of surgery compared to ESI is over the long term; its benefit compared to conservative treatment is over the short to medium term.
A recent meta-analysis by Liu et al. confirmed the beneficial effect of surgery, demonstrating significant short- and medium-term relief of radicular pain in the surgical group compared to the conservatively managed control group. The evidence supporting the conclusion that surgery yields better outcomes than conservative treatment or epidural steroid injection (ESI) is on a low level. The benefit of surgery compared to ESI is over the long term; its benefit compared to conservative treatment is over the short to medium term, with a barely detectable difference at one year. The same holds for the effect of ESI, except for the disability score (9). The interpretability of the findings of this meta-analysis is lessened by the limitations of the included studies, the lack of information on symptom duration, and limited applicability to the heterogeneous population of patients with lumbar disc herniation.
Peul et al., in a landmark study, randomized patients with sciatica of 6–12 weeks’ duration to early surgery or conservative treatment (for at least six months) and found that surgery led to faster recovery. Conservative treatment was also generally followed by recovery, but only after a delay; over the years following randomization, the outcomes converged. The high cross-over rate of 39% within 19 weeks is a critical limitation on the interpretability of the findings and leaves the actual predictive role of symptom duration before surgery unclear (16).
The German and Danish specialty societies recommend a 6– to 12-week trial of conservative treatment, except for patients with functionally impairing neurologic deficits or intractable pain, who should be offered early surgery (6, 17, e10). The NASS (North American Spine Society) points out the poor quality of the data supporting early surgery for patients with motor deficits and accordingly recommends surgery no later than 6–12 months after symptom onset for patients whose deficits are not highly acute (17, 18).
The high cross-over rate (17%–50%) in many RCTs markedly impairs the interpretability of treatment effects determined in an intention-to-treat analysis (8, 14, 19–23).
Risk factors for worse treatment outcomes
Risk factors for poorer treatment outcomes.
In most of the studies analyzed here, the duration of sciatica before treatment was found to be a negative predictor for the outcomes of both conservative and (usually) surgical treatment outcome.
In most of the studies analyzed here, the duration of sciatica before treatment was found to be a negative predictor for the outcomes of both conservative and (usually) surgical treatment outcome. This conclusion is supported by high-level evidence from several RCTs as well as multiple prospective studies from single or multiple centers. The precise figures vary considerably, however, depending on the study design, population and control group: < 12 months (11), < 8 months (12), < 6 months (8, 14, e9, 24), < 3 months (e11, e12), < 2 months (25, e13), < 6 weeks (e14). In the meta-analysis by Sabnis et al., 15 out of 19 studies with low- and medium-level evidence showed a benefit from early surgery, but the two with high-level evidence did not; the latter two studies, however, had high cross-over rates and excluded patients with manifestations requiring acute treatment (e.g. cauda equina syndrome, high-grade paresis) and patients with severe pain (10). In a recent systematic review, Rutzen et al. concluded from 10 selected studies that early treatment is advantageous, but could not supply greater precision regarding timing (beyond simply early vs. late) or any concrete recommendation, because of the heterogeneity of the data (26).
Other risk factors for an unsatisfactory result include:
chronic pain (10)
poor performance scores or neurologic deficits (10)
a recurrent prolapse (27)
social or relationship status and
Disc surgery is generally considered safe, with an intraoperative complication rate of 2.7%. The revision rate is approximately 2.1%, and the rate of readmission rate within 90 days is 2.4%. Complications are more common in elderly patients and in patients with comorbidities (28).
The most common intraoperative complication is an incidental durotomy (dural opening or tear), which occurs most commonly in revision surgery in the setting of severe lumbar spinal degenerative changes in an elderly patient (29). The most common postoperative complication is symptomatic recurrent disc herniation, which occurs in 1% to 27% of patients depending on the type of herniation, the size of the annular defect, and the patient’s age and sex.
The 27% figure applies only to the high-risk group in the study of Carragee et al. and is not to be taken as a general reference value (30, e5, e17). The reoperation rate within one year is approximately 6.4% (e18). Impaired wound healing, secondary spinal instability, and postoperative bleeding requiring treatment are rare.
Because outcomes are better with earlier surgery and the complication rate of these operations is low, surgery should be offered if pain persists after 12 weeks of conservative treatment.
The optimal timing of treatment for patients with motor deficits
The optimal timing of treatment for patients with a motor deficit.
The more severe the paresis, the greater the inclination to provide early surgery in order to prevent permanent functional impairment due to persistent weakness, particularly in younger patients (e19). Nevertheless, no recommendation on the timing of surgery has yet been made for this cohort (10, 24).
Pain that lasts for several months because of spinal nerve root compression is not known to cause any long-term physical damage, but neurologists and surgeons who operate on the spine are concerned that motor deficits might persist if surgery is not performed soon enough. The more severe the paresis, the greater the inclination to provide early surgery in order to prevent permanent functional impairment due to persistent weakness, particularly in younger patients (e19). Nevertheless, no recommendation on the timing of surgery has yet been made for this cohort (10, 24).
As 30% to 50% of patients have paresis when they present, this is a very important issue (33, 34). Paresis is more common in patients with acute onset of symptoms, a free or migrated disc herniation, and/or pre-existing spinal canal stenosis (e20). Medium- to high-level of evidence supports the conclusion that the recovery rate is lower for higher degrees of paresis. Studies including the review by Sharma et al. have identified both the degree of paresis (33, 34) and the duration of symptoms (4, 34) as risk factors for incomplete recovery (strength less than 5 on the MRC scale); the operations in question, however, were performed over a broad time span of several weeks after the onset of paralysis (35, 36, e21–25).
Risk factors for paresis.
Paresis is more common in patients with acute onset of symptoms, a free or migrated disc herniation, and/or pre-existing spinal canal stenosis
Except for patients with cauda equina syndrome, the evidence on symptom duration as a negative predictor is only moderately strong. The recovery rate varies from 30% to 75% depending on the timing and modality of treatment and the degree of paresis, many patients are left with functional impairment, which may be severe (e25–29). Because of these poor outcomes, urgent or even emergency treatment of patients with a relevant degree of paresis (MRC ≤ 3/5) is increasingly being advocated, not least because of the short time window (≤ 48 h) for cauda equina syndrome. In a meta-analysis, Ahn et al. calculated an odds ratio (OR) of 9.1 for motor recovery when surgery is performed within 48 hours (4). Although this high value must be viewed critically, one must indeed ask whether acute paresis, analogously to cauda equina syndrome, should optimally decompressed just as rapidly to prevent a disabling residual deficit.
The lack of an existing recommendation on the timing of surgery motivated us to evaluate the utility of early surgery. In a registry study with 390 patients who underwent early surgery at a spinal center for lumbar disc herniation with associated paresis, the relation of the duration and degree of paresis to the long-term outcome was studied (follow-up interval ≥ 1 year; mean, 3.5 years). For this purpose, objectifiable cut-off values for treatment recommendations were determined with the aid of the Unbiased Recursive Partitioning Conditional Inference Tree (URP-CTREE) (7). This statistical method tests the independence of predictors with a predefined outcome; like a tree diagram, it involves partitioning at the lowest p-value (including a Bonferroni correction) (e30).
Strength was measured with manual and functional testing and graded on the MRC scale (37).
Preoperative paresis was mild (MRC 4/5) in 118 patients (30.3%), moderate (MRC 3/5) in 191 (49.0%), and severe (MRC ≤ 2/5) in 81 (20.8%).
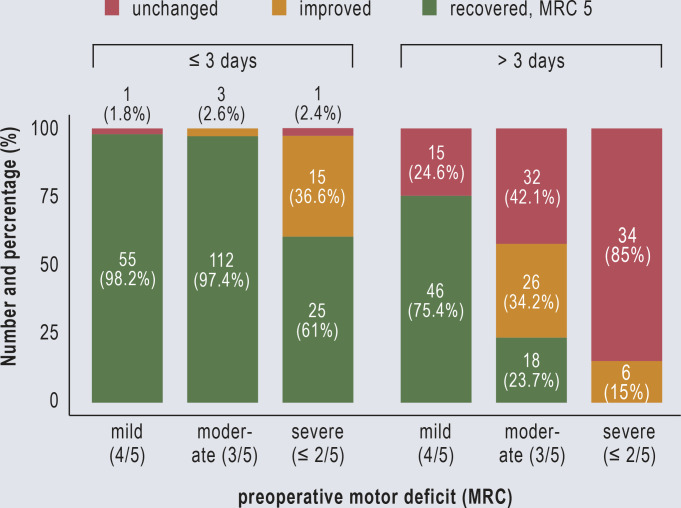
According to the URP-CTREE analysis, severe preoperative paresis (p < 0.001) was the main risk factor for incomplete recovery. The calculated time window from the onset of paresis to surgery for a significantly faster and complete recovery (= MRC 5/5) was 3 days (p = 0.022). Surgery within this time window also led to a significantly better outcome in patients with moderate paresis (p < 0.001), with a recovery rate of 97% versus 23% (Figure 1). In the mild paresis cohort, early surgery (≤ 8 days; using URP-CTREE cut-off) also yielded a superior recovery rate (98% versus 75%) (7).
Figure 1.
Motor deficit at last follow-up: Bar chart of the recovery rate as a function of the timing of treatment and the degree of preoperative paresis. Modified from the study by Thomé et al. (13)
The benefit of early surgery.
The benefit of early surgery must be critically evaluated in order not to expose patients excessively to the risks of surgery.
This study lacks a conservatively treated control group, but nevertheless demonstrates the advantage of early treatment with the statistics used and the precise information it contains on symptom duration and degree of paresis. The results are clearly superior to those of other studies in which treatment after multiple weeks or months is advocated (4, 33–35, e21, e23, e24). The authors advise against generalizing these findings to cases of paresis due to spinal stenosis, in which the pathophysiology is chronic rather than acute (34, e26). Although early surgery may intuitively seem preferable even without supporting evidence, its benefit needs to be properly studied, as the nonzero perioperative risk may be greater than the benefit for some patients. Thus, additional prospective studies to corroborate these findings would be desirable. Only on the basis of the data just cited, early (≤ 3 days) surgery is recommended for patients with acute moderate or severe paresis, as this yields a recovery rate of 97% versus 50%, according to the relevant RCT (33). Only Ahn et al. achieved a similar recovery rate with early required to be within 48 hours of symptom onset (4). Comparisons across studies are generally complicated by inconsistent definitions of the term “foot drop,” varying meanings of the word “recovery” (does a partial recovery count?), and the subjectivity (sometimes) of the determination whether a paresis is mild, moderate, or severe.
To prevent permanent deficits, patients should be referred immediately to a center where spinal surgery is performed. The general practitioners and family physicians who see the patient first should rapidly assess the degree of paresis and refer the patient for surgery if it is moderate or severe. Isolated paresis of the extensor hallucis longus muscle (weakness of great toe dorsiflexion) is not necessarily a clinically relevant indication for surgery, even if severe; this issue should be critically considered by the examiner.
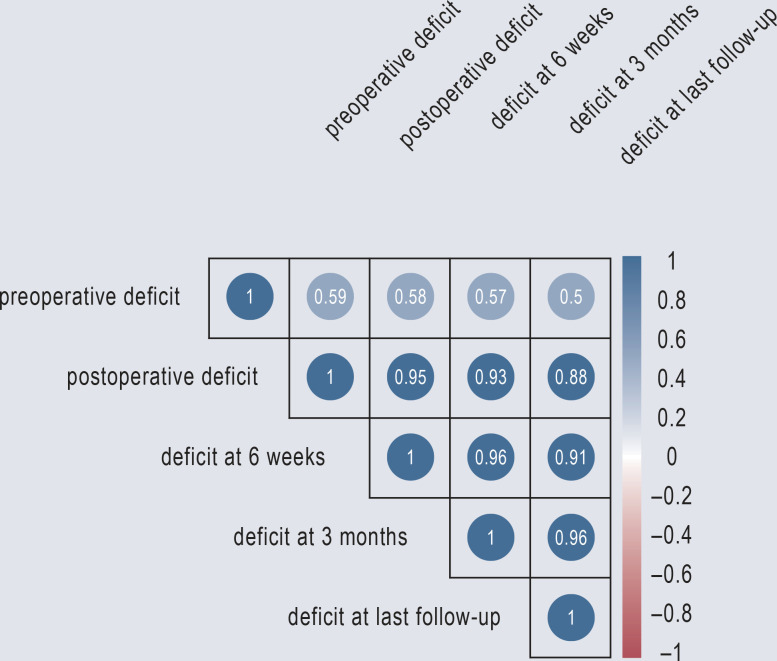
Mild (MRC 4/5) motor deficits are obviously less problematic, and the stated time window of 8 days to surgery should be considered only for patients with weakness causing functional impairment (e.g., quadriceps palsy making it impossible to climb stairs) (recovery rate of 98%). The literature implies a recovery rate of 75% in the later (> 8 days) cohort. Compensatory training of paretic muscle groups should lead to success, particularly the quadriceps; deficits in the muscles innervated by L5 and S1 are more likely to be permanent (e24). Treatment decisions must be made individually on the basis of the symptoms and signs, the affected myotome, the patient’s age and personal requirements, and the degree of functional impairment. Regular follow-up at short intervals is needed at first, so that any neurological worsening can be detected and acted upon quickly. The patient should be informed about the natural course of the problem if untreated and the improved recovery rate in the event of surgical treatment. Marked improvement of weakness can be expected within 2–4 months after surgery (the interval varies depending on the publication) (e23, e24). Our experience is similar, with a strong correlation between strength at 6–12 weeks and final strength (Figure 2). Strength at 3 months is thus a good surrogate marker for the ultimate degree of recovery (7).
Figure 2.
Plot from the study by Thomé et al. (13) showing the correlation between strength on initial examination and long-term strength after treatment.
The timing of treatment for cauda equina syndrome
The management of acute, moderate to severe weakness.
On the basis of the data cited here, early (≤ 3 days) surgery is recommended for patients with acute moderate or severe paresis, as this yields a recovery rate of 97% versus 50%, according to a relevant RCT.
The current recommendation for immediate surgery (< 48 hr after symptom onset) for patients with cauda equina syndrome is based on the findings of the meta-analysis by Ahn et al. In a long-term analysis of over 850 patients (20 retrospective and 2 prospective cohort studies), residual bladder, rectal and sexual dysfunction was found in 43%, 31% and 40%, respectively. Acute decompression (< 48 hr) led to the complete resolution of symptoms and signs in 76% of patients so treated (38). Patients who underwent surgery within 24 hours had a higher recovery rate (5). Sangondimath et al. reported improvement in all patients who underwent within 48 hours. Sexual dysfunction was still present after surgery in 70% of men and 60% of women with cauda equina syndrome (39). Other, retrospective studies, including urodynamic studies, confirmed that treatment within 48 hours leads to a favorable recovery, while the adverse consequences of delayed surgery are dramatic (e31–e33).
Saddle hypesthesia and bladder and rectal dysfunction.
Lumbar disc herniation causing bladder or bowel dysfunction (cauda equina syndrome) is a surgical emergency. This situation is present in approximately 2% of cases of lumbar disc herniation.
Take-home messages on the timing of surgery for lumbar disc herniation
Surgery should be considered 6–12 weeks after the onset of symptoms if radicular pain persists despite conservative treatment.
Emergency surgery (within 24–48 hr) is indicated for patients with bladder or rectal dysfunction.
Surgery within 3 days is indicated for patients with moderate or severe paresis (MRC ≤ 3/5).
Early surgery is indicated for patients who have mild paresis (MRC 4/5) causing functional impairment.
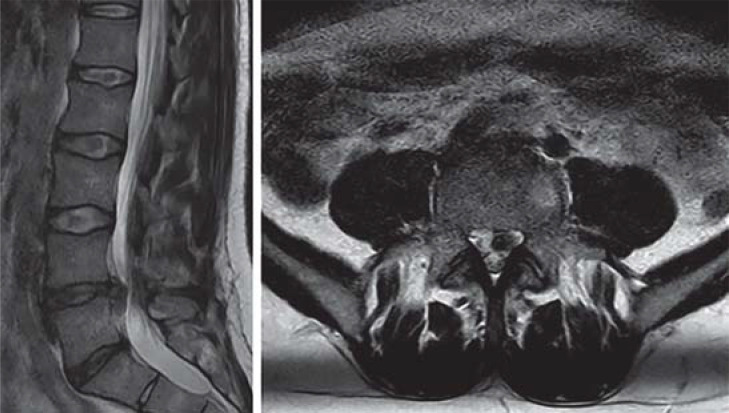
Figure 3.
Left: Sagittal T2-weighted magnetic resonance image of the lumbar spine showing disc herniation at L4/5. Right: Axial T2-weighted image showing disc herniation, more pronounced on the left side, with compression of the L5 nerve root.
Further information on CME.
Participation in the CME certification program is possible only over the Internet: cme.aerzteblatt.de. This unit can be accessed until 27 June 2025. Submissions by letter, e-mail or fax cannot be considered.
The completion time for all newly started CME units is 12 months. The results can be accessed 4 weeks following the start of the CME unit. Please note the respective submission deadline at: cme.aerzteblatt.de.
This article has been certified by the North Rhine Academy for Continuing Medical Education. CME points can be managed using the “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or entered in “Meine Daten,” and consent must be given for results to be communicated. The 15-digit EFN can be found on the CME card (8027XXXXXXXXXXX)
Questions on this article.
Participation is possible at cme.aerzteblatt.de
The submission deadline is 27 June 2025. Only one answer is possible per question.
Please select the answer that is most appropriate.
Question 1
What is the annual incidence of sciatica in the general population?
1–5%
10–15%
20–25%
30–35%
40–45%
Question 2
Which statement best describes the natural course of sciatica?
It is usually a self-limiting disease.
It is a secondary progressive disease.
85% of patients undergo surgery because of symptoms.
Most patients’ symptoms worsen after 10 days.
90% of patients treated conservatively are asymptomatic a year later.
Question 3
According to the AWMF guideline, how long after the initiation of conservative treatment for lumbar disc herniation should the indication for surgery be considered if symptoms persist?
1 to 7 days
8 days to 2 weeks
2 to 3 weeks
4 to 5 weeks
6 to 12 weeks
Question 4
What conclusion can be drawn from the meta-analysis by Liu et al. (2023)?
In case of severe back pain down the thigh dorsolaterally, surgery should be performed within one week.
Physiotherapy should be started in the acute phase of the disease.
There is little evidence that surgery yields better outcomes than conservative therapy or epidural steroid injection.
Surgery within 10 days increases the chance of pain relief (OR = 2.3 [95% CI: 1.7; 2.9]).
The risk of a neurologic deficit is twice as high if surgery is not performed within 12 weeks of the onset of severe pain.
Question 5
Which factor significantly limits the informativeness and interpretability of many RCTs on the treatment of patients with sciatica?
difficulties in the recruitment of study subjects
a drop-out rate of up to 50%
major differences in proficiency across surgeons
high cross-over rates from the conservative to the surgical treatment arm
small study sizes, so that statistical significance could not be achieved
Question 6
What is the most common intraoperative complication of lumbar disc surgery?
injury to major vessels
incidental durotomy
nerve root injury
bacterial contamination
a symptomatic recurrent herniation
Question 7
Which of the following is an important risk factor for the incomplete recovery of a motor deficit caused by a herniated disc?
a concomitant sensory deficit
moderate or severe paresis (MRC = 3/5)
the patient‘s age
inability to work for 2 weeks or more
a mild foot drop
Question 8
Which of the following is/are not a risk factor for a worse clinical outcome?
chronic pain, older age
recurrent herniation, age > 50 years
inability to work for > 4 months, depression
ongoing compensation payments, neurologic deficit
male sex, BMI > 28
Question 9
Which of the following is a common postoperative complication of lumbar disc surgery?
secondary instability
inability to work for > 4 weeks
rectal dysfunction
depression
deep venous thrombosis
Question 10
What conclusion can be drawn from the registry study by Thomé et al.?
Early surgery should be recommended for acute and high-grade paresis (MRC = 3/5).
Surgery should be performed within 48 h if spinal canal stenosis is also present.
An epidural steroid injection leads to a faster return to work than surgery.
Conservative therapy should be supported by massage and progressive muscle relaxation.
Early surgery is only indicated for patients with cauda equina syndrome.
Participation is possible only via: cme.aerzteblatt.de
CASE ILLUSTRATION.
A 43-year-old man presents to the emergency room with low back pain of two weeks’ duration that began when he changed the tires on his car. He took non-steroidal anti-inflammatory drugs (NSAIDs) insufficient pain relief. The pain in the low back improved slightly one day before presentation, but he then developed marked sciatica radiating dorsolaterally down the left thigh and leg into the left great toe.
When the patient enters the examining room, the physician notices a mild Trendelenburg sign. Neurological examination reveals that the patient cannot walk on the left heel. Strength testing with the patient supine reveals weakness of foor dorsiflexion (3/5) and of great toe dorsiflexion and foot eversion (2/5) on the left side. The Lasègue sign is positive on the left. Hypesthesia is noted along the left L5 dermatome. The patient denies bladder or bowel dysfunction, and residual urine measurement yields a value of 0 mL. Saddle hypesthesia is not present.
The remaining medical history is unremarkable, except for surgical repair of a cruciate ligament and an appendectomy in childhood. There is no history of a tick bite, a recent infection, or B symptoms.
The physician tells the patient that he has an L5 nerve root syndrome that is probably due to lumbar disc herniation.
Magnetic resonance imaging (MRI) of the lumbar spine without contrast, ordered acutely to confirm the suspected diagnosis, reveals a mediolateral, partially caudally dislocated herniated disc at the L4–5 level on the left, with compression of the L5 nerve root (see Figure 3).
The findings are discussed in detail and the treatment options explained. The patient is offered hospitalization for sequestrectomy because of the marked paresis and the associated functional impairment. He requests prompt surgery, which is performed the next day without complications. The paresis improved immediately. Except for persistent mild (4/5) weakness of great toe dorsiflexion, the motor deficits were found to have recovered completely one year after surgery.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
WL owns Novartis stock.
The other authors state that they have no conflict of interest.
References
- 1.Global Burden of Disease Study. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stafford MA, Peng P, Hill DA. Sciatica: a review of history,epidemiology, pathogenesis, and the role of epidural steroid injection in management. Br J Anaesth. 2007;99:461–473. doi: 10.1093/bja/aem238. [DOI] [PubMed] [Google Scholar]
- 3.Bauer J, Böhle E, Bork H, Broll-Zeitvogel E, Brüggemann S, et al. S2k-Leitlinie - Konservative, operative und rehabilitative Versorgung bei Bandscheibenvorfällen mit radikulärer Symptomatik. https://register.awmf.org/de/leitlinien/detail/187-057 (last accessed on 13 March 2024) [Google Scholar]
- 4.Ahn UM, Ahn NU, Buchowski JM, Garrett ES, Sieber AN, Kostuik JP. Cauda equina syndrome secondary to lumbar disc herniation: a meta-analysis of surgical outcomes. Spine (Phila Pa 1976) 2000;25:1515–1522. doi: 10.1097/00007632-200006150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Kohles SS, Kohles DA, Karp AP, Erlich VM, Polissar NL. Time-dependent surgical outcomes following cauda equina syndrome diagnosis: comments on a meta-analysis Spine (Phila Pa 1976) 2004:1281–1287. doi: 10.1097/00007632-200406010-00019. [DOI] [PubMed] [Google Scholar]
- 6.Glocker FX Deutsche Gesellschaft für Neurologie, editors. Leitlinien für Diagnostik und Therapie in der Neurologie—Lumbale Radikulopathie. 2018 [Google Scholar]
- 7.Thomé C, Kögl N, Grassner L, Vo AK, Kramer JK, Petr O. Motor recovery depends on timing of surgery in patients with lumbar disk herniation. Neurosurgery. 2022;90:347–353. doi: 10.1227/NEU.0000000000001825. [DOI] [PubMed] [Google Scholar]
- 8.Peul WC, van Houwelingen HC, van den Hout WB, et al. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245–2256. doi: 10.1056/NEJMoa064039. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Ferreira GE, Abdel Shaheed C, et al. Surgical versus non-surgical treatment for sciatica: systematic review and meta-analysis of randomised controlled trials. BMJ. 2023;381 doi: 10.1136/bmj-2022-070730. 3070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabnis AB, Diwan AD. The timing of surgery in lumbar disc prolapse: a systematic review. Indian J Orthop. 2014;48:127–135. doi: 10.4103/0019-5413.128740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng LCL, Sell P. Predictive value of the duration of sciatica for lumbar discectomy. A prospective cohort study. J Bone Joint Surg Br. 2004;86:546–549. [PubMed] [Google Scholar]
- 12.Nygaard OP, Kloster R, Solberg T. Duration of leg pain as a predictor of outcome after surgery for lumbar disc herniation: a prospective cohort study with 1-year follow up. J Neurosurg. 2000;92:131–134. doi: 10.3171/spi.2000.92.2.0131. [DOI] [PubMed] [Google Scholar]
- 13.Silverplats K, Lind B, Zoëga B, et al. Clinical factors of importance for outcome after lumbar disc herniation surgery: long-term follow-up. Eur Spine J. 2010;19:1459–1467. doi: 10.1007/s00586-010-1433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rihn JA, Hilibrand AS, Radcliff K, et al. Duration of symptoms resulting from lumbar disc herniation: effect on treatment outcomes: analysis of the Spine Patient Outcomes Research Trial (SPORT) J Bone Joint Surg Am. 2011;93:1906–1914. doi: 10.2106/JBJS.J.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher C, Noonan V, Bishop P, et al. Outcome evaluation of the operative management of lumbar disc herniation causing sciatica. J Neurosurg Spine. 2004;100:317–324. doi: 10.3171/spi.2004.100.4.0317. [DOI] [PubMed] [Google Scholar]
- 16.Peul WC, Hout WB van den, Brand R, Thomeer RTWM, Koes BW. Leiden-The Hague Spine Intervention Prognostic Study Group: Prolonged conservative care versus early surgery in patients with sciatica caused by lumbar disc herniation: two year results of a randomised controlled trial. BMJ. 2008;336:1355–1358. doi: 10.1136/bmj.a143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan ZY, Shan H, Liu TF, et al. Emerging issues questioning the current treatment strategies for lumbar disc herniation. Front Surg. 2022;9 doi: 10.3389/fsurg.2022.814531. 814531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreiner DS, Hwang SW, Easa JE, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014;14:180–191. doi: 10.1016/j.spinee.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Hofstee DJ, Gijtenbeek JMM, Hoogland PH, et al. Westeinde sciatica trial: randomized controlled study of bed rest and physiotherapy for acute sciatica. J Neurosurg. 2002;96:45–49. doi: 10.3171/spi.2002.96.1.0045. [DOI] [PubMed] [Google Scholar]
- 20.Buttermann GR. Treatment of lumbar disc herniation: epidural steroid injection compared with discectomy: a prospective, randomized study. J Bone Surg Am. 2004;86:670–679. [PubMed] [Google Scholar]
- 21.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT) Spine (Phila Pa 1976) 2008;33:2789–2800. doi: 10.1097/BRS.0b013e31818ed8f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey CS, Rasoulinejad P, Taylor D, et al. Surgery versus conservative care for persistent sciatica lasting 4 to 12 months. N Engl J Med. 2020;382:1093–1102. doi: 10.1056/NEJMoa1912658. [DOI] [PubMed] [Google Scholar]
- 23.Wilby MJ, Best A, Wood E, et al. Surgical microdiscectomy versus transforaminal epidural steroid injection in patients with sciatica secondary to herniated lumbar disc (NERVES): a phase 3, multicentre, open-label, randomised controlled trial and economic evaluation. Lancet Rheumatol. 2021;3:347–356. doi: 10.1016/S2665-9913(21)00036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoenfeld AJ, Bono CM. Does surgical timing influence functional recovery after lumbar discectomy? A systematic review. Clin Orthop Relat Res. 2015;473:1963–1970. doi: 10.1007/s11999-014-3505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothoerl RD, Woertgen C, Brawanski A. When should conservative treatment for lumbar disc herniation be ceased and surgery considered? Neurosurg Rev. 2002;25:162–165. doi: 10.1007/s101430100184. [DOI] [PubMed] [Google Scholar]
- 26.Rutzen AT, Annes RD agostin., da Silva SG. Elsevier B.V.; 2022. Clinical and functional outcomes in patients submitted to early versus late surgery for lumbar disc herniation: a systematic review. Vol. 29, Interdisciplinary Neurosurgery: Advanced Techniques and Case Management. 101550. [Google Scholar]
- 27.Gaetani P, Aimar E, Panella L, Debernardi A, Tancioni F, Rodriguez y Baena R. Surgery for herniated lumbar disc disease: factors influencing outcome measures. An analysis of 403 cases. Funct Neurol. 2004;19:43–49. [PubMed] [Google Scholar]
- 28.Fjeld OR, Grøvle L, Helgeland J, et al. Complications, reoperations, readmissions, and length of hospital stay in 34 639 surgical cases of lumbar disc herniation. Bone Joint J. 2019;101-B:470–477. doi: 10.1302/0301-620X.101B4.BJJ-2018-1184.R1. [DOI] [PubMed] [Google Scholar]
- 29.Baker GA, Cizik AM, Bransford RJ, et al. Risk factors for unintended durotomy during spine surgery: a multivariate analysis. Spine J. 2012;12:121–126. doi: 10.1016/j.spinee.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carragee EJ, Han MY, Suen PW, Kim D. Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am. 2003;85:102–108. [PubMed] [Google Scholar]
- 31.Österman H, Seitsalo S, Karppinen J, Malmivaara A. Effectiveness of microdiscectomy for lumbar disc herniation. Spine (Phila Pa 1976) 2006;31:2409–2414. doi: 10.1097/01.brs.0000239178.08796.52. [DOI] [PubMed] [Google Scholar]
- 32.Weber H. Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine (Phila Pa 1976) 1983;8:131–140. [PubMed] [Google Scholar]
- 33.Overdevest GM, Vleggeert-Lankamp CL, Jacobs WC, Brand R, Koes BW, Peul WC. Recovery of motor deficit accompanying sciatica—subgroup analysis of a randomized controlled trial. Spine J. 2014;14:1817–1824. doi: 10.1016/j.spinee.2013.07.456. [DOI] [PubMed] [Google Scholar]
- 34.Sharma H, Lee SWJ, Cole AA. The management of weakness caused by lumbar and lumbosacral nerve root compression. J Bone Joint Surg Br. 2012;94-B:1442–1447. doi: 10.1302/0301-620X.94B11.29148. [DOI] [PubMed] [Google Scholar]
- 35.Macki M, Syeda S, Kerezoudis P, Gokaslan ZL, Bydon A, Bydon M. Preoperative motor strength and time to surgery are the most important predictors of improvement in foot drop due to degenerative lumbar disease. J Neurol Sci. 2016;361:133–136. doi: 10.1016/j.jns.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Masuda S, Kanba Y, Kawai J, Ikeda N. Prognostic factors for drop foot due to lumbar degenerative diseases. Clin Spine Surg A Spine Publ. 2020;33:160–162. doi: 10.1097/BSD.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 37.Paternostro-Sluga T, Grim-Stieger M, Posch M, et al. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40:665–671. doi: 10.2340/16501977-0235. [DOI] [PubMed] [Google Scholar]
- 38.Kumar V, Baburaj V, Rajnish RK, Dhatt SS. Outcomes of cauda equina syndrome due to lumbar disc herniation after surgical management and the factors affecting it: a systematic review and meta-analysis of 22 studies with 852 cases. Eur Spine J. 2022;31:353–363. doi: 10.1007/s00586-021-07001-0. [DOI] [PubMed] [Google Scholar]
- 39.Sangondimath G, Mallepally AR, Mascharenhas A, Chhabra HS. Sexual and bladder dysfunction in cauda equina syndrome: correlation with clinical and urodynamic studies. Asian Spine J. 2020;14:782–789. doi: 10.31616/asj.2019.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318:291–300. doi: 10.1056/NEJM198802043180506. [DOI] [PubMed] [Google Scholar]
- E2.Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine (Phila Pa 1976) 2008;33:2464–2472. doi: 10.1097/BRS.0b013e318183a4a2. [DOI] [PubMed] [Google Scholar]
- E3.Grotle M, Småstuen MC, Fjeld O, et al. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-028743. e028743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the united states, 2004 to 2015. Spine (Phila Pa 1976) 2019;44:369–376. doi: 10.1097/BRS.0000000000002822. [DOI] [PubMed] [Google Scholar]
- E5.Thomé C, Klassen PD, Bouma GJ, et al. Annular closure in lumbar microdiscectomy for prevention of reherniation: a randomized clinical trial. Spine J. 2018;18:2278–2287. doi: 10.1016/j.spinee.2018.05.003. [DOI] [PubMed] [Google Scholar]
- E6.Arts MP, Peul WC Leiden-Hague Spine Intervention Prognostic Study Group L-THSIPSG. Timing and minimal access surgery for sciatica: a summary of two randomized trials. Acta Neurochir (Wien) 2011;153:967–974. doi: 10.1007/s00701-011-0983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:372. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Saal JA, Saal JS. Nonoperative treatment of herniated lumbar intervertebral disc with radiculopathy. An outcome study. Spine (Phila Pa 1976) 1989;14:431–437. doi: 10.1097/00007632-198904000-00018. [DOI] [PubMed] [Google Scholar]
- E9.Quigley MR, Bost J, Maroon JC, Elrifai A, Panahandeh M. Outcome after microdiscectomy: results of a prospective single institutional study. Surg Neurol. 1998;49:263–267. doi: 10.1016/s0090-3019(97)00448-5. discussion 267-8. [DOI] [PubMed] [Google Scholar]
- E10.NKR. Ikke-kirurgisk behandling af nylig opstået lumbal nerverodspåvirkning - Sundhedsstyrelsen. https://www.sst.dk/da/udgivelser/2016/nkr-for-ikke-kirurisk-behandling-af-nylig-opstaaet-lumbal-nerverodspaavirkning-lumbal-radikulopati (last accessed on16 March 2024) [Google Scholar]
- E11.Peul WC, Brand R, Thomeer RTWM, Koes BW. Influence of gender and other prognostic factors on outcome of sciatica. Pain. 2008;138:180–191. doi: 10.1016/j.pain.2007.12.014. [DOI] [PubMed] [Google Scholar]
- E12.Støttrup CC, Andresen AK, Carreon L, Andersen MØ. Increasing reoperation rates and inferior outcome with prolonged symptom duration in lumbar disc herniation surgery — a prospective cohort study. Spine J. 2019;19:1463–1469. doi: 10.1016/j.spinee.2019.04.001. [DOI] [PubMed] [Google Scholar]
- E13.Hurme M, Alaranta H. Factors predicting the result of surgery for lumbar intervertebral disc herniation. Spine (Phila Pa 1976) 1987;12:933, 938. doi: 10.1097/00007632-198711000-00016. [DOI] [PubMed] [Google Scholar]
- E14.Folman Y, Shabat S, Catz A, Gepstein R. Late results of surgery for herniated lumbar disk as related to duration of preoperative symptoms and type of herniation. Surg Neurol. 2008;70:398–401. doi: 10.1016/j.surneu.2007.04.022. [DOI] [PubMed] [Google Scholar]
- E15.Junge A, Dvorak J, Ahrens S. Predictors of bad and good outcomes of lumbar disc surgery. A prospective clinical study with recommendations for screening to avoid bad outcomes. Spine (Phila Pa 1976) 1995;20:460–468. doi: 10.1097/00007632-199502001-00009. [DOI] [PubMed] [Google Scholar]
- E16.Ren BO, O’Donnell JA, Anderson JT, et al. Time to surgery affects return to work rates for workers’ compensation patients with single-level lumbar disk herniation. Orthopedics. 2021;44:43–49. doi: 10.3928/01477447-20201202-06. [DOI] [PubMed] [Google Scholar]
- E17.Martens F, Vajkoczy P, Jadik S, Hegewald A, Stieber J, Hes R. Patients at the highest risk for reherniation following lumbar discectomy in a multicenter randomized controlled trial. JBJS Open Access. 2018;3 doi: 10.2106/JBJS.OA.17.00037. e0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E18.Martin BI, Mirza SK, Flum DR, et al. Repeat surgery after lumbar decompression for herniated disc: the quality implications of hospital and surgeon variation. Spine J. 2012;12:89–97. doi: 10.1016/j.spinee.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E19.Arts MP, Peul WC, Koes BW, Thomeer RTWM. Management of sciatica due to lumbar disc herniation in the Netherlands: a survey among spine surgeons. J Neurosurg Spine. 2008;9:32–39. doi: 10.3171/SPI/2008/9/7/032. [DOI] [PubMed] [Google Scholar]
- E20.Krishnan V, Rajasekaran S, Aiyer SN, Kanna R, Shetty AP. Clinical and radiological factors related to the presence of motor deficit in lumbar disc prolapse: a prospective analysis of 70 consecutive cases with neurological deficit. Eur Spine J. 2017;26:2642–2649. doi: 10.1007/s00586-017-5019-5. [DOI] [PubMed] [Google Scholar]
- E21.Aono H, Iwasaki M, Ohwada T, et al. Surgical outcome of drop foot caused by degenerative lumbar diseases. Spine (Phila Pa 1976) 2007;32:262–266. doi: 10.1097/01.brs.0000259922.82413.72. [DOI] [PubMed] [Google Scholar]
- E22.Takenaka S, Aono H. Prediction of postoperative clinical recovery of drop foot attributable to lumbar degenerative diseases, via a Bayesian Network. Clin Orthop Relat Res. 2017;475:872–880. doi: 10.1007/s11999-016-5180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E23.Ghahreman A, Ferch RD, Rao P, Chandran N, Shadbolt B. Recovery of ankle dorsiflexion weakness following lumbar decompressive surgery. J Clin Neurosci. 2009;16:1024–1027. doi: 10.1016/j.jocn.2008.10.017. [DOI] [PubMed] [Google Scholar]
- E24.Postacchini F, Giannicola G, Cinotti G. Recovery of motor deficits after microdiscectomy for lumbar disc herniation. J Bone Jt Surg. 2002;84:1040–1045. doi: 10.1302/0301-620x.84b7.12948. [DOI] [PubMed] [Google Scholar]
- E25.Davis RA. A long-term outcome analysis of 984 surgically treated herniated lumbar discs. J Neurosurg. 1994;80:415–421. doi: 10.3171/jns.1994.80.3.0415. [DOI] [PubMed] [Google Scholar]
- E26.Girardi FP, Cammisa FP, Huang RC, Parvataneni HK, Tsairis P. Improvement of preoperative foot drop after lumbar surgery. J Spinal Disord Tech. 2002;15:490–494. doi: 10.1097/00024720-200212000-00010. [DOI] [PubMed] [Google Scholar]
- E27.Jönsson B, Strömqvist B. Motor Affliction of the L5 nerve root in lumbar nerve root compression syndromes. Spine (Phila Pa 1976) 1995;20:2012–2015. doi: 10.1097/00007632-199509150-00012. [DOI] [PubMed] [Google Scholar]
- E28.Hakelius A. Prognosis in sciatica. A clinical follow-up of surgical and non-surgical treatment. Acta Orthop Scand Suppl. 1970;129:1–76. doi: 10.3109/ort.1970.41.suppl-129.01. [DOI] [PubMed] [Google Scholar]
- E29.Andersson H, Carlsson CA. Prognosis of operatively treated lumbar disc herniations causing foot extensor paralysis. Acta Chir Scand. 1966;132:501–506. [PubMed] [Google Scholar]
- E30.Tanadini LG, Steeves JD, Hothorn T, et al. Identifying homogeneous subgroups in neurological disorders: unbiased recursive partitioning in cervical complete spinal cord injury. Neurorehabil Neural Repair. 2014;28:507–515. doi: 10.1177/1545968313520413. [DOI] [PubMed] [Google Scholar]
- E31.Bečulic H, Skomorac R, Jusic A, et al. Impact of timing on surgical outcome in patients with cauda equina syndrome caused by lumbar disc herniation. Med Glas (Zenica) 2016;13:136–141. doi: 10.17392/861-16. [DOI] [PubMed] [Google Scholar]
- E32.Dave BR, Samal P, Sangvi R, Degulmadi D, Patel D, Krishnan A. Does the surgical timing and decompression alone or fusion surgery in lumbar stenosis influence outcome in cauda equina syndrome? Asian Spine J. 2019;13:198–209. doi: 10.31616/asj.2018.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E33.Kaiser R, Nasto LA, Venkatesan M, et al. Time factor and disc herniation size: are they really predictive for outcome of urinary dysfunction in patients with cauda equina syndrome? Neurosurgery. 2018;83:1193–1200. doi: 10.1093/neuros/nyx607. [DOI] [PubMed] [Google Scholar]