Abstract
Natural killer T (NKT) cells, a unique subpopulation of T cells, coexpress markers also present on NK cells and recognize the major histocompatibility complex class I-like CD1d1 molecule. We studied the effect of an acute virus infection on NKT cells. Mice were infected with the nonhepatotropic Armstrong strain of lymphocytic choriomeningitis virus (LCMV), and at various times postinfection, mononuclear cells from the liver, peritoneum, and spleen were isolated. It was found that within 2 to 3 days, there was a selective loss of NKT cells from the liver with an apparent rapid recovery within 8 to 14 days. There was no increase in peritoneal or splenic NKT cells, indicating that NKT cells did not traffic to these tissues. This loss of NKT cells was independent of gamma interferon (IFN-γ) and interleukin 12 (IL-12) production, but did occur in mice treated with poly(I-C), a classical inducer of IFN-α/β. The reduction in NKT cells was CD28 and fas/fasL independent and occurred via apoptosis. It was not observed in LCMV-infected DNA fragmentation factor 45-deficient mice, and an increase in active caspase 3-specific staining was found in liver NKT cells from LCMV-infected and poly(I-C)-treated mice compared to uninfected wild-type mice. Interestingly, it was also found that liver NKT cells from LCMV-infected mice were themselves infected. These results suggest that the loss of NKT cells following an acute LCMV infection could be due to the induction of IFN-α/β resulting in NKT-cell apoptosis and is important for the host's immune response to LCMV.
Natural killer T (NKT) cells were originally identified as T cells that express cell surface markers (e.g., NK1.1) previously thought to be found exclusively on NK cells (6). NKT cells can also be identified by their predominant use of the T-cell receptor α (TCRα) chain rearrangement, Vα14Jα281, which is associated with Vβ chains of limited diversity and are mostly CD4+ or CD4− CD8−. Very few CD8+ NKT cells have been found, and it has been reported that CD8 expression causes the negative selection of these cells in the thymus (4). The equivalent NKT-cell population in humans uses the homologous TCRα rearrangement (Vα24JαQ) and are mostly CD4− CD8− (50). We have reported that murine NKT cells recognize the nonpolymorphic major histocompatibility complex (MHC) class I-like molecule CD1d1 (5). Although CD1d1 molecules are expressed mainly on hematopoietic tissues, they are also found in the liver (6, 50)—an organ in which NKT cells are the major T-lymphocyte subpopulation. Murine and human NKT cells, upon interaction with the appropriate CD1d-expressing targets or upon stimulation with anti-CD3, promptly produce both interleukin 4 (IL-4) and gamma interferon (IFN-γ) (6, 50). Therefore, NKT-cell production of cytokines important for either Th1- or Th2-mediated responses has suggested that these cells play a role in immunoregulation. In support of this, it has been shown that in scleroderma and diabetes, a lack of (or reduction in) NKT-cell number and/or function contributes to the development of these diseases (23, 29, 37, 59, 65). It has been suggested that this prompt production of IL-4 by NKT cells plays an important role in the induction of Th2-mediated responses (37, 67), although in some systems, it appears that this is not the case (10, 15, 56). An important role for IFN-γ production by NKT cells has been found in the immune response to Nippostrongylus brasiliensis (15), Toxoplasma gondii (16), and Plasmodium yoelii (24).
It has been previously shown that infection of mice with Listeria monocytogenes (20) or Plasmodium yoelii (49) caused rapid decrease and increase, respectively, in liver NKT cells. Only two reports have implicated NKT cells in the immune response to viruses. The first showed evidence that CD4+ NKT cells may play a role in the clearance of adenovirus from the liver of mice (66). In the second, treatment of mice transgenic for the human hepatitis B virus (HBV) with the synthetic glycolipid α-galactosylceramide (α-GalCer) resulted in the inhibition of HBV replication in the liver (31). Because the liver is a major player in the host's acute-phase response to pathogens (22), we were interested in analyzing the effect of virus infection on the NKT cells themselves in the liver and other organs. In the present study, we acutely infected mice with the prototypic arenavirus and natural mouse pathogen lymphocytic choriomeningitis virus (LCMV). Our results demonstrate that LCMV infection of C57BL/6 mice causes the rapid (within 2 to 3 days) and selective loss of NKT cells from the liver, spleen, and peritoneum. This observation has implications for understanding the role that NKT cells play in the events that occur early following a viral infection (7).
MATERIALS AND METHODS
Mice.
Male and female C57BL/6 wild-type and IFN-γ-, IL-12-, and CD28-deficient mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). The knockout mice were bred in specific-pathogen-free facilities at the Indiana University School of Medicine. Female C57BL/6 mice with mutations in the fas (lpr mice) and fas ligand (fasL) (gld mice) genes were also purchased from the Jackson Laboratory. DNA fragmentation factor 45 (DFF45)-deficient mice and their wild-type controls (B6 × 129) have been described previously (69). All mice were used at 6 to 8 weeks of age. All animal procedures were approved by the Indiana University School of Medicine Animal Care and Use Committee.
Virus and infection of mice.
The Armstrong strain (nonhepatotropic) of LCMV was kindly provided by Raymond Welsh (University of Massachusetts Medical Center, Worcester). Virus stocks were prepared in BHK cells and titrated on Vero cells. Mice were infected intraperitoneally (i.p.) with 2 × 105 PFU of LCMV.
Poly(I-C) treatment of mice.
Mice were injected i.p. with 100 μg of poly(I-C) (Sigma Chemical Co., St. Louis, Mo.) in phosphate-buffered saline (PBS). One or 2 days following injection, liver mononuclear cells (MNC) were harvested and stained for NKT cells and/or for the PCR amplification of the Vα14Jα281 TCR rearrangement as described below.
Liver MNC isolation.
The procedure used to isolate liver MNC was a slight modification of that described previously (62). Briefly, at the indicated time points postinfection, mice were euthanized by CO2 asphyxiation. Livers were perfused with 10 to 15 ml of ice-cold PBS, excised, and minced. The material was then passed through a nylon mesh screen. Following two washes in ice-cold PBS, the liver cell pellet was resuspended in serum-free RPMI (BioWhittaker, Walkersville, Md.) and layered onto a 30% (vol/vol) Percoll (Sigma, St. Louis, Mo.) gradient. Following a 10-min centrifugation step (2,000 × g) at room temperature, the pellet (containing the MNC) was recovered, and erythrocytes were lysed by hypotonic shock in 0.84% NH4Cl. The remaining cells were washed once with Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) (complete medium) and then resuspended in the same medium. Aliquots were used for fluorescence-activated cell sorter (FACS) analysis or flash-frozen for PCR amplification of the canonical NKT cell TCRα chain Vα14Jα281 rearrangement (54) as described below.
PEC and splenocyte isolation. (i) Peritoneal exudate cell (PEC) isolation.
Uninfected or infected (3 days post-LCMV infection) mice were euthanized, and then 7 ml of ice-cold Hanks buffered saline solution (HBSS) was injected i.p. The peritoneal fluid was aspirated, and fluids from five mice were pooled per treatment group. Cells were collected by centrifugation at 600 × g, and cell pellets were resuspended in complete medium. Adherent cells were removed as previously described (36). The nonadherent cells were collected, washed once in complete medium, and analyzed for NK1.1 and TCRαβ surface expression by FACS as described below.
(ii) Splenocyte isolation.
For splenocyte isolation, spleens were harvested from uninfected and LCMV-infected mice at the indicated time points and processed into single-cell suspensions. Erythrocytes were lysed as for liver MNC, and NKT cells were analyzed by FACS as described below.
FACS analysis and cell sorting.
Liver MNC, splenocytes, and PEC were stained for cytofluorography with the following monoclonal antibodies (MAbs [all purchased from Pharmingen, San Diego, Calif.]): fluorescein isothiocyanate (FITC)- or CyChrome-labeled anti-mouse panTCRβ, biotinylated or phycoerythrin (PE)-conjugated anti-NK1.1, biotinylated anti-DX5, and PE-conjugated anti-mouse CD28. The biotinylated MAbs were subsequently stained with either Red670-conjugated streptavidin (Life Technologies, Gaithersburg, Md.) or CyChrome-labeled streptavidin (Pharmingen). FITC-conjugated rabbit anti-active caspase 3 was kindly provided by H. Broxmeyer, Y.-J. Kim, and C. Mantel (Indiana University School of Medicine, Indianapolis, Ind.). Liver MNC, PEC, and spleen cells were pretreated with hybridoma supernatant from the 2.4G2 cell line (anti-mouse FcRγ specific), kindly provided by J. Yewdell and J. Bennink (National Institutes of Health, Bethesda, Md.). Cells were stained for cytofluorography as previously described (11). For active caspase 3-specific staining, liver MNC from uninfected, LCMV-infected (day 3 postinfection), or poly(I-C)-treated (day 1 postinjection) C57BL/6 mice were stained with CyChrome anti-TCRβ and PE-NK1.1, fixed and permeabilized with CytoPerm/Cytofix (Pharmingen), and stained with FITC-conjugated anti-active caspase 3. Analysis was by cytofluorography as described above. For cell sorting, liver MNC from LCMV-infected (day 3) C57BL/6 mice were stained with TCRβ- and NK1.1-specific antibodies and sorted into the double-positive (TCRα/β+ NK1.1+) population by a FACStar Plus (Becton Dickinson) for RNA isolation for PCR amplification of the canonical NKT-cell TCR Vα14Jα281 rearrangement or LCMV glycoprotein (GP) sequences as described below.
PCR analysis.
RNA was extracted from liver MNC (whole or sorted TCRαβ+ NK1.1+ populations) from uninfected, LCMV Armstrong-infected (day 3 postinfection), or poly(I-C)-treated (day 2 following treatment) mice by using TriReagent (Molecular Research Center, Inc., Cincinnati, Ohio). RNA was reverse transcribed into cDNA with a first-strand cDNA synthesis kit (Boehringer Mannheim, Indianapolis, Ind.). Amplification of Vα14Jα281-specific (54) and LCMV-GP-specific (21) sequences was performed as previously described. As a control, cDNA was amplified with previously described primers for actin (46). PCR products were analyzed on a 1% agarose gel and stained with ethidium bromide.
RESULTS
Infection of mice with LCMV causes a reduction in liver NKT cells.
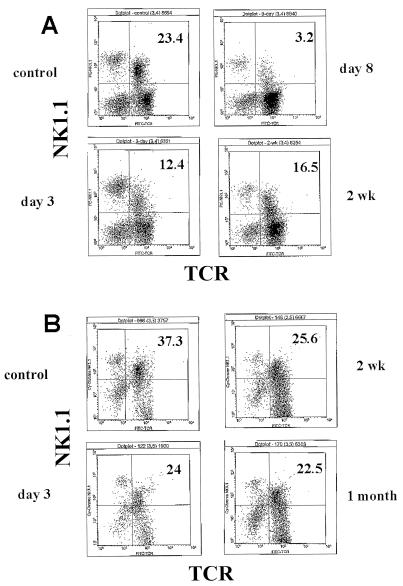
C57BL/6 mice were infected with LCMV for various lengths of time, and liver MNC were stained for TCRα/β and NK1.1. As shown in Fig. 1 and Table 1, LCMV infection caused a substantial decrease (in both percentage and absolute number) in liver NK1.1+ TCRαβ+ cells, detectable as early as 2 to 3 days postinfection. As expected and as previously reported (44), most experiments showed a clear increase in the number of NK cells in the liver at that time point (Fig. 1A) (data not shown). Despite a remarkable decrease in the percentage of NKT cells in total liver MNC (as assessed by TCRαβ- and NK1.1-specific staining) on day 8 postinfection (Fig. 1; the apex of the LCMV-specific cytotoxic T-lymphocye CTL response (64), there was in fact a substantial increase in the absolute number of NK1.1+ T cells (Table 1). However, this also corresponds to the very dramatic increase in virus-specific T cells, which have been shown to also express NK cell markers (2, 55). Thus, the apparent increase in NK1.1+ T cells on day 8 post-LCMV infection and beyond may simply reflect an increase in mainstream T (and not NKT) cells.
FIG. 1.
Kinetics of liver NKT-cell loss following infection with LCMV. Liver MNC were isolated from uninfected or LCMV-infected mice at various times postinfection: 3 days, 8 days, and 2 weeks (A) or 3 days, 2 weeks, and 1 month (B). The cells were then stained with MAbs specific for NK1.1 and TCRαβ. Analysis was by cytofluorography. Infected mice were compared to uninfected mice (control). Liver MNC from four mice were pooled per group. Values in the upper right quadrant represent the percentage of NKT cells.
TABLE 1.
Analysis of liver NKT cells from uninfected, poly(I-C)-treated, and LCMV-infected wild-type and mutant micea
| Group | Total no. of MNC | No. of NKT cellsb | % NKT-cell decrease (increase)c |
|---|---|---|---|
| Kinetics expt. 1 | |||
| Control | 4.3 × 106 | 5.1 × 105 | |
| Day 1 | 4.1 × 106 | 3.6 × 105 | 29.4 |
| Day 2 | 1.5 × 106 | 2.6 × 104 | 94.9 |
| Day 3 | 2.1 × 106 | 7.1 × 104 | 86.1 |
| Kinetics expt. 2 | |||
| Control | 3.9 × 106 | 7.9 × 105 | |
| Day 3 | 2.7 × 106 | 2.3 × 105 | 70.9 |
| Day 8 | 6.6 × 107 | 1.9 × 106 | (141) |
| 2 wk | 8.1 × 106 | 1.1 × 106 | (39.2) |
| Kinetics expt. 3 | |||
| Control | 5.3 × 106 | 9.9 × 105 | |
| Day 3 | 8.7 × 106 | 4.0 × 105 | 59.6 |
| 2 wk | 12.2 × 106 | 2.1 × 106 | (112) |
| 1 mo | 6.0 × 106 | 8.5 × 105 | 14.1 |
| lpr control | 4.6 × 106 | 5.6 × 105 | |
| lpr + LCMV | 3.9 × 106 | 1.1 × 105 | 80.4 |
| gld control | 2.4 × 106 | 4.7 × 105 | |
| gld + LCMV | 3.6 × 106 | 4.7 × 104 | 90.0 |
| IFN-γ KO control | 1.8 × 106 | 5.5 × 105 | |
| IFN-γ KO + LCMV | 1.6 × 106 | 2.1 × 105 | 61.8 |
| C57BL/6 control | 1.6 × 106 | 4.1 × 105 | |
| C57BL/6 + poly(I-C)d | 1.2 × 106 | 1.3 × 105 | 68.3 |
| (B6 × 129) controle | 6.1 × 106 | 1.0 × 106 | |
| (B6 × 129) + LCMV | 2.0 × 106 | 7.9 × 105 | 21.0 |
| DFF45 KO control | 2.5 × 106 | 3.2 × 105 | |
| DFF45 KO + LCMV | 4.7 × 106 | 1.3 × 106 | (306) |
| C57BL/6 controlf | 9.5 × 105 | 1.4 × 105 | |
| C57BL/6 + poly (I-C) | 6.0 × 105 | 4.8 × 104 | 65.7 |
| IL-12 KO controls | 1.1 × 106 | 2.8 × 105 | |
| IL-12 KO + poly(I-C) | 6.9 × 105 | 9.2 × 104 | 67.7 |
Liver MNC were obtained from uninfected or LCMV-infected C57BL/6 wild-type or indicated mutant mice on day 3 postinfection or as indicated. MNC were pooled from four mice per group. Data are representative of experiments performed at least two times.
NKT cells were identified by double staining with TCRβ- and NK1.1-specific MAbs except as specified.
Percent decrease (increase) in NKT-cell numbers post-LCMV infection or poly (I-C) treatment.
Wild-type C57BL/6 mice were treated i.p. with 100 μg of poly (I-C) 2 days before liver MNC harvest (pooled from four mice per group).
NKT cells were identified by double staining with TCRβ- and DX5-specific MAbs.
Wild-type and IL-12-deficient (IL-12 knockout [KO]) C57BL/6 mice were treated i.p. with 100 μg of poly(I-C) 2 days before liver MNC harvest (pooled from two mice per group).
Liver NKT cells do not traffic to the peritoneal cavity or spleen.
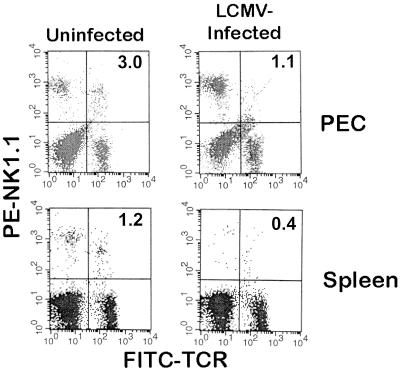
In order to determine if the loss of liver NKT cells following LCMV infection was due to their trafficking to extrahepatic sites, PEC and spleen cells were analyzed for any changes in NKT cells following infection. As shown in Fig. 2 and as previously reported (6, 28), PEC and spleen cells from uninfected mice contain few (approximately 1 to 2%) NKT cells. Three days following LCMV infection, there was no increase in the number of NKT cells in the peritoneal cavity or spleen. In fact, the NKT cells actually decreased, as occurred in the liver (Fig. 1 and Table 1). Therefore, these data suggest that following LCMV infection, liver NKT cells do not traffic to the peritoneal cavity or spleen and most likely die in situ.
FIG. 2.
FACS analysis of PEC and spleen MNC following infection with LCMV. Pooled PEC and splenic MNC (five mice per group) were isolated from uninfected or LCMV-infected (day 3 postinfection) C57BL/6 mice. The cells were stained and analyzed by cytofluorography as in Fig. 1. Values in the upper right quadrant indicate the percentage of NKT cells. The experiment shown was performed three times.
Liver Vα14Jα281+ NKT cells are lost following LCMV infection.
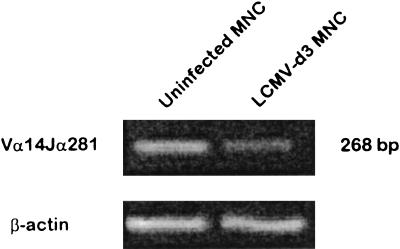
It has been shown that NKT cells from NK1.1+ mice (e.g., C57BL/6) lose NK1.1 expression upon activation in culture (14). Therefore, it was possible that the apparent loss of liver NKT cells following LCMV infection was simply due to a loss of NK1.1 expression. Although NKT cells predominantly express a Vα14Jα281 rearrangement (6), no MAb specific for this TCRα chain rearrangement is available. As another approach to identify the presence of liver NKT cells, we amplified cDNA from liver MNC obtained from uninfected and LCMV-infected mice with PCR primers that will amplify only the canonical NKT-cell Vα14Jα281 rearrangement (54) by semiquantitative PCR analysis. As shown in Fig. 3, a band corresponding to the canonical NKT-cell Vα14Jα281 rearrangement could be amplified from liver MNC cDNA derived from uninfected mice. In contrast, a substantial reduction in the Vα14Jα281-specific band was found in the liver MNC cDNA from mice infected 3 days previously with LCMV. As a PCR control, β-actin sequences were amplified at comparable levels from all samples. Therefore, these results suggest that the apparent loss of liver NKT cells following LCMV infection is indeed a loss and was not simply due to a down-regulation of NK1.1 expression.
FIG. 3.
PCR amplification of the NKT-cell canonical TCR Vα14Jα281 rearrangement from liver MNC in uninfected and LCMV-infected mice. Liver MNC were isolated from uninfected or day 3 post-LCMV-infected C57BL/6 mice and pooled (four mice per group). RNA was isolated from liver MNC and reverse transcribed into cDNA. The cDNA generated was amplified by PCR with primer pairs specific for the canonical NKT-cell TCR Vα14Jα281 rearrangement or actin as a control. PCR products were analyzed on a 1% agarose gel stained with ethidium bromide. The experiment shown is representative of three performed.
Loss of NKT cells is CD28 independent.
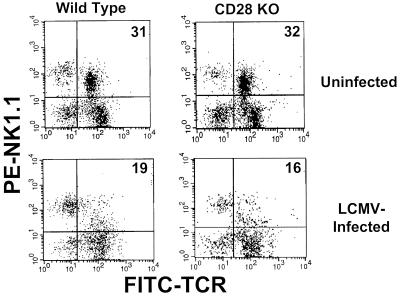
In order to determine if the observed loss in liver NKT cells following LCMV infection was dependent upon the classical T-cell costimulatory molecule CD28 (53), wild-type and CD28-deficient C57BL/6 mice were infected with LCMV, and 3 days later, liver MNC were isolated and analyzed by cytofluorography as described above. As shown in Fig. 4, liver NKT cells from uninfected wild-type and CD28-deficient C57BL/6 mice comprised approximately 31 and 32% of the liver MNC, respectively. This percentage decreased to 19 and 16%, respectively, on day 3 following LCMV infection. Therefore, these results suggest that the reduction in liver NKT cells following infection with LCMV is independent of CD28-mediated costimulation.
FIG. 4.
FACS analysis of liver NKT cells from wild-type and CD28-deficient mice following LCMV infection. Liver MNC were isolated from uninfected or LCMV-infected (day 3 postinfection) wild-type or CD28-deficient C57BL/6 mice, stained with TCRβ- and NK1.1-specific MAbs, and analyzed by cytofluorography as in Fig. 1. Liver MNC from four mice were pooled per group. The experiment shown is representative of two performed.
Loss of NKT cells does not occur via the Fas-FasL pathway.
It was possible that NKT cells were lost by apoptosis. One mechanism by which this might occur is by Fas-FasL interactions, because it has been shown extensively that T cells are highly susceptible to activation-induced cell death via this pathway (38). Although LCMV-specific T cells do not die in significant numbers via the Fas-FasL pathway following an acute LCMV infection (40), bystander (and not LCMV-specific) memory T cells are susceptible to activation-induced cell death by this pathway (68). NKT cells possess an activated, memory phenotype (6). Therefore, in order to determine if the loss of liver NKT cells following an LCMV infection was due to Fas-FasL interactions, C57BL/6 mice with defects in fas (lpr mice) or in fasL (gld mice) were infected with LCMV, and the liver NKT cells were analyzed 3 days later by FACS. As shown in the representative experiments presented in Table 1, NKT cells in both lpr and gld mice were lost at levels comparable to those seen in wild-type mice (80 and 90% decreases from uninfected lpr and gld mice, respectively). Therefore, the loss of NKT cells following LCMV infection is independent of the Fas-FasL pathway.
Liver NKT cells are directly infected with LCMV in vivo and ultimately die by apoptosis.
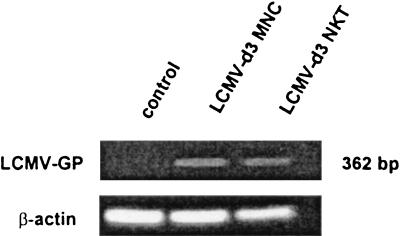
Because we did not see an increase in the number of NKT cells in the peritoneum or spleen (Fig. 2), this indicated that NKT cells did not traffic to these tissues following LCMV infection. With the concomitant loss of resident NKT cells in these tissues following LCMV infection and in spite of the lack of Fas-FasL interactions as shown above, it seemed likely nonetheless that the loss of liver NKT cells following LCMV infection was due to apoptosis. It is known that a number of viruses are able to induce apoptosis in infected cells or make them more susceptible to apoptosis by outside stimulation (51, 60). Although the strain of LCMV that we used (Armstrong) is not hepatotropic (44), it can grow in T lymphocytes (1, 35). In order to determine if liver NKT cells from LCMV-infected mice were themselves infected, liver MNC were isolated from uninfected and LCMV Armstrong-infected (day 3) mice. It should be pointed out again here that the Armstrong strain of LCMV does not grow in hepatocytes and that the liver was perfused before isolation. Thus, peripheral blood contamination (possibly bringing with it LCMV) would not be an issue, and as a result, only the resident liver MNC cells were harvested. Liver MNC from LCMV-infected mice were extensively washed following harvest and either left unsorted or sorted into TCRαβ+ NK1.1+ double-positive cells. The cells were then assessed for expression of LCMV-GP by PCR analysis of amplified cDNA generated from these cells by using LCMV-specific primers as described previously (21). As shown in Fig. 5, LCMV-GP could be amplified from unsorted liver MNC from LCMV-infected mice. Interestingly, LCMV-GP could also be amplified in sorted (TCRαβ+ NK1.1+) NKT cells from LCMV-infected mice. As expected, LCMV-GP-specific sequences could not be amplified from liver MNC isolated from uninfected mice. In all cases, for a PCR control, β-actin sequences were amplified at comparable levels from uninfected and LCMV-infected mice. Therefore, these results indicate that liver NKT cells from LCMV-infected mice are directly infected in vivo.
FIG. 5.
PCR amplification of LCMV-GP in liver NKT cells. Liver MNC were isolated from uninfected or day 3 post-LCMV-infected C57BL/6 mice and pooled (four mice per group). The MNC were either unsorted or sorted into an NKT-cell population by TCRβ- and NK1.1-specific MAbs by electronic cell sorting. RNA was extracted from liver MNC (whole or sorted TCRαβ+ NK1.1+ populations) from uninfected or LCMV Armstrong-infected (day 3 postinfection) wild-type C57BL/6 mice (four mice per group), reverse transcribed into cDNA, and amplified by PCR with primer pairs for LCMV-GP or actin as a control. PCR products were analyzed as in Fig. 3. The data shown are representative of two independent experiments.
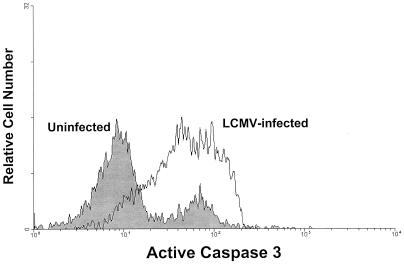
In order to determine if the liver MNC cells were dying by apoptosis, two approaches were taken. In the first, we infected mice deficient in DFF45, an integral component of a complex causing DNA fragmentation during apoptosis (69), with LCMV Armstrong. T cells from these mice are more resistant to apoptosis than wild-type T cells (70). Thus, as a control, we used their wild-type counterparts. Because the background strain of the DFF45-deficient mice is of a mixed (B6 × 129) background, we could not use the NK1.1 marker reliably (129 strain mice are NK1.1−). Therefore, we used the pan-NK cell marker (also present on NKT cells), DX5 (17, 28). The DX5 marker overlaps the TCRαβ+ NK1.1+ population in NK1.1+ strains of mice, although it is not perfect (17, 28). As shown in Table 1, infection of wild-type (B6 × 129) mice 3 days previously with LCMV Armstrong resulted in a 21% reduction in the absolute numbers of NKT cells. In contrast, liver NKT cells from DFF45-deficient mice actually increased following infection. Therefore, these results suggest that the death of liver NKT cells in LCMV-infected wild-type mice required a functional DFF45. The results presented above also suggest that the direct infection of liver NKT cells may have contributed to their death in situ. As a second approach to determine whether the liver NKT cells were dying by apoptosis, liver MNC isolated from uninfected and LCMV-infected (day 3 postinfection) C57BL/6 mice were stained for TCRαβ and NK1.1. Additionally, these cells were stained intracellularly with an antibody specific for active caspase 3. The caspase 3 proenzyme becomes cleaved into its active form during apoptosis and is thus a good marker for programmed cell death (38). As shown in Fig. 6, a significant increase in active caspase 3-specific staining was observed in liver NKT cells obtained from LCMV-infected mice as compared to those from uninfected mice. Therefore, taken together, the above results suggest that LCMV infects liver NKT cells and that this directly (or indirectly) results in their rapid death by apoptosis.
FIG. 6.
FACS analysis of active caspase 3 expression in liver NKT cells following LCMV infection. Liver MNC were isolated from uninfected or LCMV-infected (day 3 postinfection) wild-type C57BL/6 mice and stained for cell surface NK1.1 and TCRαβ and intracellular active caspase 3. The cells were then analyzed by cytofluorography as in Fig. 1. The histogram represents active caspase 3-specific staining in TCRαβ+ NK1.1+-gated NKT cells. Liver MNC from four mice were pooled per group. The data shown are representative of two independent experiments.
Loss of NKT cells is IFN-γ independent but occurs following poly(I-C) treatment.
It has recently been shown that the loss of liver NKT cells in HBV transgenic mice injected with the synthetic glycolipid α-GalCer, occurs by the induction of IFN-γ or IFN-α/β (31). In order to determine if the reduction in NKT cells observed following LCMV infection was due to IFN-γ induction, IFN-γ-deficient mice of the C57BL/6 background were infected with LCMV for 3 days, and the liver NKT cells were analyzed by FACS and compared to those of uninfected mice as described above. As shown in Table 1, NKT cells in IFN-γ-deficient mice were substantially reduced (61.8% reduction in the representative experiment shown) 3 days following LCMV infection, as was observed in wild-type C57BL/6 mice. Therefore, these results suggest that the LCMV-induced loss of liver NKT cells is independent of IFN-γ production.
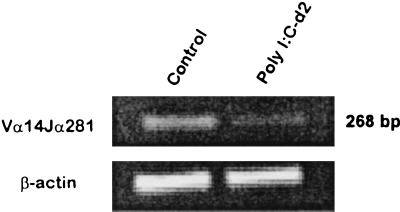
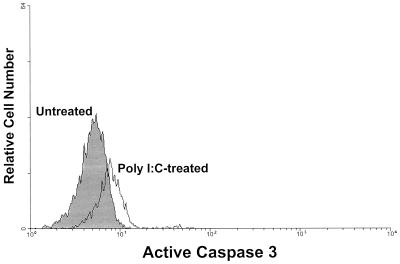
Poly(I-C) is a classical inducer of IFN-α/β, an important component of the immune response following LCMV infection (reviewed in reference 7). In order to determine if IFN-α/β can affect the number of NKT cells, wild-type C57BL/6 mice were treated with poly(I-C), and liver MNC were analyzed for NKT cells (as compared to untreated control mice) by FACS. As shown in Table 1, poly(I-C) treatment of C57BL/6 wild-type mice caused a substantial (68.3%) loss in liver NKT cells 2 days postinjection with regard to percent loss and overall decline in NKT-cell numbers. Figure 7 shows that there was a substantial reduction in classical NKT cells 2 days following the poly(I-C) injection as detected by PCR amplification of Vα14Jα281-specific sequences. As shown in Fig. 8, the loss of liver NKT cells following poly(I-C) injection was concomitant with an increase in active caspase 3 expression, as was seen in LCMV-infected mice, suggesting that the mechanism of NKT cell loss caused by either of these two agents is the same. Therefore, these results are consistent with the idea that IFN-α/β induction by LCMV infection causes the NKT-cell loss observed following infection.
FIG. 7.
Reduction in canonical Vα14Jα281+ liver NKT cells following poly(I-C) injection. Liver MNC were harvested from untreated and poly(I-C)-treated mice (day 2 postinjection). RNA was extracted and reverse transcribed into cDNA. The cDNA was amplified by primers specific for the TCR Vα14Jα281 rearrangement or actin as a control. PCR products were analyzed as in Fig. 3. The data shown are representative of two independent experiments.
FIG. 8.
FACS analysis of active caspase 3-specific staining of NKT cells from poly(I-C)-treated mice. Liver MNC were isolated from untreated or poly(I-C)-treated (day 1 postinjection) wild-type C57BL/6 mice and stained for cell surface NK1.1 and TCRαβ and intracellular active caspase 3. The cells were then analyzed by cytofluorography. The histogram represents active caspase 3-specific staining in TCRαβ+ NK1.1+-gated liver MNC. Liver MNC from four mice were pooled per group. The data shown are representative of two independent experiments.
Poly(I-C)-induced loss of liver NKT cells is IL-12-independent.
Although poly(I-C) is a classical IFN-α/β inducer, it can also stimulate IL-12 production (41, 61), and IL-12 treatment of mice has been shown to result in the rapid and selective loss of NKT cells by apoptosis (18). Thus, it was possible that the loss of liver NKT cells following poly(I-C) injection was due solely to IL-12 (rather than IFN-α/β) induction in vivo. In order to rule out this possibility, wild-type and IL-12-deficient C57BL/6 mice were injected with 100 μg of poly(I-C), and 2 days later, liver MNC were harvested and stained with antibodies specific for NK1.1 and TCRβ. As observed in our other experiments, the FACS analyses showed that poly(I-C) treatment caused a substantial decrease in the number of liver NKT cells in C57BL/6 wild-type mice [untreated, 1.4 × 105 NKT cells; poly(I-C) treated, 4.8 × 104 NKT cells; 66% decrease]. Interestingly, a comparable loss in liver NKT cells was also observed in poly(I-C)-treated IL-12-deficient C57BL/6 mice [untreated, 2.8 × 105 NKT cells; poly(I-C) treated, 9.2 × 104 NKT cells; 68% decrease). It should also be noted that, although LCMV does not induce IL-12 production in vivo (47), we also infected IL-12-deficient C57BL/6 mice with LCMV and saw a loss in NKT cells similar to that found in wild-type mice (data not shown). Therefore, these results suggest that the loss of liver NKT cells by the classical IFN-α/β inducer poly(I-C) (or LCMV) is not due to IL-12 production.
DISCUSSION
We have presented evidence in this report demonstrating that an acute infection with LCMV results in a selective loss of NKT cells and suggests that this occurs via apoptosis in a Fas- or FasL-independent manner, does not require IFN-γ production, but may be dependent upon IFN-α/β induction. Furthermore, this reduction does not require classical costimulatory molecules, because the loss of NKT cells was also observed in LCMV-infected CD28-deficient mice. The last observation is perhaps not too surprising, because some human CD1b-restricted T cells have been shown to recognize their targets in a CD28- and B7–1-independent fashion (3). The loss of NKT cells was not simply due to a down-regulation of NK1.1 that occurs following sustained culture in vitro (14), but was an actual loss (Fig. 3). Furthermore, the reduction in NKT cells following LCMV infection also occurred in the peritoneum and spleen (Fig. 2). Therefore, rather than NKT cells trafficking to extrahepatic sites, it is likely that the NKT cells simply die by apoptosis. This is supported by the result with LCMV-infected DFF45-deficient mice, in which the loss of liver NKT cells was not observed (Table 1) and an increase in active caspase 3-specific staining in liver NKT cells from LCMV-infected mice was noted (Fig. 6).
Viral clearance mechanisms in which NKT cells might play a role can be cytotoxic to hepatocytes, such as with adenovirus (66) or noncytopathic, as has been observed in HBV transgenic mice or in mice infected with hepatotropic strains of LCMV (25–27). Thus, viral clearance has multiple mechanisms by which it can occur and is different depending upon the virus. We have found that even in an NKT-cell-deficient (i.e., CD1d1 knockout mice) environment, the in vivo control of LCMV is comparable to that in wild-type mice (57). Thus, in that model, either the lack of NKT cells is beneficial, by preventing an NKT-cell-mediated cytotoxic response affecting hepatocytes and other tissues, or, alternatively, NKT cells simply play no role in the control of that virus infection. We prefer the former possibility. In support of that, there is a high percentage of NKT cells in the normal liver (6), and it has been previously suggested that hepatic NKT cells are responsible for liver damage following infection with pathogens such as Salmonella (30). Mice that are deficient in NKT cells, such as β2-microglobulin- or Jα281-knockout mice, did not develop the Salmonella-induced liver damage that was observed in wild-type mice (30). Furthermore, in an experimental model of autoimmune hepatitis induced by the polyclonal T-cell mitogen concanavalin A, NKT cells were responsible for the hepatocyte damage (32). Interestingly, a recent report by Chisari and colleagues (31) found that the activation of NKT cells in HBV transgenic mice in vivo by α-GalCer (a glycolipid that is presented by CD1d1 to NKT cells) (8, 9, 12, 33) caused the rapid loss of NKT cells, recruited NK cells, and inhibited HBV replication in the mice in an IFN-α/β- and IFN-γ-dependent manner (31). Others have also seen such a reduction in liver NKT cells following treatment of mice with α-GalCer (19, 43, 48) or anti-CD3 (18) and have reported a role for NKT-cell-produced IFN-γ in the induction of NK cell activity (13, 19). In the case of LCMV, we have found that the virus-induced NK cell activity is actually normal in an NKT-cell-deficient environment (i.e., CD1d1-deficient mice) (57), and LCMV infection also resulted in the loss of NKT cells in IFN-γ-deficient mice (Table 1). This suggests that the importance of NKT cells in various infections will be dependent not only on the pathogens, but also the cytokines that they induce. Although infection with LCMV does induce IFN-γ production, it occurs later, ∼2 weeks postinfection (58): by that time, the NKT cells have already recovered from their loss (Fig. 1 and Table 1). LCMV is also a classic inducer of α/β IFNs (7, 45), and IFN-α/β was found to contribute to the loss of NKT cells in α-GalCer-treated HBV-transgenic mice (31). In the current study, we found that injection of mice with poly(I-C), a well-known and commonly used inducer of IFN-α/β, also caused a substantial loss of NKT cells (Table 1), suggesting that IFN-α/β induction following LCMV infection may have been responsible for the NKT cell loss, at least indirectly. However, poly(I-C) can also induce the production of IL-12 (41, 61) and IL-15 (71), both of which can have effects on NKT cells. With regard to IL-15, mice deficient in IL-15 or the IL-15 receptor α chain have a substantial reduction in both NK and NKT cells (34, 39), and IL-15 can induce the generation of pre-NKT cells into mature NKT cells (52), making it unlikely that an increase in IL-15 would cause a selective loss of NKT cells. IL-12 has been reported to cause a rapid reduction in NKT cells (18). Because poly(I-C) also induces IL-12 production (41, 61), it was important to rule out this cytokine in the NKT-cell loss observed in the present study. Thus, IL-12-deficient mice were treated with poly(I-C)—or infected with LCMV—which does not induce IL-12 production (47)—and the status of liver NKT cells was compared to C57BL/6 wild-type cells treated similarly. Treatment with poly(I-C) (or infection with LCMV) resulted in the loss of liver NKT cells from IL-12-deficient mice at levels comparable to those in wild-type mice (Table 1) (data not shown). Therefore, the results from those experiments are consistent with the notion that α/β IFNs play a role in the observed reduction in NKT cells following an LCMV infection or poly(I-C) treatment. The putative role for IFN-α/β in the loss of NKT cells was actually somewhat surprising in light of the evidence suggesting that α/β IFNs can protect activated T cells from apoptosis (42) and NKT cells possess an activated memory phenotype (6). Interestingly, we also found that liver NKT cells from LCMV-infected mice were themselves infected (Fig. 5), and there are data suggesting that IFN-α/β promotes apoptosis of virus-infected cells (60). Furthermore, many viruses can induce apoptosis in the cells that they infect (51). Thus, the most plausible mechanism by which NKT cells are lost following an LCMV infection is via the induction of α/β IFNs, and this contributes to NKT-cell death by apoptosis. Exactly why NKT cells appear to be particularly susceptible to apoptotic death following LCMV infection and whether the effects of IFN-α/β are direct or indirect are currently unknown and obviously represent an important area of investigation.
Along with the fact that there is a large proportion of NKT cells in the liver, the NKT-cell population appears to be quite dynamic in response to pathogens and other disease processes. In this report, we have shown that NKT cells are selectively lost following an acute virus infection. As with infection by other pathogens (20, 30–32), it is possible that the LCMV-induced loss of NKT cells is advantageous to the host. In fact, this hypothesis is supported by a recent review by Welsh and McNally (63), in which it is argued that the elimination of both virus-specific and non-virus-specific T cells may actually help shape the antiviral T-cell response. Experiments aimed at answering questions that address this hypothesis are currently under way.
ACKNOWLEDGMENTS
J.A.H. and S.C. contributed equally to this work.
We thank Jon Yewdell and Jack Bennink for the 2.4G2 cell line; Raymond Welsh for the Armstrong strain of LCMV; Hal Broxmeyer, Young-June Kim, and Charlie Mantel for the anti-active caspase 3 antibody; and Arun Srivastava and Susan Brutkiewicz for helpful comments on the manuscript.
This work was supported in part by an award from the Indiana University School of Medicine Biomedical Research Committee to R.R.B. and by grants from the National Institutes of Health (RO1 AI 46455 to R.R.B. and DA 11005 to M.X.).
ADDENDUM IN PROOF
While the manuscript was under review, it was reported (K. A. Daniels, G. Devora, W. C. Lai, C. L. O'Donnell, M. Bennett, and R. M. Welsh, J. Exp. Med. 194:29–44, 2001) that several different viruses (in addition to a variant of LCMV that causes a persistent infection) can also cause a reduction in liver NKT cells, as we have found.
REFERENCES
- 1.Ahmed R, King C C, Oldstone M B. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J Virol. 1987;61:1571–1576. doi: 10.1128/jvi.61.5.1571-1576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assarsson E, Kambayashi T, Sandberg J K, Hong S, Taniguchi M, Van Kaer L, Ljunggren H G, Chambers B J. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J Immunol. 2000;165:3673–3679. doi: 10.4049/jimmunol.165.7.3673. [DOI] [PubMed] [Google Scholar]
- 3.Behar S M, Porcelli S A, Beckman E M, Brenner M B. A pathway of costimulation that prevents anergy in CD28− T cells: B7-independent costimulation of CD1-restricted T cells. J Exp Med. 1995;182:2007–2018. doi: 10.1084/jem.182.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendelac A, Killeen N, Littman D R, Schwartz R H. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 5.Bendelac A, Lantz O, Quimby M E, Yewdell J W, Bennink J R, Brutkiewicz R R. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 6.Bendelac A, Rivera M N, Park S H, Roark J H. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 7.Biron C A. Role of early cytokines, including alpha and beta interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 8.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brossay L, Naidenko O, Burdin N, Matsuda J, Sakai T, Kronenberg M. Structural requirements for galactosylceramide recognition by CD1-restricted NK T cells. J Immunol. 1998;161:5124–5128. [PubMed] [Google Scholar]
- 10.Brown D R, Fowell D J, Corry D B, Wynn T A, Moskowitz N H, Cheever A W, Locksley R M, Reiner S L. β2-Microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J Exp Med. 1996;184:1295–1304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brutkiewicz R R, Bennink J R, Yewdell J W, Bendelac A. TAP-independent, β2-microglobulin-dependent surface expression of functional mouse CD1.1. J Exp Med. 1995;182:1913–1919. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdin N, Brossay L, Koezuka Y, Smiley S T, Grusby M J, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. Selective ability of mouse CD1 to present glycolipids: α-galactosylceramide specifically stimulates Vα14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 13.Carnaud C, Lee D, Donnars O, Park S H, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 14.Chen H, Huang H, Paul W E. NK1.1+ CD4+ T cells lose NK1.1 expression upon in vitro activation. J Immunol. 1997;158:5112–5119. [PubMed] [Google Scholar]
- 15.Cui J, Watanabe N, Kawano T, Yamashita M, Kamata T, Shimizu C, Kimura M, Shimizu E, Koike J, Koseki H, Tanaka Y, Taniguchi M, Nakayama T. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J Exp Med. 1999;190:783–792. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denkers E Y, Gazzinelli R T, Martin D, Sher A. Emergence of NK1.1+ cells as effectors of IFN-γ dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med. 1993;178:1465–1472. doi: 10.1084/jem.178.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberl G, Lees R, Smiley S T, Taniguchi M, Grusby M J, MacDonald H R. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- 18.Eberl G, MacDonald H R. Rapid death and regeneration of NKT cells in anti-CD3ɛ- or IL-12-treated mice: a major role for bone marrow in NKT homeostasis. Immunity. 1998;9:345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 19.Eberl G, MacDonald H R. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–992. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Emoto M, Emoto Y, Kaufmann S H. Interleukin-4-producing CD4+ NK1.1+ TCR α/β intermediate liver lymphocytes are down-regulated by Listeria monocytogenes. Eur J Immunol. 1995;25:3321–3325. doi: 10.1002/eji.1830251218. [DOI] [PubMed] [Google Scholar]
- 21.Evans C F, Borrow P J, de la Torre C, Oldstone M B. Virus-induced immunosuppression: kinetic analysis of the selection of a mutation associated with viral persistence. J Virol. 1994;68:7367–7373. doi: 10.1128/jvi.68.11.7367-7373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauldie J, Lamontagne L, Stadnyk A. Acute phase response in infectious disease. Surv Synth Pathol Res. 1985;4:126–151. doi: 10.1159/000156970. [DOI] [PubMed] [Google Scholar]
- 23.Gombert J M, Tancrede-Bohin E, Hameg A, Leite-de-Moraes M C, Vicari A, Bach J F, Herbelin A. IL-7 reverses NK1+ T cell-defective IL-4 production in the non-obese diabetic mouse. Int Immunol. 1996;8:1751–1758. doi: 10.1093/intimm/8.11.1751. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, Taniguchi M, Bendelac A, Van Kaer L, Koezuka Y, Tsuji M. α-Galactosylceramide-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–8466. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guidotti L G, Borrow P, Brown A, McClary H, Koch R, Chisari F V. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J Exp Med. 1999;189:1555–1564. doi: 10.1084/jem.189.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 27.Guidotti L G, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F V. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 28.Hammond K J, Pelikan S B, Crowe N Y, Randle-Barrett E, Nakayama T, Taniguchi M, Smyth M J, van Driel I R, Scollay R, Baxter A G, Godfrey D I. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999;29:3768–3781. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 29.Hammond K J L, Poulton L D, Palmisano L J, Silveira P A, Godfrey D I, Baxter A G. α/β-T cell receptor (TCR)+CD4−CD8− (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishigami M, Nishimura H, Naiki Y, Yoshioka K, Kawano T, Tanaka Y, Taniguchi M, Kakumu S, Yoshikai Y. The roles of intrahepatic Vα14+ NK1.1+ T cells for liver injury induced by Salmonella infection in mice. Hepatology. 1999;29:1799–1808. doi: 10.1002/hep.510290605. [DOI] [PubMed] [Google Scholar]
- 31.Kakimi K, Guidotti L G, Koezuka Y, Chisari F V. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaneko B Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M. Augmentation of Vα14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy M K, Glaccum M, Brown S N, Butz E A, Viney J L, Embers M, Matsuki N, Charrier K, Sedger L, Willis C R, Brasel K, Morrissey P J, Stocking K, Schuh J C, Joyce S, Peschon J J. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King C C, Jamieson B D, Reddy K, Bali N, Concepcion R J, Ahmed R. Viral infection of the thymus. J Virol. 1992;66:3155–3160. doi: 10.1128/jvi.66.5.3155-3160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumagai K, Itoh K, Hinuma S, Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29:17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- 37.Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach J F, Monteiro R C. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–1839. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenardo M, Chan K M, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 39.Lodolce J P, Boone D L, Chai S, Swain R E, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 40.Lohman B L, Razvi E S, Welsh R M. T-lymphocyte downregulation after acute viral infection is not dependent on CD95 (Fas) receptor-ligand interactions. J Virol. 1996;70:8199–8203. doi: 10.1128/jvi.70.11.8199-8203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manetti R, Annunziato F, Tomasevic L, Gianno V, Parronchi P, Romagnani S, Maggi E. Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-α and interleukin-12. Eur J Immunol. 1995;25:2656–2660. doi: 10.1002/eji.1830250938. [DOI] [PubMed] [Google Scholar]
- 42.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsuda J L, Naidenko O V, Gapin L, Nakayama T, Taniguchi M, Wang C R, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McIntyre K W, Welsh R M. Accumulation of natural killer and cytotoxic T large granular lymphocytes in the liver during virus infection. J Exp Med. 1986;164:1667–1681. doi: 10.1084/jem.164.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muller U, Steinhoff U, Reis L F, Hemmi S, Pavlovic J, Zinkernagel R M, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 46.Naiki Y, Nishimura H, Kawano T, Tanaka Y, Itohara S, Taniguchi M, Yoshikai Y. Regulatory role of peritoneal NK1.1+ αβ T cells in IL-12 production during Salmonella infection. J Immunol. 1999;163:2057–2063. [PubMed] [Google Scholar]
- 47.Orange J S, Biron C A. An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 48.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of α-galactosylceramide. Eur J Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Pied S, Roland J, Louise A, Voegtle D, Soulard V, Mazier D, Cazenave P A. Liver CD4−CD8− NK1.1+ TCRαβ intermediate cells increase during experimental malaria infection and are able to exhibit inhibitory activity against the parasite liver stage in vitro. J Immunol. 2000;164:1463–1469. doi: 10.4049/jimmunol.164.3.1463. [DOI] [PubMed] [Google Scholar]
- 50.Porcelli S A, Modlin R L. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 51.Roulston A, Marcellus R C, Branton P E. Viruses and apoptosis. Annu Rev Microbiol. 1999;53:577–628. doi: 10.1146/annurev.micro.53.1.577. [DOI] [PubMed] [Google Scholar]
- 52.Sato H, Nakayama T, Tanaka Y, Yamashita M, Shibata Y, Kondo E, Saito Y, Taniguchi M. Induction of differentiation of pre-NKT cells to mature Vα14 NKT cells by granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1999;96:7439–7444. doi: 10.1073/pnas.96.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharpe A H. Costimulatory signals and viral immunity. Semin Virol. 1996;7:103–111. [Google Scholar]
- 54.Shimamura M, Ohteki T, Beutner U, MacDonald H R. Lack of directed Vα14-Jα281 rearrangements in NK1+ T cells. Eur J Immunol. 1997;27:1576–1579. doi: 10.1002/eji.1830270638. [DOI] [PubMed] [Google Scholar]
- 55.Slifka M K, Pagarigan R R, Whitton J L. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol. 2000;164:2009–2015. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- 56.Smiley S T, Kaplan M H, Grusby M J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 57.Spence, P. M., V. Sriram, L. Van Kaer, J. A. Hobbs, and R. R. Brutkiewicz. Generation of cellular immunity to lymphocytic choriomeningitis virus is independent of CD1d1 expression. Immunology, in press. [DOI] [PMC free article] [PubMed]
- 58.Su H C, Cousens L P, Fast L D, Slifka M K, Bungiro R D, Ahmed R, Biron C A. CD4+ and CD8+ T cell interactions in IFN-γ and IL-4 responses to viral infections: requirements for IL-2. J Immunol. 1998;160:5007–5017. [PubMed] [Google Scholar]
- 59.Sumida T, Sakamoto A, Murata H, Makino Y, Takahashi H, Yoshida S, Nishioka K, Iwamoto I, Taniguchi M. Selective reduction of T cells bearing invariant Vα24 JαQ antigen receptor in patients with systemic sclerosis. J Exp Med. 1995;182:1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka N, Sato M, Lamphier M S, Nozawa H, Oda E, Noguchi S, Schreiber R D, Tsujimoto Y, Taniguchi T. Type I interferons are essential mediators of apoptotic death in virally infected cells. Genes Cells. 1998;3:29–37. doi: 10.1046/j.1365-2443.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 61.Verdijk R M, Mutis T, Esendam B, Kamp J, Melief C J, Brand A, Goulmy E. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- 62.Watanabe H, Ohtsuka K, Kimura M, Ikarashi Y, Ohmori K, Kusumi A, Ohteki T, Seki S, Abo T. Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods. 1992;146:145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- 63.Welsh R M, McNally J M. Immune deficiency, immune silencing, and clonal exhaustion of T cell responses during viral infections. Curr Opin Microbiol. 1999;2:382–387. doi: 10.1016/s1369-5274(99)80067-8. [DOI] [PubMed] [Google Scholar]
- 64.Welsh R M., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978;148:163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson S B, Kent S C, Patton K T, Orban T, Jackson R A, Exley M, Porcelli S, Schatz D A, Atkinson M A, Balk S P, Strominger J L, Hafler D A. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 66.Yang Y, Wilson J M. Clearance of adenovirus-infected hepatocytes by MHC class I-restricted CD4+ CTLs in vivo. J Immunol. 1995;155:2564–2570. [PubMed] [Google Scholar]
- 67.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul W E. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 68.Zarozinski C C, McNally J M, Lohman B L, Daniels K A, Welsh R M. Bystander sensitization to activation-induced cell death as a mechanism of virus-induced immune suppression. J Virol. 2000;74:3650–3658. doi: 10.1128/jvi.74.8.3650-3658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J, Liu X, Scherer D C, van Kaer L, Wang X, Xu M. Resistance to DNA fragmentation and chromatin condensation in mice lacking the DNA fragmentation factor 45. Proc Natl Acad Sci USA. 1998;95:12480–12485. doi: 10.1073/pnas.95.21.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J, Wang X, Bove K E, Xu M. DNA fragmentation factor 45-deficient cells are more resistant to apoptosis and exhibit different dying morphology than wild-type control cells. J Biol Chem. 1999;274:37450–37454. doi: 10.1074/jbc.274.52.37450. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]