Abstract
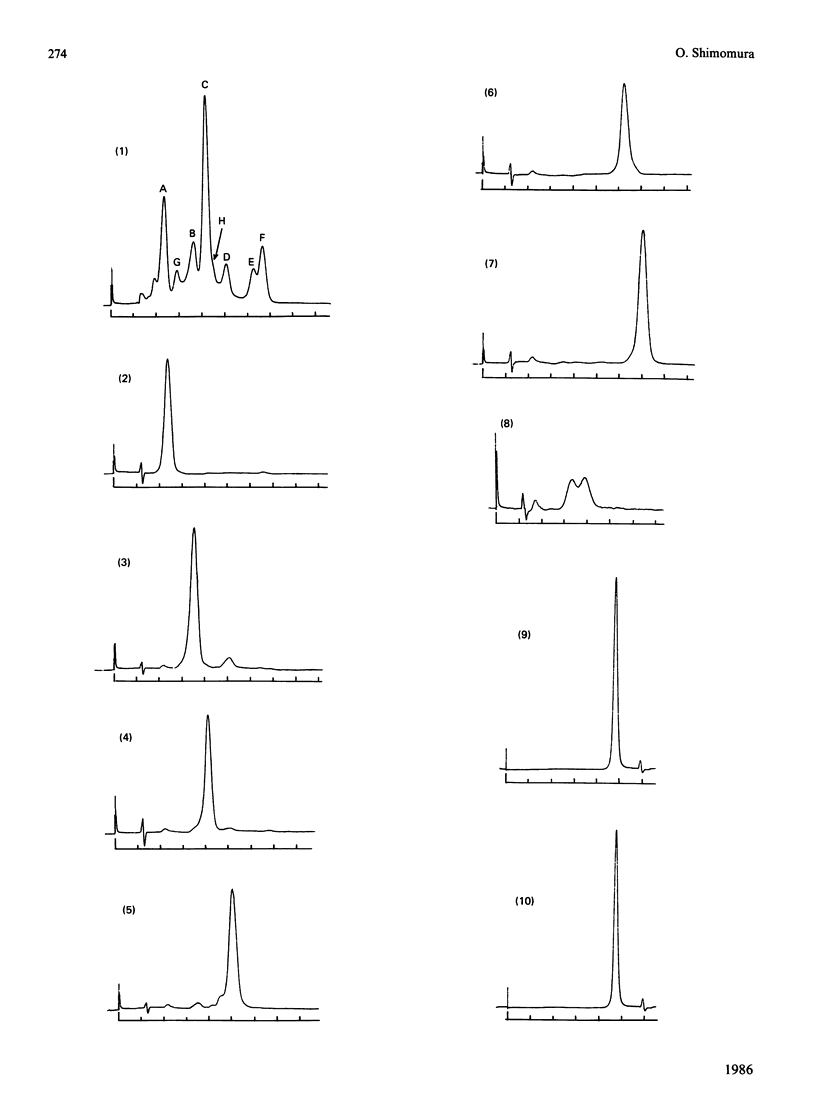
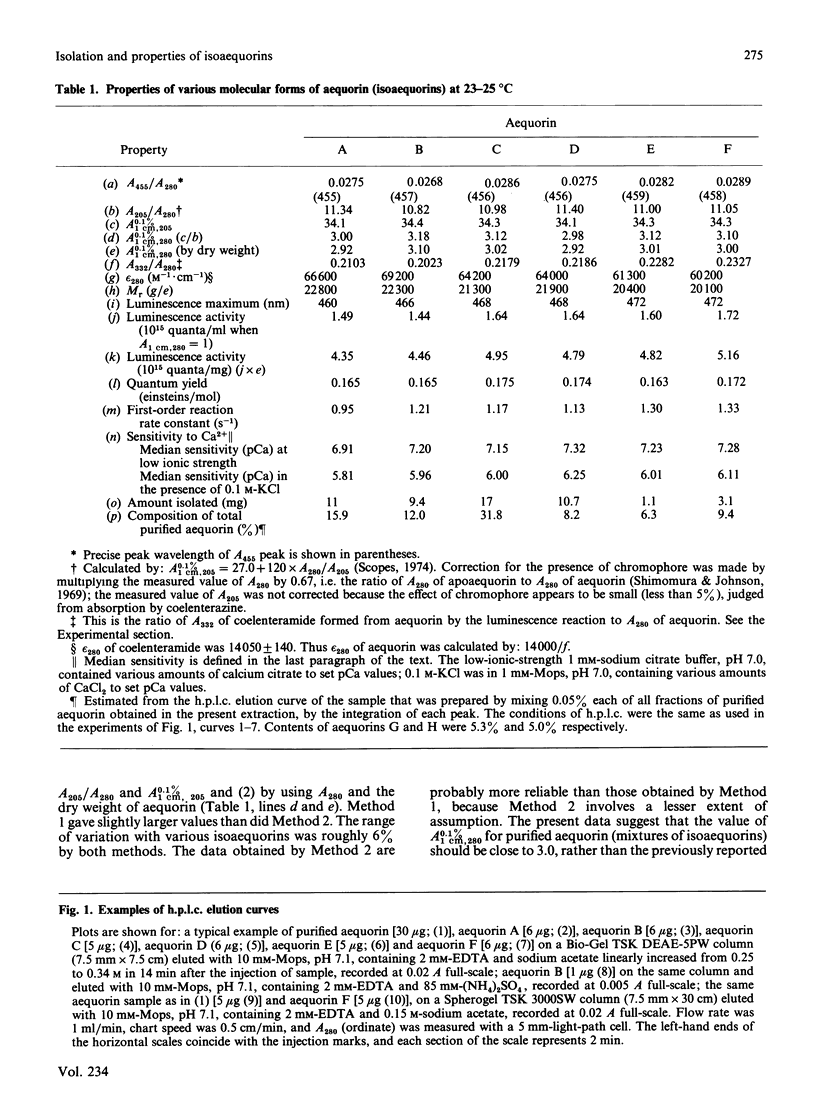
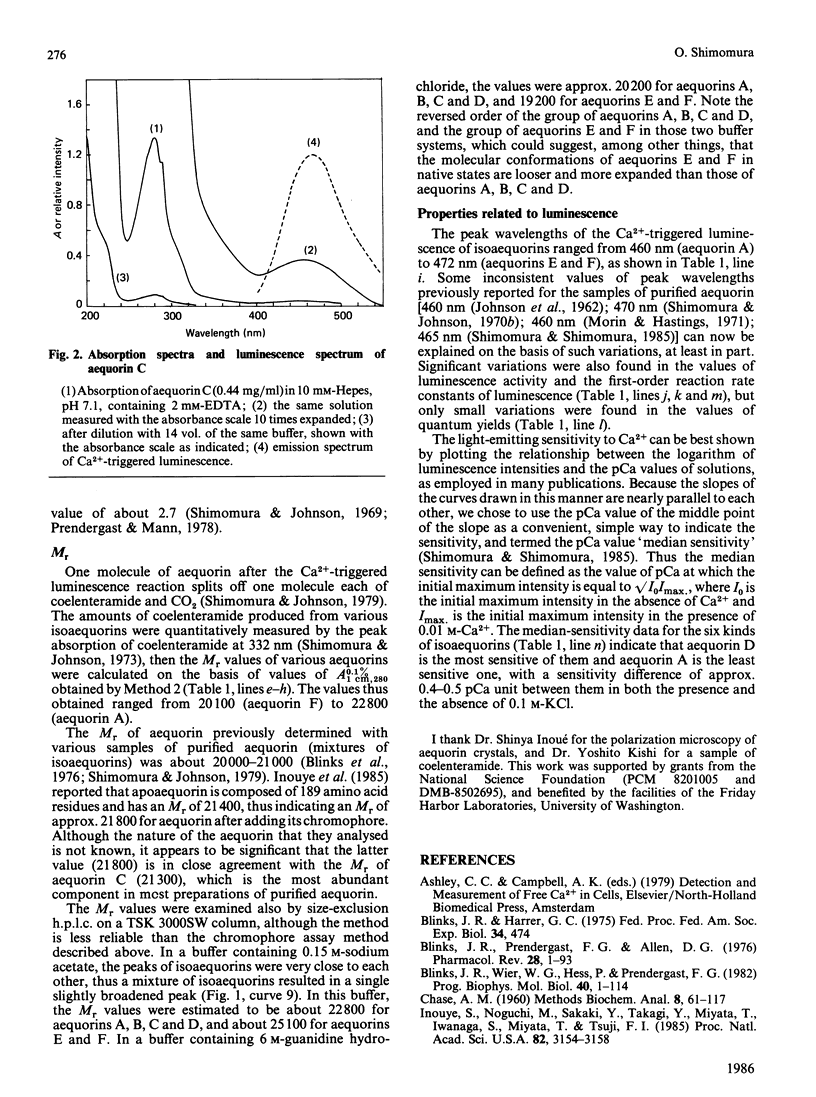
The photoprotein aequorin emits light by an intramolecular reaction when a trace of Ca2+ is added. The samples of aequorin that were purified by the conventional methods of column chromatography were separated by high-performance liquid chromatography into eight molecular forms (isoaequorins), which were designated aequorins A-H. Aequorins A, C and F were obtained in crystalline states. A wide range of properties were studied with aequorins A-F, which were essentially pure. These six isoaequorins showed relatively small differences in their spectroscopic properties, but their values of A0.1%/1 cm, 280 were found to be close to 3.0, about 10% more than the previously reported value of 2.70-2.71 that was obtained with the samples of conventionally purified aequorin. The Mr values ranged from 20,100 (aequorin F) to 22,800 (aequorin A), the luminescence activities ranged from 4.35 X 10(15) photons/mg (aequorin A) to 5.16 X 10(15) photons/mg (aequorin F), and the first-order reaction rate constants of luminescence ranged from 0.95 s-1 (aequorin A) to 1.33 s-1 (aequorin F). As regards sensitivity to Ca2+, aequorin D was the most sensitive, having a sensitivity about 0.4-0.5 pCa unit above that of the least sensitive kind (aequorin A).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blinks J. R., Prendergast F. G., Allen D. G. Photoproteins as biological calcium indicators. Pharmacol Rev. 1976 Mar;28(1):1–93. [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- CHASE A. M. The measurement of luciferin and luciferase. Methods Biochem Anal. 1960;8:61–117. doi: 10.1002/9780470110249.ch2. [DOI] [PubMed] [Google Scholar]
- Inouye S., Noguchi M., Sakaki Y., Takagi Y., Miyata T., Iwanaga S., Miyata T., Tsuji F. I. Cloning and sequence analysis of cDNA for the luminescent protein aequorin. Proc Natl Acad Sci U S A. 1985 May;82(10):3154–3158. doi: 10.1073/pnas.82.10.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin J. G., Hastings J. W. Biochemistry of the bioluminescence of colonial hydroids and other coelenterates. J Cell Physiol. 1971 Jun;77(3):305–312. doi: 10.1002/jcp.1040770304. [DOI] [PubMed] [Google Scholar]
- Prendergast F. G., Mann K. G. Chemical and physical properties of aequorin and the green fluorescent protein isolated from Aequorea forskålea. Biochemistry. 1978 Aug 22;17(17):3448–3453. doi: 10.1021/bi00610a004. [DOI] [PubMed] [Google Scholar]
- SHIMOMURA O., JOHNSON F. H., SAIGA Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962 Jun;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Calcium binding, quantum yield, and emitting molecule in aequorin bioluminescence. Nature. 1970 Sep 26;227(5265):1356–1357. doi: 10.1038/2271356a0. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Mechanisms in the quantum yield of Cypridina bioluminescence. Photochem Photobiol. 1970 Oct;12(4):291–295. doi: 10.1111/j.1751-1097.1970.tb06061.x. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Peroxidized coelenterazine, the active group in the photoprotein aequorin. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2611–2615. doi: 10.1073/pnas.75.6.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Properties of the bioluminescent protein aequorin. Biochemistry. 1969 Oct;8(10):3991–3997. doi: 10.1021/bi00838a015. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Regeneration of the photoprotein aequorin. Nature. 1975 Jul 17;256(5514):236–238. doi: 10.1038/256236a0. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Shimomura A. EDTA-binding and acylation of the Ca2+-sensitive photoprotein aequorin. FEBS Lett. 1982 Feb 22;138(2):201–204. doi: 10.1016/0014-5793(82)80441-9. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Shimomura A. Effect of calcium chelators on the Ca2+-dependent luminescence of aequorin. Biochem J. 1984 Aug 1;221(3):907–910. doi: 10.1042/bj2210907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O., Shimomura A. Halistaurin, phialidin and modified forms of aequorin as Ca2+ indicator in biological systems. Biochem J. 1985 Jun 15;228(3):745–749. doi: 10.1042/bj2280745. [DOI] [PMC free article] [PubMed] [Google Scholar]


