Abstract
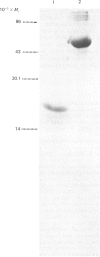
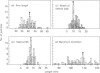
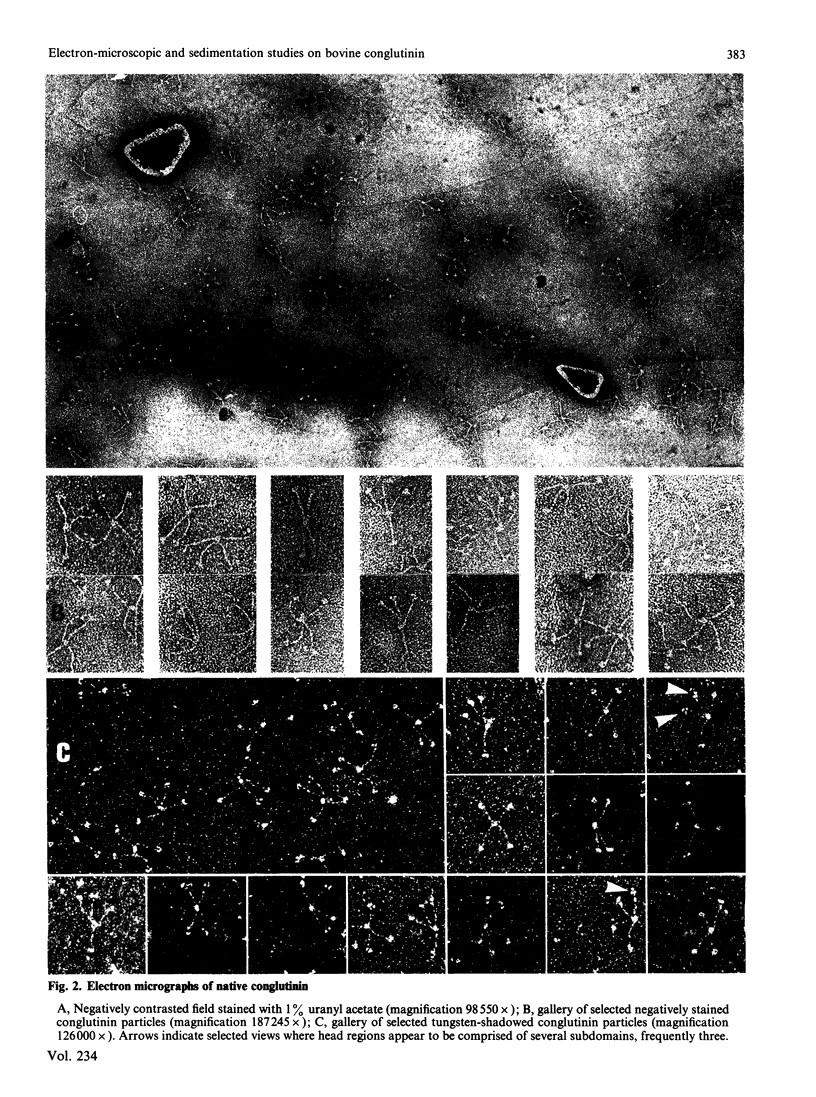
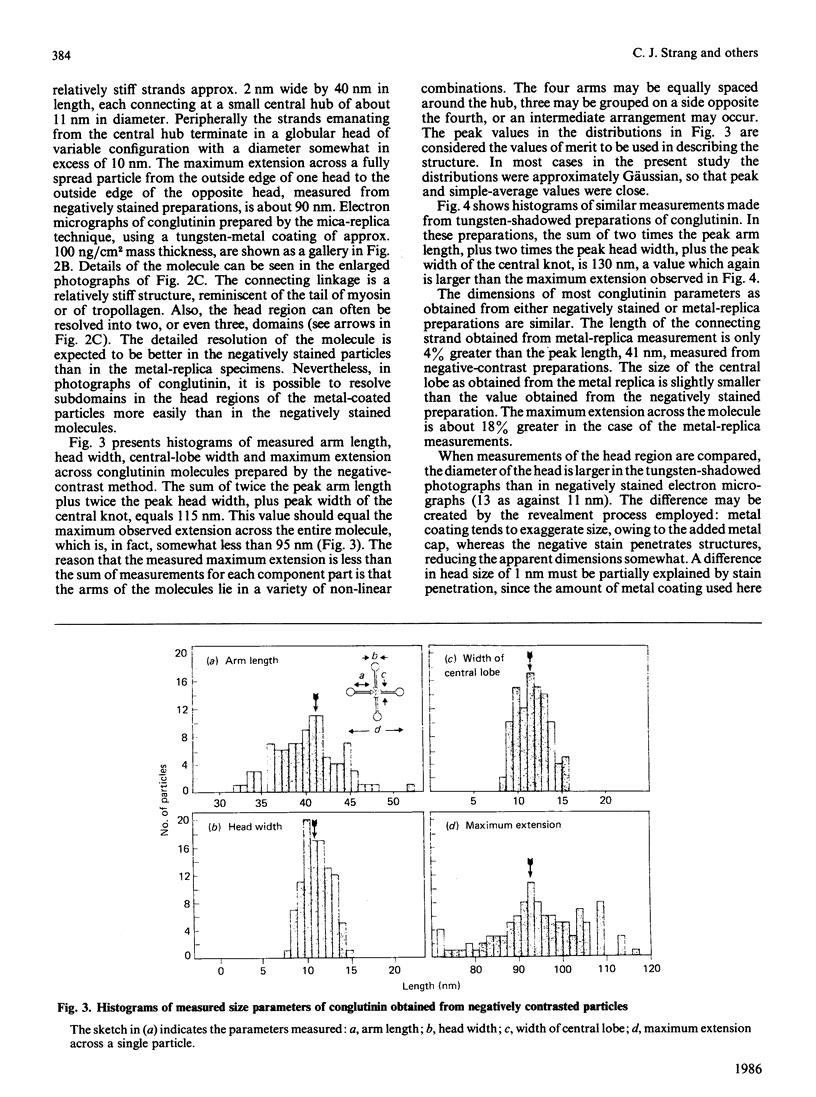
Conglutinin binds in a Ca2+-dependent manner to the carbohydrate portion of zymosan and cell-bound iC3b (complement subcomponent C3b cleaved by Factor I in the presence of factor H) similarly to lectin-like proteins that participate in the clearance of plasma glycoproteins. This carbohydrate-binding protein has been found to include both collagenous and non-collagenous domains. Electron micrographs of bovine conglutinin are presented in which conglutinin appears as a tetramer of four 'lollipop' structures emanating from a central hub. The stem region, linking each head to the central hub, is quite stiff, whereas the hub-stem junction is a flexible hinge. From electron micrographs of a pepsin digest of conglutinin, the linkage region is identified as the collagenous portion of the macromolecule. Conglutinin is a multimer of a single polypeptide chain. From sedimentation equilibria of unreduced as compared with reduced and alkylated conglutinin, there are determined to be three disulphide-linked chains. These data, combined with information on the subunit polypeptide of conglutinin, suggest a model for conglutinin in which four disulphide-linked trimers are associated via the N-termini to form the intact macromolecule as viewed in the electron microscope. The ultrastructure of conglutinin appears ideally suited to its lectin-like function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodsky-Doyle B., Leonard K. R., Reid K. B. Circular-dichroism and electron-microscopy studies of human subcomponent C1q before and after limited proteolysis by pepsin. Biochem J. 1976 Nov;159(2):279–286. doi: 10.1042/bj1590279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coligan J. E., Slayter H. S. Structure of thrombospondin. J Biol Chem. 1984 Mar 25;259(6):3944–3948. [PubMed] [Google Scholar]
- Crowther R. A., Pearse B. M. Assembly and packing of clathrin into coats. J Cell Biol. 1981 Dec;91(3 Pt 1):790–797. doi: 10.1083/jcb.91.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Smith C. A., Müller-Eberhard H. J. Visualization of human C4b-binding protein and its complexes with vitamin K-dependent protein S and complement protein C4b. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3461–3465. doi: 10.1073/pnas.80.11.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Lachmann P. J. Bovine conglutinin is a collagen-like protein. Biochemistry. 1984 May 8;23(10):2139–2144. doi: 10.1021/bi00305a006. [DOI] [PubMed] [Google Scholar]
- Engel J., Odermatt E., Engel A., Madri J. A., Furthmayr H., Rohde H., Timpl R. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 1981 Jul 25;150(1):97–120. doi: 10.1016/0022-2836(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Kirchhausen T. Clathrin, cages, and coated vesicles. Cell. 1983 Jul;33(3):650–652. doi: 10.1016/0092-8674(83)90007-7. [DOI] [PubMed] [Google Scholar]
- Hirani S., Lambris J. D., Müller-Eberhard H. J. Localization of the conglutinin binding site on the third component of human complement. J Immunol. 1985 Feb;134(2):1105–1109. [PubMed] [Google Scholar]
- INGRAM D. G. The conglutination phenomenon. XIII. In vivo interactions of conglutinin and experimental bacterial infection. Immunology. 1959 Oct;2:322–333. [PMC free article] [PubMed] [Google Scholar]
- INGRAM D. G. The conglutination phenomenon. XIV. The resistance enhancing effect of conglutinin and immuno-conglutinin in experimental bacterial infections. Immunology. 1959 Oct;2:334–345. [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C. Structural domains of clathrin heavy chains. J Cell Biol. 1984 Nov;99(5):1725–1734. doi: 10.1083/jcb.99.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobel H. R., Villiger W., Isliker H. Chemical analysis and electron microscopy studies of human C1q prepared by different methods. Eur J Immunol. 1975 Jan;5(1):78–82. doi: 10.1002/eji.1830050119. [DOI] [PubMed] [Google Scholar]
- Kozutsumi Y., Kawasaki T., Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit serum. Biochem Biophys Res Commun. 1980 Jul 31;95(2):658–664. doi: 10.1016/0006-291x(80)90836-0. [DOI] [PubMed] [Google Scholar]
- LACHMANN P. J., RICHARDS C. B. AN ESTIMATE OF SOME MOLECULAR PARAMETERS OF BOVINE CONGLUTININ. Immunochemistry. 1964 Apr;1:37–41. doi: 10.1016/0019-2791(64)90054-0. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Müller-Eberhard H. J. The demonstration in human serum of "conglutinogen-activating factor" and its effect on the third component of complement. J Immunol. 1968 Apr;100(4):691–698. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linscott W. D., Ranken R., Triglia R. P. Evidence that bovine conglutinin reacts with an early product of C3b degradation, and an improved conglutination assay. J Immunol. 1978 Aug;121(2):658–664. [PubMed] [Google Scholar]
- Mizuno Y., Kozutsumi Y., Kawasaki T., Yamashina I. Isolation and characterization of a mannan-binding protein from rat liver. J Biol Chem. 1981 May 10;256(9):4247–4252. [PubMed] [Google Scholar]
- Poon P. H., Schumaker V. N., Phillips M. L., Strang C. J. Conformation and restricted segmental flexibility of C1, the first component of human complement. J Mol Biol. 1983 Aug 15;168(3):563–577. doi: 10.1016/s0022-2836(83)80302-7. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I., Tuderman L., Guzman N. A. The biosynthesis of collagen and its disorders (first of two parts). N Engl J Med. 1979 Jul 5;301(1):13–23. doi: 10.1056/NEJM197907053010104. [DOI] [PubMed] [Google Scholar]
- Reid K. B., Lowe D. M., Porter R. R. Isolation and characterization of C1q, a subcomponent of the first component of complement, from human and rabbit sera. Biochem J. 1972 Dec;130(3):749–763. doi: 10.1042/bj1300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Sim R. B., Faiers A. P. Inhibition of the reconstitution of the haemolytic activity of the first component of human complement by a pepsin-derived fragment of subcomponent C1q. Biochem J. 1977 Feb 1;161(2):239–245. doi: 10.1042/bj1610239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L., Barnett P., Mays C. Acetylcholinesterase. Methods Enzymol. 1982;82(Pt A):325–339. doi: 10.1016/0076-6879(82)82070-3. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Lambris J. D. Identification of a C3bi-specific membrane complement receptor that is expressed on lymphocytes, monocytes, neutrophils, and erythrocytes. J Exp Med. 1982 Jan 1;155(1):96–110. doi: 10.1084/jem.155.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker V. N., Calcott M. A., Spiegelberg H. L., Müller-Eberhard H. J. Ultracentifuge studies of the binding of IgG of different subclasses to the Clq subunit of the first component of complement. Biochemistry. 1976 Nov 16;15(23):5175–5181. doi: 10.1021/bi00668a035. [DOI] [PubMed] [Google Scholar]
- Shelton E., Yonemasu K., Stroud R. M. Ultrastructure of the human complement component, Clq (negative staining-glutamine synthetase-biologically active Clq). Proc Natl Acad Sci U S A. 1972 Jan;69(1):65–68. doi: 10.1073/pnas.69.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter H. S. Electron microscopic studies of fibrinogen structure: historical perspectives and recent experiments. Ann N Y Acad Sci. 1983 Jun 27;408:131–145. doi: 10.1111/j.1749-6632.1983.tb23241.x. [DOI] [PubMed] [Google Scholar]
- Slayter H. S. High-resolution metal replication of macromolecules. Ultramicroscopy. 1976 Sep-Oct;1(4):341–357. doi: 10.1016/0304-3991(76)90050-4. [DOI] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Strang C. J., Siegel R. C., Phillips M. L., Poon P. H., Schumaker V. N. Ultrastructure of the first component of human complement: electron microscopy of the crosslinked complex. Proc Natl Acad Sci U S A. 1982 Jan;79(2):586–590. doi: 10.1073/pnas.79.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R., Stahl P. Isolation and characterization of a mannose/N-acetylglucosamine/fucose-binding protein from rat liver. Biochem J. 1981 Jan 15;194(1):209–214. doi: 10.1042/bj1940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W., Piez K. A. The chemistry and structure of collagen. Adv Protein Chem. 1971;25:243–352. doi: 10.1016/s0065-3233(08)60281-8. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Branton D. Assembly units of clathrin coats. Nature. 1981 Jan 29;289(5796):420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]