Abstract
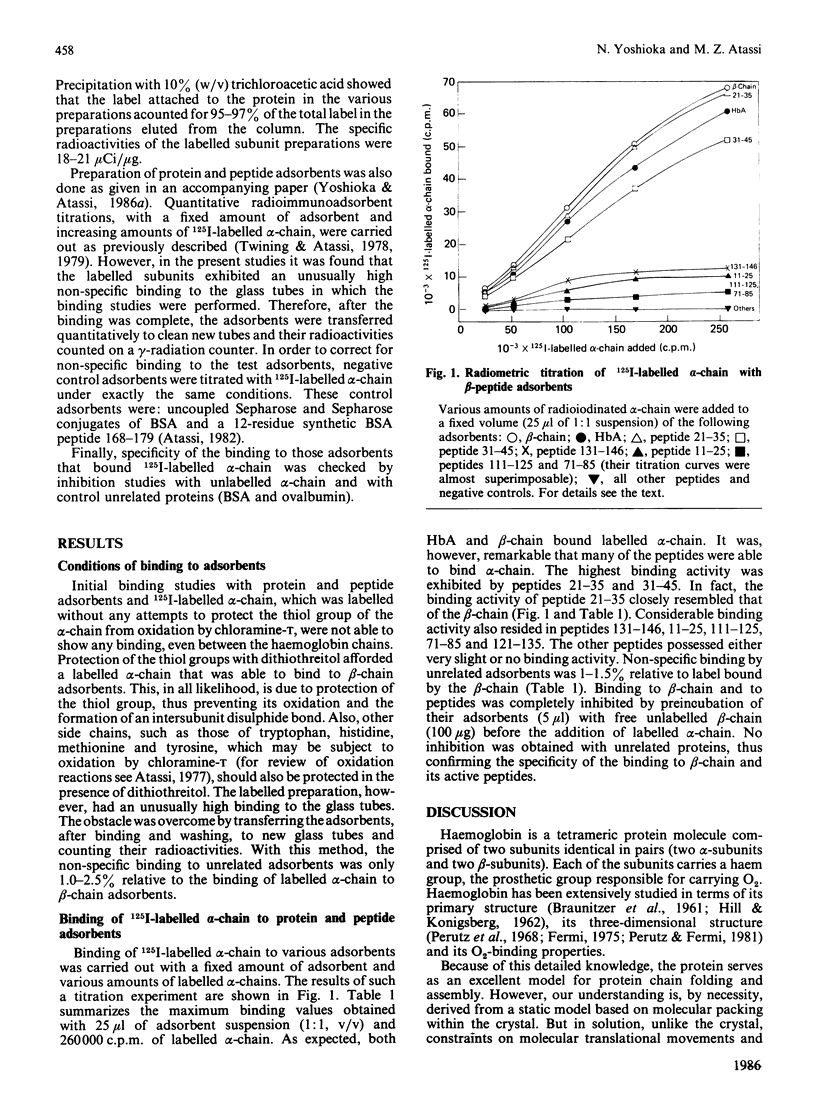
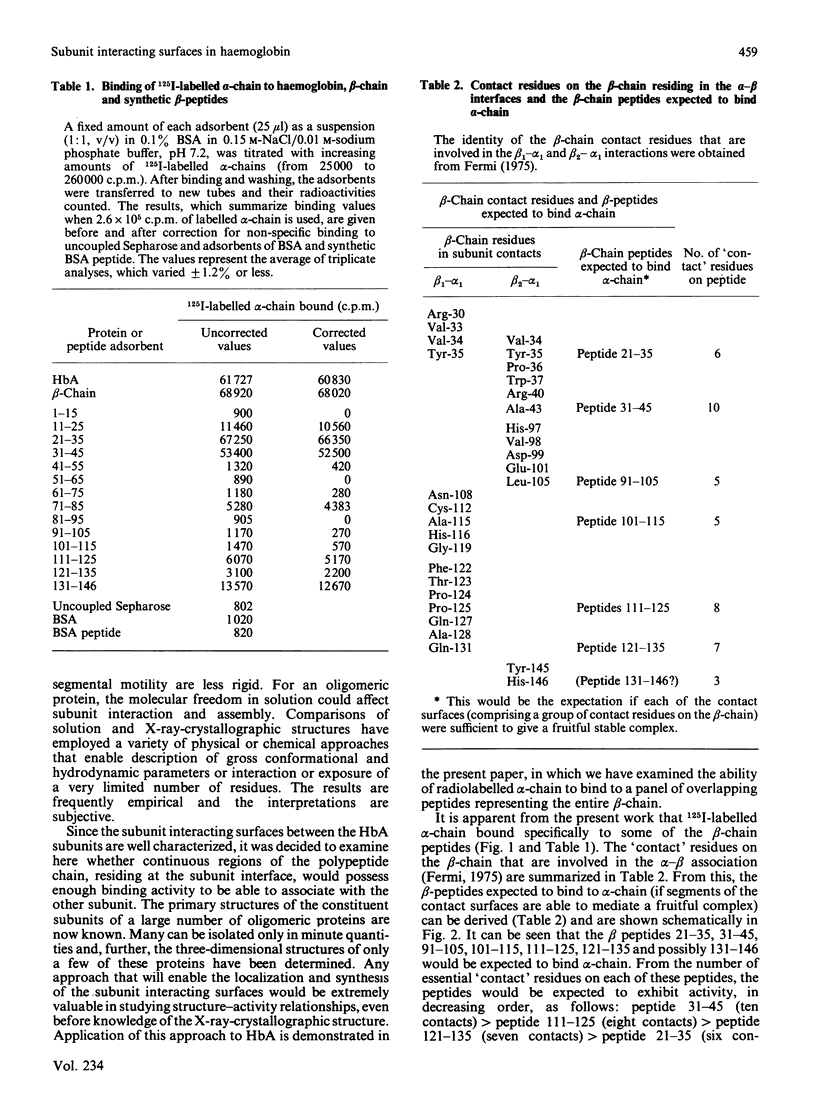
A synthetic approach is introduced for localization of subunit interacting surfaces in oligomeric proteins. It consists of studying the binding activity of consecutive uniform overlapping peptides encompassing an entire subunit to the other, radiolabelled, subunit. This permits the establishment of the full profile of peptides that bind the other intact subunit. This approach has been demonstrated with haemoglobin, and its application here with the beta-chain peptides has enabled the localization on the beta-chain of the submolecular regions responsible for its binding to alpha-chain in solution. There was good agreement between the binding surfaces found here in solution and those expected from the crystal structure. There were also, however, some significant differences in the levels of binding found in solution and those expected from the crystal. Peptide 21-35 possessed much higher binding activity than would be expected from its contribution to subunit association in the crystal. Conversely, other regions expected to possess considerable binding capacity for alpha-chain either showed low (peptides 111-125 and 121-135) or almost no binding (peptides 91-105 and 101-115) capacity. On the other hand, two interacting surfaces (within peptides 11-25 and 71-85) that make a contribution in solution do not appear to play a role in the crystal. It is concluded that the regions of subunit association in solution are close to, but not identical with, those in the crystal. The approach should serve as an effective method for localization of subunit interacting surfaces of unknown proteins, even those that can be isolated only in traces.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atassi M. Z. Chemical studies on haemoglobins A1 and A0. Biochem J. 1964 Oct;93(1):189–197. doi: 10.1042/bj0930189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Immune recognition of serum albumin.15. Localization by synthesis of antigenic site 4 of bovine serum albumin to the region around the disulfide bond 166-175. Biochim Biophys Acta. 1982 Jun 24;704(3):552–555. doi: 10.1016/0167-4838(82)90081-4. [DOI] [PubMed] [Google Scholar]
- BRAUNITZER G., GEHRING-MUELLER R., HILSCHMANN N., HILSE K., HOBOM G., RUDLOFF V., WITTMANN-LIEBOLD B. [The structure of normal adult human hemoglobins]. Hoppe Seylers Z Physiol Chem. 1961 Sep 20;325:283–286. doi: 10.1515/bchm2.1961.325.1.283. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- HILL R. J., KONIGSBERG W. The structure of human hemoglobin. IV. The chymotryptic digestion of the alpha chain of human hemoglobin. J Biol Chem. 1962 Oct;237:3151–3156. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Twining S. S., Atassi M. Z. Antibody-combining sites can be mimicked synthetically. Surface-simulation synthesis of the immunoglobulin new combining site to the gamma-hydroxyl derivative of vitamin K1. J Biol Chem. 1978 Aug 10;253(15):5259–5262. [PubMed] [Google Scholar]
- Twining S. S., Atassi M. Z. Use of immunoadsorbents for the study of antibody binding to sperm whale myoglobin and its synthetic antigenic sites. J Immunol Methods. 1979;30(2):139–151. doi: 10.1016/0022-1759(79)90088-7. [DOI] [PubMed] [Google Scholar]
- Yoshioka N., Atassi M. Z. Antigenic structure of human haemoglobin. Localization of the antigenic sites of the beta-chain in three host species by synthetic overlapping peptides representing the entire chain. Biochem J. 1986 Mar 1;234(2):441–447. doi: 10.1042/bj2340441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka N., Atassi M. Z. Haemoglobin binding with haptoglobin. Localization of the haptoglobin-binding sites on the beta-chain of human haemoglobin by synthetic overlapping peptides encompassing the entire chain. Biochem J. 1986 Mar 1;234(2):453–456. doi: 10.1042/bj2340453. [DOI] [PMC free article] [PubMed] [Google Scholar]


