Abstract
The intracellular assembly site for flaviviruses in currently not known but is presumed to be located within the lumen of the rough endoplasmic reticulum (RER). Building on previous studies involving immunofluorescence (IF) and cryoimmunoelectron microscopy of Kunjin virus (KUN)-infected cells, we sought to identify the steps involved in the assembly and maturation of KUN. Thus, using antibodies directed against envelope protein E in IF analysis, we found the accumulation of E within regions coincident with the RER and endosomal compartments. Immunogold labeling of cryosections of infected cells indicated that E and minor envelope protein prM were localized to reticulum membranes continuous with KUN-induced convoluted membranes (CM) or paracrystalline arrays (PC) and that sometimes the RER contained immunogold-labeled virus particles. Both proteins were also observed to be labeled in membranes at the periphery of the induced CM or PC structures, but the latter were very seldom labeled internally. Utilizing drugs that inhibit protein and/or membrane traffic throughout the cell, we found that the secretion of KUN particles late in infection was significantly affected in the presence of brefeldin A and that the infectivity of secreted particles was severely affected in the presence of monensin and N-nonyl-deoxynojirimycin. Nocodazole did not appear to affect maturation, suggesting that microtubules play no role in assembly or maturation processes. Subsequently, we showed that the exit of intact virions from the RER involves the transport of individual virions within individual vesicles en route to the Golgi apparatus. The results suggest that the assembly of virions occurs within the lumen of the RER and that subsequent maturation occurs via the secretory pathway.
The formation of flavivirus infectious particles (∼50 nm in diameter) involves the assembly of the nucleocapsid or core particles (∼30 nm in diameter) comprising the positive-sense RNA genome and core protein C, all enclosed in a lipid envelope containing envelope protein E and premembrane prM (which is proteolytically cleaved late during maturation to yield protein M). The core particle has never been isolated in its free state from infected cells or positively identified free within the cytoplasm by electron microscopy. Virions accumulate as large cytoplasmic aggregates, but with rare exceptions, the normal process of budding through membranes that is associated with the envelopment of viruses has not been observed with Flavivirus species (15, 37). Using Kunjin virus (KUN) as a model to study the events of flavivirus replication, we have characterized unique sets of induced membranes and their apparent replication functions by cryoimmunoelectron microscopy (CIEM). Thus, we have identified the intracellular site of KUN RNA synthesis within packets of vesicles (VP) that associate closely with another virus-induced membrane structure, termed convoluted membranes (CM) interconvertible with paracrystalline array (PC) structures (33, 59). CM or PC is presumed to be the site of proteolytic processing by the viral serine protease (NS3 and cofactor NS2B) located therein (59). The consensus composition of the KUN replicase complex in the VP comprises NS1, NS2A, NS3, NS4A, NS5, and the template double-stranded RNA or replicative form, all identified with monospecific antibodies by CIEM and radioimmunoprecipitation and by biochemical analyses of infected cells (31, 33, 59). We have proposed that this collection of membranes, along with associated virions and proliferating endoplasmic reticulum (ER), comprises virus factories in which compartmentalization within the induced membranes ensures a more efficient replication process.
While it appears that virus particles are closely associated with the KUN factories, the exact intracellular site of flavivirus assembly remains obscure. Ultrastructural studies of flavivirus-infected cells have consistently revealed apparent mature virions within distended ER, large cytoplasmic vesicles, and vacuoles (37). In addition, occasional sections have shown individual particles within Golgi cisternae, suggesting that flavivirus maturation proceeds through the Golgi apparatus (6, 14–16, 26, 32). After translation and translocation of the KUN polyprotein into the ER lumen, processing of prM and E occurs via host signal peptidase, whereas the core protein remains within the cytosol and is cleaved at a dibasic site preceding its carboxy-terminal hydrophobic domain by the KUN protease. Immunolocalization studies have indicated that the core protein translocates to the nucleus, associates in the cytosol with virus-induced CM or PC, and is incorporated into virions (58). In vitro studies with expressed recombinant proteins of other flavivirus species have shown that a heterodimeric association between prM and E is formed during the assembly and maturation processes (3, 57), and domains required for this interaction have been identified within both E and prM (3, 4). However, the interaction that occurs between the core protein and either prM or E to promote the assembly process is still unknown. Several studies have indicated that prM facilitates the correct folding of E (3, 17, 24) or, alternatively, masks a retention signal within E, although no such signal has thus far been identified. During maturation, prM appears to prevent E undergoing an acid-catalyzed conformational change required for fusion within the endosome formed during the entry of the virus (3, 12, 13, 18). During the final stages of maturation before virus release, this preventive mechanism is overcome by cleavage of the pr moiety from prM by the host protease furin (52).
Due to the orientation and topology of the structural proteins within the lipid bilayer, it is presumed that flavivirus assembly must occur via the core particle budding into the ER lumen (46). Although this process has not been convincingly shown, recent developments in cryofixation and CIEM have revealed budding of West Nile virus strain Sarafend (glycosylated in both prM and E) at the plasma and intracellular membranes (38). However, as noted above, this process does not appear to be the general mechanism for Flavivirus species. In this study, we have addressed the ultrastructural location of flavivirus assembly and the subsequent maturation pathway by using CIEM in conjunction with various inhibitors of secretion.
MATERIALS AND METHODS
Cells and virus.
Vero cells were grown and maintained in Dulbecco modified Eagle medium (DMEM) (Gibco BRL) supplemented with 5% fetal calf serum and penicillin-streptomycin. Cells were infected with KUN strain MRM61C at a multiplicity of infection (MOI) of 3 as previously described (59), and infected cells were maintained in DMEM containing 0.1% bovine serum albumin.
Reagents.
Brefeldin A (BFA), monensin (MON), and nocadazole (NOZ) were obtained from Sigma and used at concentrations of 5 μg/ml, 10 μM, and 2 μg/ml, respectively. Imino sugars, deoxynojirimycin (DNJ), and N-nonyl-DNJ (NN-DNJ) were obtained from Toronto Research Chemicals (Toronto, Ontario, Canada) and used at a concentration of 100 μM. Antibodies used were mouse monoclonal anti-KUN E antibodies (1), rabbit polyclonal anti-Murray Valley encephalitis virus E antibodies (cross-reactive with KUN E), and mouse monoclonal antibodies to KUN prM glycoprotein (1E7), all provided by R. Hall (University of Queensland, Brisbane, Queensland, Australia); monoclonal antibodies to ERGIC53 (48) and to giantin (28), provided by H.-P. Hauri (University of Basel, Basel, Switzerland), and to protein disulfide isomerase (PD1) (ID3) (55), provided by S. Fuller European Molecular Biology Laboratory, Heidelberg, Germany); rabbit polyclonal antibodies to human Lamp1 (93/B) (11), provided by M. Fukuda (La Jolla Cancer Research Foundation, La Jolla, Calif.); goat polyclonal antibodies to mannose-6-phosphate receptor (M6PR) (Zi I–2), provided by A. Hille (Department of Biochemistry, Göttingen, Germany); and donkey antibodies specific for rabbit or goat immunoglobulin G and conjugated to fluorescein isothiocyanate (FITC) or Texas Red, purchased from Edward Keller (Hallam, Victoria, Australia).
Radiolabeling.
Subconfluent monolayers of Vero cells were infected with KUN at an MOI of 3. At 17 h postinfection (p.i.), cells were incubated in methionine- and cysteine-deficient medium for 1 h. Cells were then pulse-labeled with 50 μCi of [35S]methionine-cysteine (Trans-label; ICN) per ml for 120 min in the presence of 3 μg of actinomycin D per ml. When chase experiments were performed, the labeling medium was removed and the cells were washed twice with phosphate-buffered saline (PBS) before incubation with DMEM supplemented with 0.1% bovine serum albumin and a 10× excess of unlabeled methionine and cysteine (150 and 32 μg/ml, respectively). After either labeling or chase periods (4 h), the tissue culture fluid was collected and clarified by centrifugation at low speed; the cell monolayer was harvested in coimmunoprecipitation buffer (10 mM Tris [pH 8.0], 150 mM NaCl, 5 mM EDTA, 1% Triton X-100) containing protease inhibitors and incubated on ice for 20 min before clarification by centrifugation. Both the tissue culture fluid and lysates were used for radioimmunoprecipitation (RIP) experiments with mouse anti-E antibodies as previously described (22).
Immunofluorescence (IF).
Vero cell monolayers on coverslips were infected with KUN at an MOI of approximately 3 to 5 and incubated at 37°C for 24 h. The cells were subsequently washed with PBS, fixed with 4% paraformaldehyde for 10 min at 20°C, and permeabilized with 0.2% Triton X-100 in 4% paraformaldehyde for 10 min at 20°C. The cells were washed with PBS, and aldehyde groups were quenched with 0.5 M NH4Cl for 7 min at 20°C. The cells were subsequently washed twice with PBS before incubation with antibodies.
Electron microscopy.
Methods for resin embedding, cryofixation, preparation of cryosections, and immunolabeling have been described elsewhere (31, 32, 59). However, for the current experiments, the cell blocks used for sectioning were embedded in 10% gelatin and postfixed with 1% paraformaldehyde. Sections were cut with a Diatome Cryo-P diamond knife and retrieved from the cryochamber with a droplet of 14:1 2.3 M sucrose–2% methylcellulose.
RESULTS
IF analysis of the subcellular localization of KUN E protein.
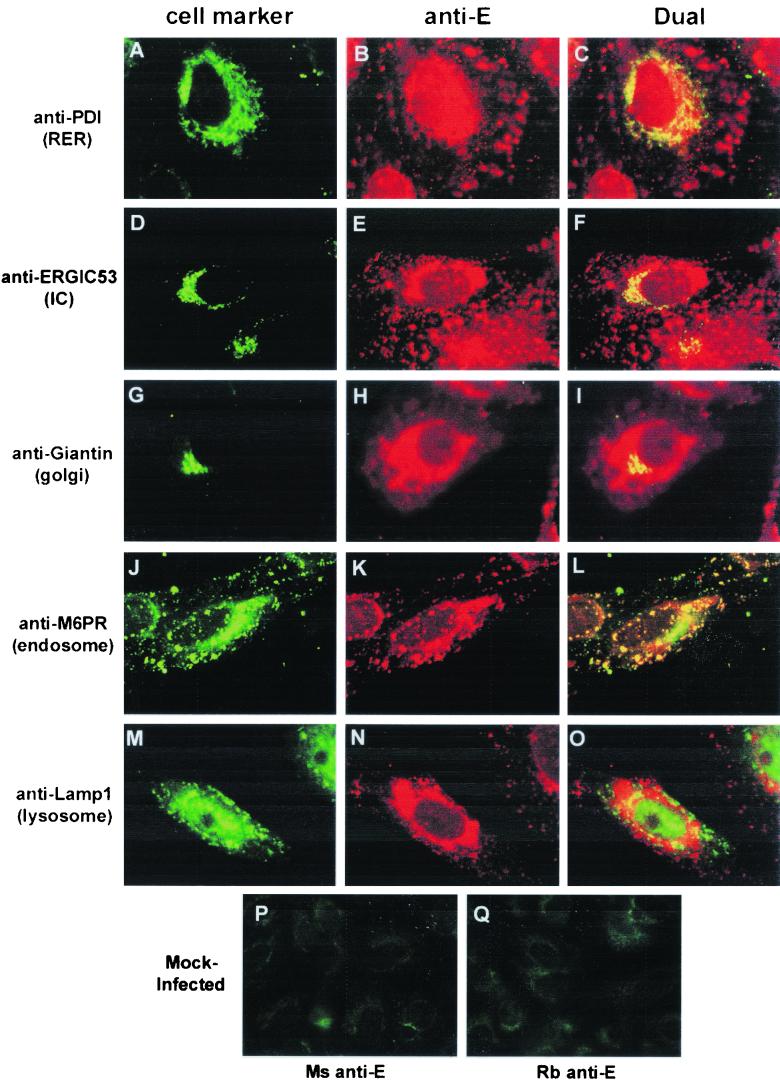
To monitor the distribution of the KUN E protein in relation to cellular organelles possibly involved in assembly or maturation of virions, we examined the immunolocalization of E with antibodies to various cell markers. The KUN strain (MRM61C) used for these experiments encodes a nonglycosylated E protein (60). At 24 h postinfection (p.i.), KUN-infected cells were fixed and immunostained. The overall distribution of KUN E consisted of diffuse perinuclear staining that often included several large densely staining foci and small isolated foci that were widespread throughout the cytoplasm. Dual-labeling experiments revealed extensive colocalization of KUN E with the marker for the rough ER (RER) (anti-PDI) in the perinuclear region (Fig. 1A to C), suggesting the accumulation of KUN E in this region. The perinuclear staining pattern of E also overlapped that of the Golgi apparatus (detected with antigiantin) (Fig. 1G to I), but precise colocalization within this organelle could not be determined by light microscopy. However, some costained cells displayed quite distinct staining patterns with anti-E and antigiantin antibodies (results not shown). Some anti-E foci associated with the perinuclear region were colabeled with a marker for the intermediate compartment (IC) (anti-ERGIC53) (Fig. 1D to F). A similar IF staining pattern with anti-ERGIC53 antibodies and anti-KUN NS3 antibodies was previously found (30), suggesting that the anti-E-labeled foci may be associated with KUN CM or PC. In contrast, the smaller anti-E-stained foci distributed within the cytoplasm were strongly colabeled with a marker for endosomes (anti-M6PR) (Fig. 1J to L). This coincidental labeling of E within endosomes is unfortunately unable to differentiate between incoming or outgoing E-labeled virions. E appeared to also show some coincidental labeling with lysosomes in the perinuclear region (Fig. 1M to O).
FIG. 1.
Markers for the RER, IC, and endosomal region are associated by IF with the KUN E protein. KUN-infected Vero cells were fixed at 24 h p.i. and processed for IF with anti-E antibodies conjugated to Texas Red (B, E, H, K, and N) for comparisons with FITC-labeled of antibodies to various cellular compartments (A, D, G, J, and M) (same cells). Apparent partial coincidence in the dual labels was observed as a yellow hue in panels C, F, and L. No coincidence with KUN E was apparent for the marker for lysosomes (Lamp1), but some partial overlap was observed with the marker for the Golgi apparatus (giantin). (P and Q) Mock-infected Vero cells incubated with mouse and rabbit anti-E antibodies, respectively.
In summary, KUN E protein appears to accumulate within both the RER and endosomal compartments. Limited coincidental staining within the Golgi region suggests that either the immature virion does not traverse to this organelle or transport through the Golgi apparatus is very rapid.
Ultrastructural analysis shows that KUN E and prM are primarily associated with reticular membranes and the KUN-induced CM or PC structures within infected cells.
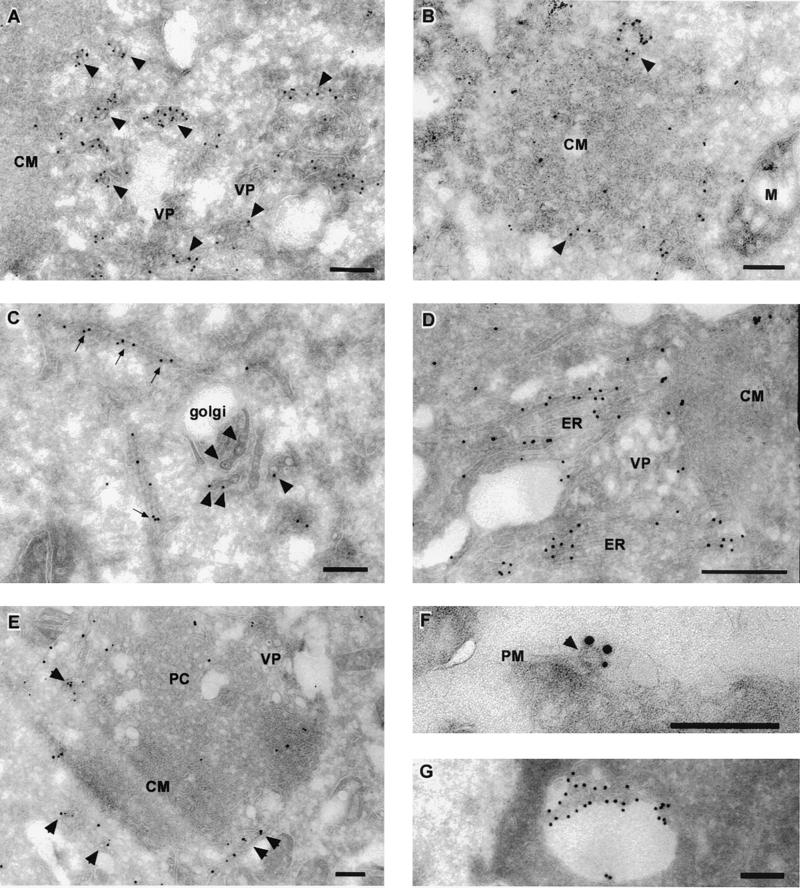
To further define the intracellular localization of KUN during its assembly and maturation, we immunolabeled cryosections from KUN-infected cells at 24 h p.i. with monoclonal antibodies raised against the E and prM proteins. As shown in Fig. 2, gold particles were primarily associated with reticular membranes present within the cytoplasm of infected cells. Some of these reticular membranes appeared to show direct connections with KUN-induced CM or PC structures (Fig. 2D and E). In most sections, labeling with anti-E and/or anti-prM antibodies within the reticular membranes was associated with accumulated virus particles (Fig. 2A, B, C, and E); however, in some sections labeling did not appear to be associated with any particular structure other than the ER or virus particles (Fig. 2D) and therefore may have represented either the accumulation of E at assembly sites or the detection of E translocated into the ER during its synthesis. Both KUN E and prM were also immunolabeled at similar sites on reticular membranes, at or around the periphery of induced CM or PC (Fig. 2E) and on virus particles that were either extracellular (Fig. 2F) or intracellular, within a vacuole (Fig. 2G). Gold particles appeared to sparsely label the inner components of some CM or PC structures (Fig. 2B and E), but gold particles were never observed to label VP structures. Interestingly, previous immunoelectron microscopy observations with anticore antibodies showed a labeling pattern (of the periphery of induced membranes and virions [58]) very similar to that of anti-E and anti-prM antibodies presented here (Fig. 2A, B, C, and E). Notably, gold particles within the Golgi apparatus were always associated with virions (Fig. 2C), implying that the E protein alone does not traverse this organelle.
FIG. 2.
KUN E and prM proteins localize to reticulum membranes, associate with the periphery of KUN-induced CM or PC structures, and label both intra- and extracellular KUN virions. Infected Vero cells were harvested at 24 h p.i. and processed for cryoelectron microscopy and immunolabeling. Ultrathin cryosections were cut and probed with antibodies to KUN E and prM (10- or 15-nm protein-A gold particles respectively, supplied by Utrecht University, Utrecht, The Netherlands). Enrichment of anti-prM antibodies was observed within distended reticulum membranes containing virus particles (A and B, arrowheads) and also with membranes on the periphery of the induced CM structures (B, arrowheads). Anti-E antibodies were located on virions associated with the Golgi apparatus (C, arrowheads) and on ER continuous with the induced CMs structures (D). Arrows in panel C highlight E labeling on reticulum membranes adjacent to the Golgi apparatus. Cryosections were dually labeled in panels E and F with anti-E antibodies (15-nm gold particles) and anti-prM antibodies (10-nm gold particles). Panel E shows labeling with both anti-E and anti-prM antibodies of reticulum membranes and enclosed virions (arrowheads) that are in close association with the induced CM or PC structures. In panel F, extracellular virions were also dually labeled (arrowhead). In panel G, intracellular virions in a vacuole were labeled with anti-E antibodies. Abbreviations: M, mitochondria; PM, plasma membrane. Bars, 200 nm.
In summary, we have observed both anti-E and anti-prM gold-labeled antibodies associated with the Golgi apparatus and more strongly with reticular membranes that appeared to be directly connected to KUN-induced CM or PC structures, and they labeled predominantly the periphery rather than the interior of the CM or PC structures. Both intra- and extracellular virus particles were also clearly labeled with both antibodies.
Intracellular distribution of the KUN E protein after treatment of infected cells with metabolic inhibitors.
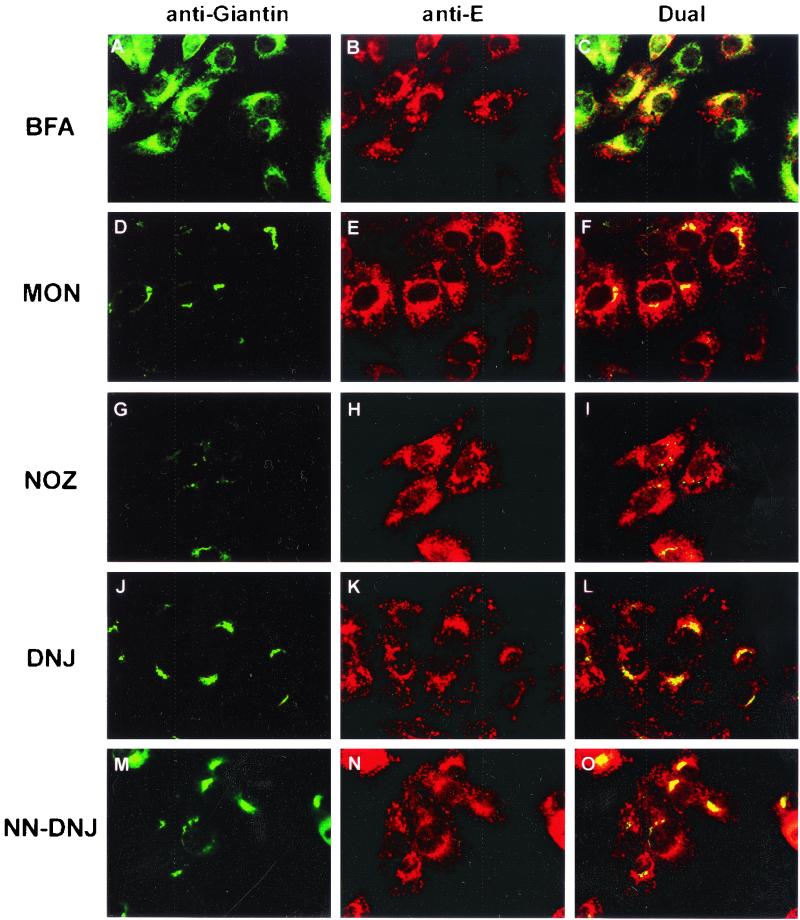
As part of the studies on the intracellular location of flavivirus assembly, we sought to determine the effects of metabolic inhibitors on this process late in infection. Our initial screening was performed by dual IF with antibodies to KUN E and the Golgi apparatus (antigiantin). Antigiantin antibodies were used to determine the effects of the inhibitors on cellular morphology, as most of them have an impact on the Golgi apparatus. BFA affects many cellular functions, but its primary target is the G-coupled ADP-ribosylation factors (10, 19). The effect of BFA on ADP-ribosylation factors leads to an interruption in the vesicular flow from the IC to the Golgi apparatus, resulting in Golgi apparatus disassembly (9, 49). MON arrests protein movement within the medial Golgi compartment (44), whereas NOZ disrupts microtubules and thus microtubule-dependent traffic. DNJ and NN-DNJ are inhibitors of the α-glucosidases, involved in the removal of terminal glucose residues from the glycans of nascent glycoproteins in the RER, and thus cause some viral glycoproteins to be misfolded and retained within the ER (8, 36, 62).
For these experiments, the inhibitors were added to infected cells at 18 h p.i., and cells were maintained in the medium for an additional 6 h. As shown in Fig. 3A to C and 3J to O, BFA, DNJ and NN-DNJ had negligible effects on the apparent intracellular distribution of E seen by IF. Both the thickened perinuclear staining and the cytoplasmic foci of E observed in Fig. 1 were also seen in the presence of these inhibitors. Staining of the Golgi apparatus showed drastic dispersal in the presence of BFA (compare with Fig. 1), but no obvious effect on Golgi apparatus distribution was observed with either DNJ or NN-DNJ. Much of the dispersed antigiantin staining in the presence of BFA was surprisingly colocalized with unchanged staining of E (Fig. 3A to C). This apparent colocalization probably represents redistribution of the Golgi apparatus back into the RER, induced by the BFA treatment, to a location similar to that of accumulated E protein (Fig. 1A to C). NOZ induced an expansion of amorphous cytoplasmic staining of E and a scattered distribution of the Golgi apparatus, most likely representing a collapse in the cell ultrastructure when microtubules were disrupted (Fig. 3G to I). Treatment of infected cells with MON induced the Golgi apparatus to condense into small foci in the perinuclear region and also caused the distribution of E to become condensed into a more punctate pattern of small foci in the perinuclear region; some of these foci overlapped the altered Golgi foci (Fig. 3D to F).
FIG. 3.
Effect of inhibitors on the subcellular distribution of KUN E protein. KUN-infected Vero cells were incubated with the appropriate inhibitors from 18 to 24 h p.i. and then fixed for IF analysis. The location of KUN E protein was visualized with anti-E monoclonal antibodies conjugated to Texas Red (B, E, H, K, and N) and compared with that of the Golgi apparatus marker giantin conjugated to FITC (A, D, G, J, and M). Coincidental labeling was observed as a yellow hue in panels C, F, I, L, and O. Inhibitors are indicated at left.
Effects of metabolic inhibitors on the assembly, secretion, and maturation of KUN.
To extend the above study, we used radiolabeling in pulse-chase experiments to evaluate both assembly and secretion of KUN in the presence of inhibitors. For the assembly studies, we reasoned that the coprecipitation of core protein from treated lysates or infected culture fluid during RIP with anti-E antibodies would suggest that the formation of virus particles had occurred in the presence of inhibitors. This presumption stems from the observations of Khromykh et al. (22), who found that core protein interacted with the other structural proteins only in the presence of replicating KUN RNA. Our aims were to attempt inhibition of assembly at specific cellular sites, namely, RER (with DNJ or NN-DNJ), IC (with BFA), and medial Golgi compartment (with MON), and to look for the presence of pulse-labeled core in anti-E immunoprecipitates. The effects of the addition of inhibitors from 18 h p.i. on virus secretion when approaching the maximum period of virus release were analyzed by collecting radiolabeled culture fluids for RIP and plaque assay analyses.
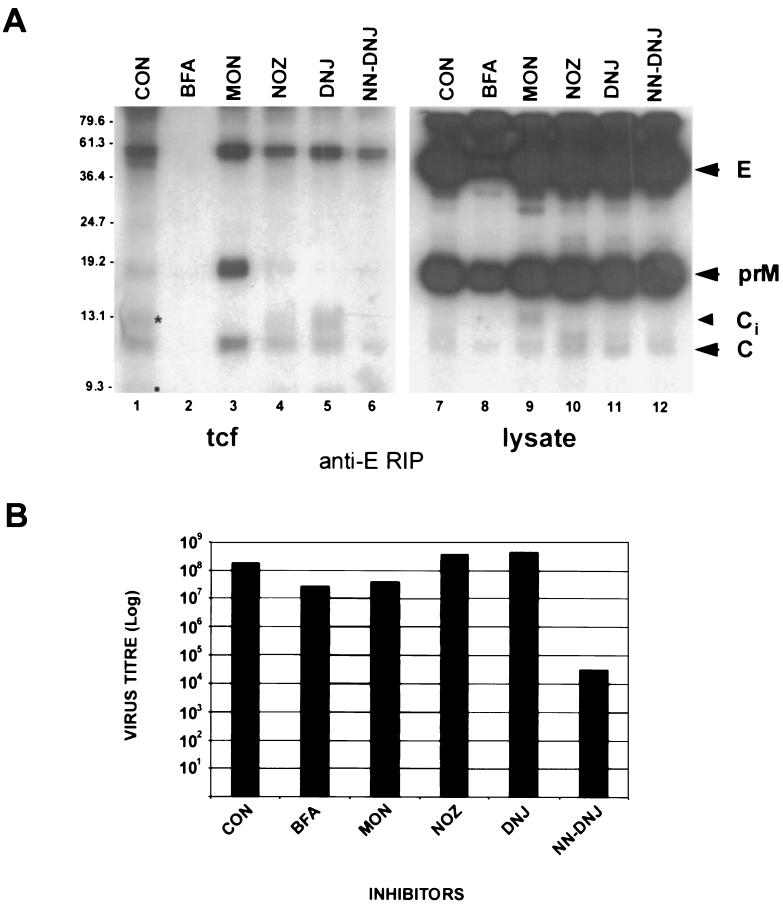
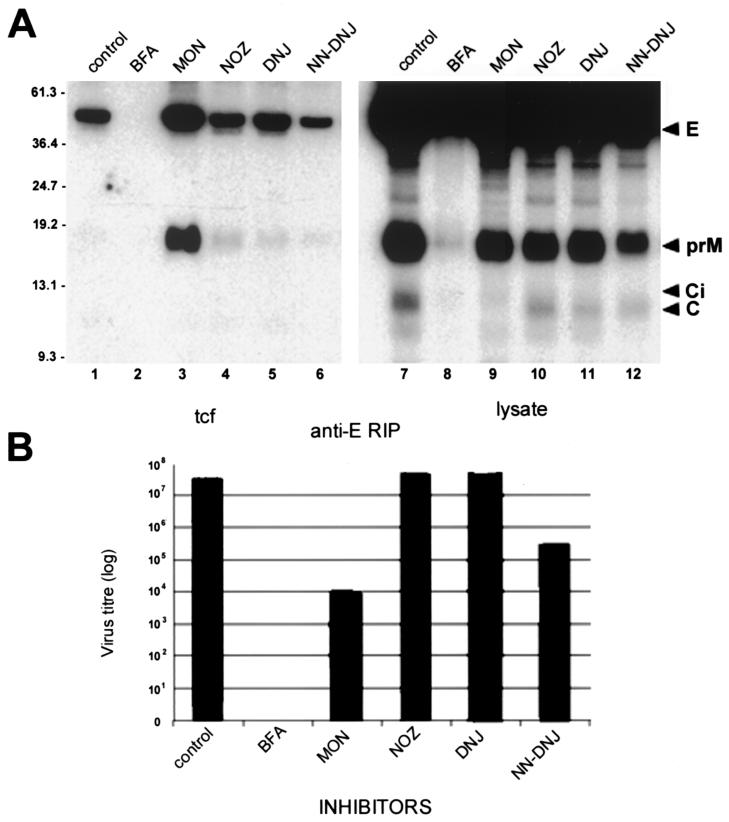
In all lysates, incorporation of the radiolabel in E, C, and prM from 18 to 20 h p.i. appeared efficient and did not appear to be inhibited by the presence of inhibitors (Fig. 4A); however, in lysates of MON-treated cells, the immature precursor Ci of virion C appeared to be inefficiently cleaved because normal product C (61) was only weakly labeled (Fig. 4A, lane 9). Control (untreated) samples revealed that the KUN virion was efficiently secreted into the culture fluid during the chase period of 4 h, and most of the labeled prM glycoprotein in lysates was proteolytically processed to pr and M in the tcf (Fig. 4A, compare lanes 1 and 9). In stark contrast, incubation of infected cells in the presence of BFA drastically reduced the amounts of all secreted structural proteins to undetectable amounts, yet the level of incorporation of radiolabel in BFA-treated cells was only slightly reduced (Fig. 4A, lanes 2 and 8) compared to the results obtained for other lysates associated with each inhibitor treatment. Treatment of cells late in infection with MON allowed efficient secretion of particles comprising E, prM, and C; notably, however, prM glycoprotein was not further processed (Fig. 4A, lanes 3 and 9), suggesting that either these particles did not pass through the trans-Golgi compartment or treatment with MON affected the pH-dependent cleavage of prM to M. Treatment of cells with either NOZ, DNJ, or NN-DNJ did not appear to have a significant influence on the secretion of virus particles or on the normal processing of prM, although the amounts of immunoprecipitated secreted structural proteins were reduced in each of these samples compared with MON-treated cells (Fig. 4A, lanes 3 to 6). In all samples other than the BFA culture fluid, core protein appeared to be coprecipitated with anti-E antibodies, indicating that particle formation had occurred.
FIG. 4.
Effect of inhibitors on the secretion of KUN structural proteins, the assembly of KUN virions, and the infectivity of secreted particles when inhibitors were added late in infection. (A) Infected Vero cells were radiolabeled (in the presence of inhibitors) from 18 to 20 h p.i. and chased in medium containing excess methionine-cysteine (and inhibitors) from 20 to 24 h p.i. Subsequently, cells and tissue culture fluid (tcf) were harvested in coimmunoprecipitation buffer for RIP with anti-E antibodies. The control lane (CON) represents tcf and lysates of infected cells labeled in the absence of inhibitors. Arrowheads on the right indicate the KUN structural proteins E, prM, and C. Ci appears to be the immature form of C that is normally converted to C by cleavage of the carboxy-terminal hydrophobic sequence of 18 amino acids. Proteolytically processed products pr (asterisk) and M (small square) are highlighted in lane 1. Sizes of molecular mass markers (in kilodaltons) are shown on the left. Proteins were separated on a 15% polyacrylamide–sodium dodecyl sulfate gel and visualized by autoradiography. (B) Tissue culture fluid (tcf) collected from the above experiment was assessed for infectivity by a plaque assay with Vero cells. Cell monolayers were incubated at 37°C for 4 days under 2% carboxymethyl cellulose overlay medium before plaques were visualized by staining of cells with 0.2% crystal violet.
Most of the above results were reflected in the amounts of released infectious virus, as measured by a plaque assay (Fig. 4B). NOZ- and DNJ-treated infected cells appeared to secrete viruses at equivalent titers compared to the untreated control. Titers of infectious virus released from MON- and BFA-treated cells were reduced by about 6- and 10-fold, respectively, during the 6-h treatment period. This result is as expected for BFA treatment, where the secretion of radiolabeled virions is drastically inhibited and the apparent small decrease in virus titer is explained by the apparent release of preassembled but unlabeled virions present during the pulse and chase periods. The MON effect can be explained by inefficient processing of labeled prM to M in the secreted virions and the release of virions assembled prior to MON treatment. Surprisingly, the titer of virus released from NN-DNJ-treated cells was reduced by about 3.5 log units, despite the observations that radiolabeling of lysates was not inhibited by NN-DNJ treatment, radiolabel in secreted particles was readily detected, and no accumulation of prM occurred in these particles. The conclusion is that although NN-DNJ did not affect viral protein synthesis, assembly, or secretion, impairment in the glycosylation of prM reduced the infectivity of progeny virions by 99.9%. The different effects observed with DNJ and NN-DNJ indicate that the inhibition of α-glucosidase activity by NN-DNJ was unique in that it had a profound effect on the infectivity of secreted virus, even though prM appeared to be cleaved.
When BFA was added as early as 1.5 h p.i., viral protein synthesis and release of infectious virus late in infection were drastically impaired (Fig. 5), compared with the small effects when BFA was added late (Fig. 4). These results indicate that the early addition of BFA severely restricts expression, probably by inhibiting the membrane induction (involving the trans-Golgi membranes) required for optimal RNA synthesis (30), as suggested for the inhibitory effects of BFA on poliovirus replication (35). The effects of the other inhibitors were similar when they were added either early or late (compare Fig. 4 and 5). Notably, the early disruption of microtubules by NOZ had no effect on virus replication and release.
FIG. 5.
Effect of inhibitors on virus assembly and on secretion and infectivity of released KUN virions when inhibitors were added early during the infectious cycle. In this experiment complementary to that shown in Fig. 4, inhibitors were added at 1.5 h p.i. and maintained in the medium until the cells were harvested at 24 h p.i., Experimental procedures were the same as those described in the legend to Fig. 4.
In summary, it appears that none of the specific inhibitors prevented KUN assembly, as demonstrated by the observed coprecipitation of core protein with anti-E antibodies after treatment with inhibitors, but secretion (with BFA) and processing of prM in the immature virion (with MON) and infectivity (with NN-DNJ) were severely affected. The high level of infectivity of virus released after NOZ and DNJ treatments could not represent an intracellular accumulation of virus at 18 h p.i. (i.e., prior to late treatment) because of the observed low level of infectivity of virus released after parallel NN-DNJ treatment.
Exit of KUN virions from the RER occurs via transport vesicles.
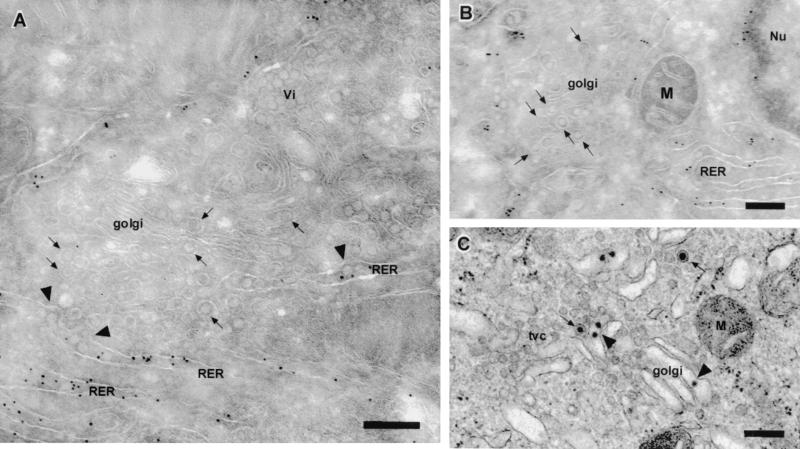
The observations presented in this article and those of others (46) suggest that the assembly of the flavivirus virion occurs within the RER. No direct evidence of budding of nucleocapsids into the lumen of the RER has been published, indicating that this event either is extremely rapid or occurs infrequently. We therefore carefully scanned cryosections for any evidence of a maturation pathway for KUN virions involving transport from the RER via individual vesicles to the Golgi apparatus. Figure 6A shows quite clearly KUN virions apparently exiting the RER (gold labeled with antibodies against PDI) via individual protuberant vesicles emerging from the RER membrane. It is apparent that each virion is being transported to the Golgi apparatus via a separate vesicle carrier (Fig. 6B and C). These vesicles most likely represent transport vesicles associated with the IC, as tubular-vesicular clusters (tvc) synonymous with the IC (29, 54) were frequently observed associated with virus-carrier vesicles (Fig. 6C). We propose that subsequent transport proceeds through the Golgi apparatus (Fig. 6) and that the accumulation of “mature” virions occurs in the trans-Golgi region or endosomal region. KUN virions do not appear to accumulate within the Golgi organelle, indicating that transit through the stacks must be a rapid event.
FIG. 6.
Transport of assembled KUN virions from the RER to the Golgi apparatus involves individual transport vesicles. (A and B) Infected cryosections were immunolabeled with anti-PDI antibodies and 10-nm anti-immunoglobulin G gold (Biocell, Cardiff, United Kingdom). Arrowheads in panel A highlight apparent KUN virions exiting from the RER, whereas the arrows in all panels indicate individual virions within individual transport vesicles en route to the golgi apparatus. (C) A resin-embedded section more clearly defines the Golgi apparatus containing a virion (arrowhead) and the individual vesicles transporting KUN virions (arrows). Note that the individual virions in the clusters (Vi) in panel A are not enclosed in the membrane associated with the transport vesicles. Abbreviations: M, mitochondria; Nu, nucleus; tvc, tubular-vesicular clusters. Bars, 200 nm.
Thus, our results are compatible with the notion that KUN assembly occurs by budding into the RER or by condensation of the nucleocapsid with associated structural proteins within membranes of the RER. The virion is subsequently transported within individual transport vesicles to the Golgi apparatus via the IC and emerges from the trans-Golgi apparatus for eventual release via the secretory pathway.
DISCUSSION
The experiments described in this paper have attempted to define the intracellular site of assembly and the maturation pathway for the flavivirus KUN. For these analyses, we used antibody markers to cellular compartments and to the KUN structural proteins E and prM for both dual IF (Fig. 1 and 3) and immunolabeling of cryosections (Fig. 2, 5, and 6). We showed that KUN virions appear to accumulate within the RER and endosomal vesicles (Fig. 1 and 6), that the E and prM proteins localize to distended ER and also to the periphery of the induced CM or PC structures (sometimes associated with virions) (Fig. 2), and that the KUN virion appears to initiate maturation by exiting the RER via individual transport vesicles (Fig. 6) and probably fuses with the cis-Golgi compartment. Although no direct evidence for virus assembly was observed, the accumulation of apparently intact virions within the RER and the assembly of infectious virions in the presence of BFA (Fig. 4) suggest that this event occurs pre-Golgi apparatus, presumably within the RER.
IF analyses revealed that KUN E was distributed within the perinuclear region in a diffuse manner and in large densely staining foci and accumulated throughout the cytoplasm as small isolated foci (Fig. 1). When compared to locations of cell markers, the staining pattern of KUN E indicated accumulation largely within the RER and endosomal compartments, plus some coincidence with the IC marker. Interestingly, a minor overlap with the Golgi apparatus was observed (Fig. 1G to I) and persisted in the presence of the inhibitor MON, thought to arrest transport within this organelle (Fig. 3D to F). CIEM results suggested that E transversed the Golgi apparatus only when incorporated in a virus particle (Fig. 2C), suggesting that assembly occurs pre-Golgi apparatus. Virus particles were not frequently observed within the Golgi apparatus, implying that transport through the individual stacks occurs quite rapidly.
The use of metabolic inhibitors to study aspects of flavivirus assembly has not been investigated extensively. Sreenivasan et al. (51) added BFA at 1 h p.i. to investigate its effects on West Nile virus strain Sarafend replication in Vero cells. The results showed some reduction in protein synthesis and severe impairment of [3H] mannose incorporation in viral glycoproteins E and prM in the presence of 1 μg of BFA per ml, and these effects were associated with a reduction of 3 log units in the release of infectious extracellular virus at 20 h p.i. We performed similar experiments and found that the presence of 5 μg of BFA per ml early in infection severely hampered the metabolic incorporation of radiolabel into KUN structural proteins (Fig. 5), most likely by restricting membrane induction and thus KUN replication (30). Courageot et al. (8) incubated dengue 1 virus-infected cells in the presence of 30 μM DNJ and 300 μM castanospermine (both α-glucosidase inhibitors) from 19 to 25 h p.i. and found a 50 to 80% reduction in the titer of extracellular virus. In contrast, our similar experiments with KUN and 100 μM DNJ showed no effect on infectious virus release. However, our observed effect of NN-DNJ on KUN infectivity is in agreement with the results of Zitzmann et al. (62), who found a similar effect of NN-DNJ on the infectivity of bovine viral diarrhea virus (also a member of the Flaviviridae). Currently, the only explanation we have for the discrepancy in the lack of effect of DNJ on infectious KUN release is that the strain of KUN that we are currently using encodes an unglycosylated E protein. In fact, preliminary experiments using a mutated strain of KUN with glycosylated E (1) suggest this may be the case, as in these experiments DNJ reduced the release of infectious virus by 50% whereas NN-DNJ surprisingly had no effect on the infectious virus titer (unpublished results).
The results obtained with MON are intriguing. First, incubation of cells with MON is thought to arrest viral glycoprotein movement within the Golgi apparatus (20, 21, 42, 44), yet our RIP analysis after pulse-chase experiments (Fig. 4A) indicated that KUN structural proteins are still secreted under these conditions. Second, during treatment with MON, a large amount of prM associated with E and C was secreted into the medium (Fig. 4A), suggesting that movement of virus particles within the medial Golgi compartment was not arrested and that subsequent furin cleavage of prM was either not occurring or was very inefficient. This deficiency in cleavage is primarily responsible for the reduction in infectious virus titer by not allowing the conformational change in the E protein (of immature particles containing prM) that is required for uncoating and fusion with endosomal membranes after infection (2, 18, 53), rather than arrest of particles within the Golgi apparatus. Similar results were observed when acidotropic amines (such as NH4Cl, chloroquine, or methylamine) were added to the culture fluid of flavivirus-infected cells (45, 47). Based on these earlier results and our present observations, it would appear that the ionophore MON causes an increase in the pH of endosomal compartments, disabling the low-pH-dependent action of furin that normally occurs just prior to virus secretion (52). In addition, anchored or immature core protein (Ci) appeared to accumulate in lysates (Fig. 4A, lane 9) in lieu of normal end-product virion C and was not secreted. This accumulation of nonassembled Ci may have also enhanced the formation and release of noninfectious slowly sedimenting hemagglutinin particles, comprising predominantly E and prM (50), into the tissue culture fluid, and this factor would also contribute to the observed reduction in infectivity. Thus, in addition to the obvious inhibition of cleavage of prM, MON also appears in some unknown way to inhibit the cleavage of Ci that generates virion C.
The results obtained with NOZ indicated that microtubules play no role in the release of KUN from infected cells (Fig. 4 and 5), similar to the findings of Ng et al. (39), who found reductions of about only 10-fold in extracellular virus titers after treatment of KUN-infected cells with the cytoskeletal disrupting agents vinblastine sulfate, colchicine, and cytochalasin B.
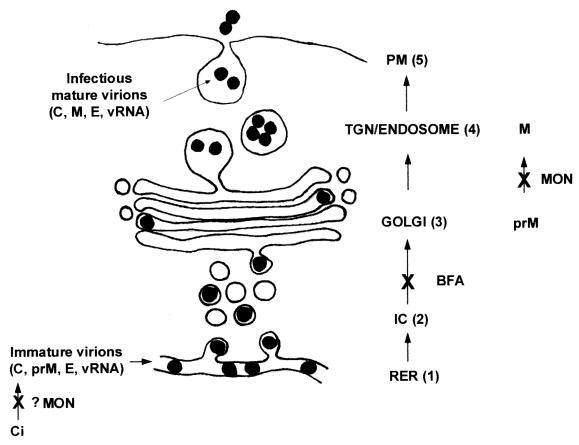
Previous localization data obtained with anticore antibodies (58) revealed a distribution in the cytoplasm similar to that obtained here with anti-E and anti-prM antibodies (Fig. 2). Thus, all three structural proteins appeared to be labeled on the periphery of the induced CM or PC structures in association with the RER, which is involved in their sequential translocation and posttranslational cleavage. We have suggested that the induced CM or PC structures are the sites of viral protease cleavage (59); thus, the posttranslational cleavage of C from prM by NS2B-NS3 (5, 27, 41, 61) may also occur at this location. In addition, as VP are the intracellular sites of KUN RNA synthesis (59) and are also closely associated with the CM or PC structures, it is not surprising that the structural proteins would also remain closely associated with these structures and package the newly synthesized genomic RNA. One could speculate that assembly thus occurs at or near the ER membranes continuous with the virus-induced membrane structures. The subsequent steps involve maturation and secretion of virions that exit the RER via individual transport vesicles en route to the Golgi apparatus (Fig. 6). Once these vesicles fuse with the Golgi apparatus, the virions appear to move through the individual stacks into the trans-Golgi region, where they appear to cluster and accumulate within large vesicles until exocytosis occurs. This process is schematically depicted in Fig. 7.
FIG. 7.
Proposed model of maturation of flavivirus KUN involves a single vesicular transport step from the RER to the Golgi apparatus and subsequent exocytosis. Although not observed, assembly of the flavivirus virion is presumed to occur within the lumen of the RER (step 1). Subsequently, transport from the RER involves the formation of individual transport vesicles that carry individual virions to the Golgi apparatus (step 2). After virions are transported through the Golgi apparatus (step 3), they accumulate within an endosomal compartment (step 4) to await exocytosis (step 5). Each proposed site of action of the inhibitors BFA and MON on this pathway is indicated. Abbreviations: TGN, trans-Golgi network; PM, plasma membrane; vRNA, virion RNA.
Questions arise as to how virions exit the RER via vesicles for transport to the Golgi apparatus and what signals allow interactions with the coatomer proteins (COP [34]) required for vesicle formation. Obviously, prM plays a role in this process, as the expression of Japanese encephalitis virus E alone led to the retention of the protein within the ER (24). It was additionally shown that the ectodomain of the E protein was responsible for the interaction with prM to allow assembly and secretion of subviral particles of tick-borne encephalitis and dengue viruses (3, 57). These results suggest either that a retention signal within the ectodomain of E is masked in the presence of prM or that prM is required for the correct folding of E. Generally, membrane proteins destined for secretion have a cytoplasmic tail with encoded motifs, such as DxE, that interact with coatomer components to facilitate vesicle formation (40). However, after virus assembly, this type of signal within either E or prM would necessarily interact with the nucleocapsid within the lumen of the ER. Martinez-Menarguez et al. (34) very elegantly showed that soluble secretory proteins were concentrated in noncoated tubular clusters associated with ER membranes containing COPI components, however, after further transport, enrichment of the soluble cargo was observed within COPII-bearing structures and the secretory proteins were then excluded from COPI-bearing structures. Unfortunately, the exact mechanism for this exclusion is still under investigation, but it would be interesting to investigate the distributions of COPI and COPII within flavivirus-infected cells. In addition, because KUN E is nonglycosylated, some as-yet-undefined peptide signal(s) within the protein may exist to target the virion to the trans-Golgi apparatus to allow furin cleavage of pr from prM Another aspect of viral exit is the observed budding of the E-glycosylated strain of West Nile virus (Sarafend) at the plasma membrane (38). Because West Nile virus strains and KUN share 93 to 98% amino acid identity (7, 25, 43), it is surprising that glycosylation of E in the Sarafend strain may induce a distinctly different maturation pathway. Budding of flavivirus nucleocapsids from intracellular membranes has been observed very infrequently and appears to be virus strain dependent and also cell type dependent (14, 15).
The results presented in this paper have highlighted the maturation pathway of the flavivirus KUN. Some limited evidence suggests that assembly occurs within the RER in close association with the KUN-induced CM or PC and VP structures. Molecular approaches, such as the use of the KUN replicon (23), with the opportunity to vary the addition of structural proteins, should enable further investigation of the interactions of structural proteins during the assembly process and aid in visualizing possible assembly sites within the RER.
ACKNOWLEDGMENTS
We thank R. Hall, S. Fuller, H.-P. Hauri, A. Hille, and M. Fukuda for generously providing antibodies. We also thank A. Khromykh for critical reading of the manuscript and for helpful discussions.
Funding for this work was provided by the National Health and Medical Research Council of Australia (grant 102481).
Footnotes
SASVRC publication 134.
REFERENCES
- 1.Adams S C, Broom A K, Sammels L M, Hartnett A C, Howard M J, Coelen R J, Mackenzie J S, Hall R A. Glycosylation and antigenic variation among Kunjin virus isolates. Virology. 1995;206:49–56. doi: 10.1016/s0042-6822(95)80018-2. [DOI] [PubMed] [Google Scholar]
- 2.Allison S L, Schalich J, Stiasny K, Mandl C W, Kunz C, Heinz F X. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J Virol. 1995;69:695–700. doi: 10.1128/jvi.69.2.695-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison S L, Stadler K, Mandl C W, Kunz C, Heinz F X. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate forms. J Virol. 1995;69:5816–5820. doi: 10.1128/jvi.69.9.5816-5820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison S L, Stiasny K, Stadler K, Mandl C W, Heinz F X. Mapping of functional elements in the stem-anchor region of tick-borne encephalitis virus envelope protein E. J Virol. 1999;73:5605–5612. doi: 10.1128/jvi.73.7.5605-5612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amberg S M, Rice C M. Mutagenesis of the NS2B-NS3-mediated cleavage site in the flavivirus capsid protein demonstrates a requirement for coordinated processing. J Virol. 1999;73:8083–8094. doi: 10.1128/jvi.73.10.8083-8094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barth O M. Ultrastructural aspects of the dengue virus (flavivirus) particle morphogenesis. J Submicrosc Cytol Pathol. 1999;31:407–412. [PubMed] [Google Scholar]
- 7.Coia G, Parker M D, Speight G, Byrne M E, Westaway E G. Nucleotide and complete amino acid sequences of Kunjin virus: definitive gene order and characteristics of the virus-specified proteins. J Gen Virol. 1988;69:1–21. doi: 10.1099/0022-1317-69-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Courageot M P, Frenkiel M P, Dos Santos C D, Deubel V, Despres P. Alpha-glucosidase inhibitors reduce dengue virus production by affecting the initial steps of virion morphogenesis in the endoplasmic reticulum. J Virol. 2000;74:564–572. doi: 10.1128/jvi.74.1.564-572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dascher C, Balch W E. Dominant inhibitory mutants of ARFI block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J Biol Chem. 1994;269:1437–1448. [PubMed] [Google Scholar]
- 10.Donaldson J G, Finazzi D, Klausner R D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda M, Viitala J, Matteson J, Carlsson S R. Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Comparison of their deduced amino acid sequences. J Biol Chem. 1988;263:18920–18928. [PubMed] [Google Scholar]
- 12.Guirakhoo F, Bolin R A, Roehrig J T. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology. 1992;191:921–931. doi: 10.1016/0042-6822(92)90267-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guirakhoo F, Heinz F X, Mandl C W, Holzmann H, Kunz C. Fusion activity of flaviviruses: comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J Gen Virol. 1991;72:1323–1329. doi: 10.1099/0022-1317-72-6-1323. [DOI] [PubMed] [Google Scholar]
- 14.Hase T, Summers P L, Eckels K H, Baze W B. An electron and immunoelectron microscopic study of dengue-2 virus infection of cultured mosquito cells: maturation events. Arch Virol. 1987;92:273–291. doi: 10.1007/BF01317484. [DOI] [PubMed] [Google Scholar]
- 15.Hase T, Summers P L, Eckels K H, Baze W B. Maturation process of Japanese encephalitis virus in cultured mosquito cells in vitro and mouse brain cells in vivo. Arch Virol. 1987;96:135–151. doi: 10.1007/BF01320956. [DOI] [PubMed] [Google Scholar]
- 16.Hase T, Summers P L, Eckels K H, Putnak J R. Morphogenesis of flaviviruses. Subcell Biochem. 1989;15:275–305. doi: 10.1007/978-1-4899-1675-4_9. [DOI] [PubMed] [Google Scholar]
- 17.Heinz F X, Auer G, Stiasny K, Holzmann H, Mandl C, Guirakhoo F, Kunz C. The interactions of the flavivirus envelope proteins: implications for virus entry and release. Arch Virol Suppl. 1994;9:339–348. doi: 10.1007/978-3-7091-9326-6_34. [DOI] [PubMed] [Google Scholar]
- 18.Heinz F X, Stiasny K, Puschner-Auer G, Holzmann H, Allison S L, Mandl C W, Kunz C. Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology. 1994;198:109–117. doi: 10.1006/viro.1994.1013. [DOI] [PubMed] [Google Scholar]
- 19.Helms J B, Rothman J E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- 20.Johnson D C, Schlesinger M J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980;103:407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- 21.Kaariainen L, Hashimoto K, Saraste J, Virtanen I, Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980;87:783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khromykh A A, Varnavski A N, Westaway E G. Encapsidation of the flavivirus Kunjin virus replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J Virol. 1998;72:5967–5977. doi: 10.1128/jvi.72.7.5967-5977.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khromykh A A, Westaway E G. Subgenomic replicons of the flavivirus Kunjin virus: construction and applications. J Virol. 1997;71:1497–1505. doi: 10.1128/jvi.71.2.1497-1505.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konishi E, Mason P W. Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. J Virol. 1993;67:1672–1675. doi: 10.1128/jvi.67.3.1672-1675.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanciotti R S, Roehrig J T, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe K E, Crabtree M B, Scherret J H, Hall R A, MacKenzie J S, Cropp C B, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage H M, Stone W, McNamara T, Gubler D J. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 26.Leary K, Blair C D. Sequential events in the morphogenesis of Japanese encephalitis virus. J Ultrastruct Res. 1980;72:123–129. doi: 10.1016/s0022-5320(80)90050-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee E, Stocks C E, Amberg S M, Rice C M, Lobigs M. Mutagenesis of the signal sequence of yellow fever virus prM protein: enhancement of signalase cleavage in vitro is lethal for virus production. J Virol. 2000;74:24–32. doi: 10.1128/jvi.74.1.24-32.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linstedt A D, Foguet M, Renz M, Seelig H P, Glick B S, Hauri H P. A C-terminally-anchored Golgi protein is inserted into the endoplasmic reticulum and then transported to the Golgi apparatus. Proc Natl Acad Sci USA. 1995;92:5102–5105. doi: 10.1073/pnas.92.11.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotti L V, Torrisi M R, Pascale M C, Bonatti S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the Golgi complex. J Cell Biol. 1992;118:43–50. doi: 10.1083/jcb.118.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackenzie J M, Jones M K, Westaway E G. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J Virol. 1999;73:9555–9567. doi: 10.1128/jvi.73.11.9555-9567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackenzie J M, Jones M K, Young P R. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology. 1996;220:232–240. doi: 10.1006/viro.1996.0307. [DOI] [PubMed] [Google Scholar]
- 32.Mackenzie J M, Jones M K, Young P R. Improved membrane preservation of flavivirus-infected cells with cryosectioning. J Virol Methods. 1996;56:67–75. doi: 10.1016/0166-0934(95)01916-2. [DOI] [PubMed] [Google Scholar]
- 33.Mackenzie J M, Khromykh A A, Jones M K, Westaway E G. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology. 1998;245:203–215. doi: 10.1006/viro.1998.9156. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Menarguez J A, Geuze H J, Slot J W, Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- 35.Maynell L A, Kirkegaard K, Klymkowsky M W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992;66:1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta A, Zitzmann N, Rudd P M, Block T M, Dwek R A. Alpha-glucosidase inhibitors as potential broad based anti-viral agents. FEBS Lett. 1998;430:17–22. doi: 10.1016/s0014-5793(98)00525-0. [DOI] [PubMed] [Google Scholar]
- 37.Murphy F A. Morphology and morphogenesis. In: Monath T P, editor. St. Louis encephalitis. Washington, D.C.: American Public Health Association Inc; 1980. pp. 65–103. [Google Scholar]
- 38.Ng M L, Howe J, Sreenivasan V, Mulders J J. Flavivirus West Nile (Sarafend) egress at the plasma membrane. Arch Virol. 1994;137:303–313. doi: 10.1007/BF01309477. [DOI] [PubMed] [Google Scholar]
- 39.Ng M L, Pedersen J S, Toh B H, Westaway E G. Immunofluorescent sites in Vero cells infected with the flavivirus Kunjin. Arch Virol. 1983;78:177–190. doi: 10.1007/BF01311313. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura N, Bannykh S, Slabough S, Matteson J, Altschuler Y, Hahn K, Balch W E. A di-acidic (DXE) code directs concentration of cargo during export from the endoplasmic reticulum. J Biol Chem. 1999;274:15937–15946. doi: 10.1074/jbc.274.22.15937. [DOI] [PubMed] [Google Scholar]
- 41.Nowak T, Farber P M, Wengler G. Analyses of the terminal sequences of West Nile virus structural proteins and of the in vitro translation of these proteins allow the proposal of a complete scheme of the proteolytic cleavages involved in their synthesis. Virology. 1989;169:365–376. doi: 10.1016/0042-6822(89)90162-1. [DOI] [PubMed] [Google Scholar]
- 42.Pesonen M, Kaariainen L. Incomplete complex oligosaccharides in semliki forest virus envelope proteins arrested within the cell in the presence of monensin. J Mol Biol. 1982;158:213–230. doi: 10.1016/0022-2836(82)90430-2. [DOI] [PubMed] [Google Scholar]
- 43.Poidinger M, Hall R A, Mackenzie J S. Molecular characterization of the Japanese encephalitis serocomplex of the flavivirus genus. Virology. 1996;218:417–421. doi: 10.1006/viro.1996.0213. [DOI] [PubMed] [Google Scholar]
- 44.Qiu Z, Tufaro F, Gillam S. Brefeldin A and monensin arrest cell surface expression of membrane glycoproteins and release of rubella virus. J Gen Virol. 1995;76:855–863. doi: 10.1099/0022-1317-76-4-855. [DOI] [PubMed] [Google Scholar]
- 45.Randolph V B, Winkler G, Stollar V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology. 1990;174:450–458. doi: 10.1016/0042-6822(90)90099-d. [DOI] [PubMed] [Google Scholar]
- 46.Rice C M. Flaviviridae: the viruses and their replication. In: Fields B N, Knipe D N, Howley P M, Chanock R M, Melnick J L, Monath T P, editors. Virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 931–959. [Google Scholar]
- 47.Schalich J, Allison S L, Stiasny K, Mandl C W, Kunz C, Heinz F X. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J Virol. 1996;70:4549–4557. doi: 10.1128/jvi.70.7.4549-4557.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schweizer A, Fransen J A, Bachi T, Ginsel L, Hauri H P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sciaky N, Presley J, Smith C, Zaal K J, Cole N, Moreira J E, Terasaki M, Siggia E, Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shapiro D, Brandt W E, Cardiff R D, Russell P K. The proteins of Japanese encephalitis virus. Virology. 1971;44:108–124. doi: 10.1016/0042-6822(71)90158-9. [DOI] [PubMed] [Google Scholar]
- 51.Sreenivasan V, Ng K L, Ng M L. Brefeldin A affects West Nile virus replication in Vero cells but not C6/36 cells. J Virol Methods. 1993;45:1–17. doi: 10.1016/0166-0934(93)90135-e. [DOI] [PubMed] [Google Scholar]
- 52.Stadler K, Allison S L, Schalich J, Heinz F X. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stiasny K, Allison S L, Marchler-Bauer A, Kunz C, Heinz F X. Structural requirements for low-pH-induced rearrangements in the envelope glycoprotein of tick-borne encephalitis virus. J Virol. 1996;70:8142–8147. doi: 10.1128/jvi.70.11.8142-8147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stinchcombe J C, Nomoto H, Cutler D F, Hopkins C R. Anterograde and retrograde traffic between the rough endoplasmic reticulum and the Golgi complex. J Cell Biol. 1995;131:1387–1401. doi: 10.1083/jcb.131.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaux D, Tooze J, Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990;345:495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, He R, Anderson R. PrM- and cell-binding domains of the dengue virus E protein. J Virol. 1999;73:2547–2551. doi: 10.1128/jvi.73.3.2547-2551.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wengler G, Wengler G. Cell-associated West Nile flavivirus is covered with E+pre-M protein heterodimers which are destroyed and reorganized by proteolytic cleavage during virus release. J Virol. 1989;63:2521–2526. doi: 10.1128/jvi.63.6.2521-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westaway E G, Khromykh A A, Kenney M T, Mackenzie J M, Jones M K. Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virology. 1997;234:31–41. doi: 10.1006/viro.1997.8629. [DOI] [PubMed] [Google Scholar]
- 59.Westaway E G, Mackenzie J M, Kenney M T, Jones M K, Khromykh A A. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA and of NS2B with NS3 in virus-induced membrane structures. J Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright P J. Envelope protein of the flavivirus Kunjin is apparently not glycosylated. J Gen Virol. 1982;59:29–38. doi: 10.1099/0022-1317-59-1-29. [DOI] [PubMed] [Google Scholar]
- 61.Yamshchikov V F, Compans R W. Processing of the intracellular form of the West Nile virus capsid protein by the viral NS2B-NS3 protease: an in vitro study. J Virol. 1994;68:5765–5771. doi: 10.1128/jvi.68.9.5765-5771.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zitzmann N, Mehta A S, Carrouee S, Butters T D, Platt F M, McCauley J, Blumberg B S, Dwek R A, Block T M. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: implications for the development of broad spectrum anti-hepatitis virus agents. Proc Natl Acad Sci USA. 1999;96:11878–11882. doi: 10.1073/pnas.96.21.11878. [DOI] [PMC free article] [PubMed] [Google Scholar]