Abstract
Background
There are scant data relating to prognostic biomarkers for chronic kidney disease (CKD) complicating type 1 diabetes. The aim of this study was to assess the performance of the plasma protein biomarker-based PromarkerD test developed and validated for predicting renal decline in type 2 diabetes in the context of type 1 diabetes.
Methods
The baseline PromarkerD test score was determined in 91 community-based individuals (mean age 46.2 years, 56.5% males) with confirmed type 1 diabetes recruited to the longitudinal observational Fremantle Diabetes Study Phase II. The performance of the PromarkerD test in predicting the risk of incident CKD (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2 in people without CKD at baseline) or an eGFR decline of ≥ 30% over the next four years was determined. The score can range from 0 to 100%, and is categorized as representing low (< 10%), moderate (10% to < 20%) or high (≥ 20%) risk.
Results
The area under the receiver operating characteristic curve was 0.93 (95% confidence interval 0.87–0.99) for the composite renal endpoint, indicating strong predictive accuracy. The positive and negative predictive values at moderate (10% to < 20%) and high (≥ 20%) risk PromarkerD cut-offs were 46.7–50.0% and ≥ 92.0%, respectively.
Conclusions
These preliminary data suggest that PromarkerD is at least as good a prognostic test for renal decline in type 1 as type 2 diabetes.
Keywords: Type 1 diabetes, Chronic kidney disease, Protein biomarkers, Prediction
Introduction
Around one third of people with diabetes will develop chronic kidney disease (CKD) [1] and diabetes has emerged as the largest single cause of end-stage renal disease in developed and developing countries [2]. CKD can remain asymptomatic for years before diagnosis, but it is strongly associated with cardiovascular disease (CVD) and premature death [3]. Population-based studies have shown that the risk of CKD appears greater in type 1 versus type 2 diabetes across all age strata [4, 5], and there is evidence of relative underutilization of renoprotective therapies in type 1 diabetes [4].
These observations argue for validated tests that reliably identify the risk of progressive renal disease at an early stage in type 1 diabetes and pre-empt preventive management strategies. Unfortunately, most studies of candidate prognostic biomarkers have been conducted in type 2 diabetes [6] and few biomarker tests are available in clinical practice [6, 7]. We have developed a validated plasma protein biomarker-based prognostic test (PromarkerD®, Proteomics International, Perth, Australia) which has excellent performance characteristics for predicting incident CKD and rapid renal decline complicating type 2 diabetes including independent validation in the in the Canagliflozin Cardiovascular Assessment Study (CANVAS) cohort [8–10]. The aim of the present study was to determine whether PromarkerD has similar clinical utility in type 1 diabetes.
Methods
The PromarkerD test was developed using samples and data from individuals with type 2 diabetes participating Phases I and II of the representative, community-based, longitudinal, observational Fremantle Diabetes Study (FDS) [11]. There were 1,732 participants in Phase II (FDS2) who were recruited from an urban Australian population base of 150,000 between 2008 and 2011 [11]. Of these, 139 (8.0% of the total FDS2 sample) had type 1 diabetes based on clinical criteria and laboratory confirmation including islet autoantibody status and genotyping for monogenic diabetes [12]. All FDS2 participants were invited to detailed face-to-face assessments conducted biennially which comprised comprehensive questionnaires, a physical examination, and fasting biochemical tests performed in a single nationally accredited laboratory [11, 13].
The PromarkerD test was performed using baseline plasma samples. The test algorithm combines the plasma concentrations of three protein biomarkers, apolipoprotein A-IV (ApoA4), CD5 antigen-like (CD5L) and insulin-like growth factor-binding protein 3 (IGFBP3) which, together with the concomitant age, serum HDL-cholesterol concentration and estimated glomerular filtration rate (eGFR) [14], generate an estimate of the risk of incident CKD (eGFR < 60 mL/min/1.73m2 in people without CKD at baseline) or an eGFR decline of ≥ 30% over the next four years [8–10]. An enzyme-linked immunosorbent assay was used to measure baseline concentrations of the three biomarkers, as previously described [15]. PromarkerD scores are predicted probabilities of renal outcomes ranging from 0 to 100% and can be categorized as low (< 10%), moderate (10% to < 20%) or high (≥ 20%) risk as determined by pre-specified cut-offs for optimal sensitivity and specificity [8]. Performance was assessed using the area under the receiver operating characteristic curve (ROC AUC). Additionally, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated.
Results
Of the 139 FDS2 participants with type 1 diabetes, 92 (66%) had renal function assessed at the four-year review. Nine of these eligible participants (9.8%) had incident CKD or an eGFR decline ≥ 30%. The baseline clinical and demographic characteristics of the participants categorized by renal outcome are summarized in Table 1. The baseline eGFR and urinary albumin:creatinine ratio differed significantly between the two groups, and the use of antihypertensive medications by those with renal outcomes was double in those without. In bivariable analysis, plasma concentrations of the biomarkers ApoA4 and CD5L were significantly elevated in the people with prespecified renal outcomes while those of IGFBP3 showed no significant difference. PromarkerD scores were substantially higher in those with incident renal outcomes.
Table 1.
Bivariable analysis showing differences between those with no incident CKD or eGFR decline ≥ 30% over 4 years and those with incident CKD or eGFR decline ≥ 30% over four years
| Variables at baseline | No incident CKD or eGFR decline ≥ 30% over 4 years | Incident CKD or eGFR decline ≥ 30% over 4 years | P-value |
|---|---|---|---|
| Number (%) | 83 (90.2) | 9 (9.8) | |
| Age at FDS entry (years) | 45.3 ± 16.4 | 54.3 ± 13.5 | 0.117 |
| Sex (% male) | 59.0 | 33.0 | 0.170 |
| Age at diabetes diagnosis (years) | 23.9 ± 12.3 | 24.7 ± 12.3 | 0.860 |
| Diabetes duration (years) | 20.0 [9.9–31.3] | 31.0 [22.5–37.0] | 0.056 |
| Body mass index (kg/m2) | 25.6 ± 4.3 | 31.0 ± 7.6 | 0.066 |
| Systolic blood pressure (mmHg) | 137 ± 23 | 147 ± 17 | 0.202 |
| Diastolic blood pressure (mmHg) | 78 ± 12 | 76 ± 12 | 0.704 |
| HbA1c (%) | 7.8 [7.2–8.8] | 7.8 [6.9–8.2] | 0.737 |
| HbA1c (mmol/mol) | 62 [55–73] | 62 [52–66] | 0.737 |
| eGFR (mL/min/1.73m2) | 97.8 ± 21.4 | 54.8 ± 20.9 | < 0.001 |
| eGFR (%) | < 0.001 | ||
| ≥ 90 mL/min/1.73m2 | 73.5 | 0.0 | |
| 60–89 mL/min/1.73m2 | 18.1 | 55.6 | |
| 45–59 mL/min/1.73m2 | 3.6 | 11.1 | |
| < 45 mL/min/1.73m2 | 4.8 | 33.3 | |
| Total serum cholesterol (mmol/L) | 4.6 ± 1.0 | 5.0 ± 0.8 | 0.324 |
| Serum triglycerides (mmol/L) | 1.0 (0.6–1.6) | 1.0 (0.7–1.7) | 0.629 |
| Serum HDL-cholesterol (mmol/L) | 1.59 ± 0.49 | 1.78 ± 0.47 | 0.261 |
| Urinary albumin:creatinine ratio (mg/mmol) | 1.6 (0.7–3.7) | 14.7 (1.7–126.3) | 0.014 |
| Antihypertensive medication (%) | 32.5 | 77.8 | 0.012 |
| Renin-angiotensin system blocking drugs (%) | 31.3 | 66.7 | 0.060 |
| Lipid-modifying medication (%) | 28.9 | 44.4 | 0.447 |
| Proteomic biomarkers (μg/mL) | |||
| APOA4 | 25.9 (13.2–50.8) | 46.6 (26.9–80.9) | 0.007 |
| CD5L | 3.1 (1.3–7.3) | 7.3 (4.6–11.7) | 0.004 |
| IGFBP3 | 2.1 (1.5–3.0) | 2.6 (1.3–4.9) | 0.155 |
| PromarkerD Scores (%) | 0.16 (0.01–2.96) | 12.18 (6.02–24.65) | < 0.001 |
Data are presented as percentages, mean ± standard deviation (SD), geometric mean (SD range), or median [interquartile range]. Two-way comparisons were performed using Fisher’s exact test, Student’s t-test or Mann–Whitney U-test as appropriate
The performance characteristics of PromarkerD are summarized in Table 2. At the moderate risk cut-off, the sum of sensitivity plus specificity was 168.2%, indicating good clinical utility [16]. At the high-risk cut-off, this figure was lower at 119.8% but there were only small number of individuals in this category. At both cut-offs, PPV was moderate (46.7–50.0%) but NPV was very high at ≥ 92.0%. The ROC curve is shown Fig. 1. The ROC AUC was 0.93 (95% confidence interval (CI) 0.87–0.99), consistent with excellent predictive accuracy.
Table 2.
Predicted versus actual incident CKD or eGFR decline ≥ 30% over four years in individuals with type 1 diabetes and performance metrics at the two PromarkerD risk cut-offs
| No adverse renal outcomes (predicted) | Incident CKD or ≥ 30% 4-year eGFR decline (predicted) | Total (predicted) |
Performance metrics | ||||
|---|---|---|---|---|---|---|---|
| Sensitivity (%) |
Specificity (%) | PPV (%) | NPV (%) | ||||
| Moderate risk cut-off (10%) | |||||||
| No adverse renal outcomes (actual) | 75 | 8 | 83 | 77.8 | 90.4 | 46.7 | 97.4 |
| Incident CKD/ ≥ 30% eGFR decline (actual) | 2 | 7 | 9 | ||||
| Total (actual) | 77 | 15 | 92 | ||||
| High risk cut-off (20%) | |||||||
| No adverse renal outcomes (actual) | 81 | 2 | 83 | 22.2 | 97.6 | 50.0 | 92.0 |
| Incident CKD/ ≥ 30% eGFR decline (actual) | 7 | 2 | 9 | ||||
| Total (actual) | 88 | 4 | 92 | ||||
Fig. 1.
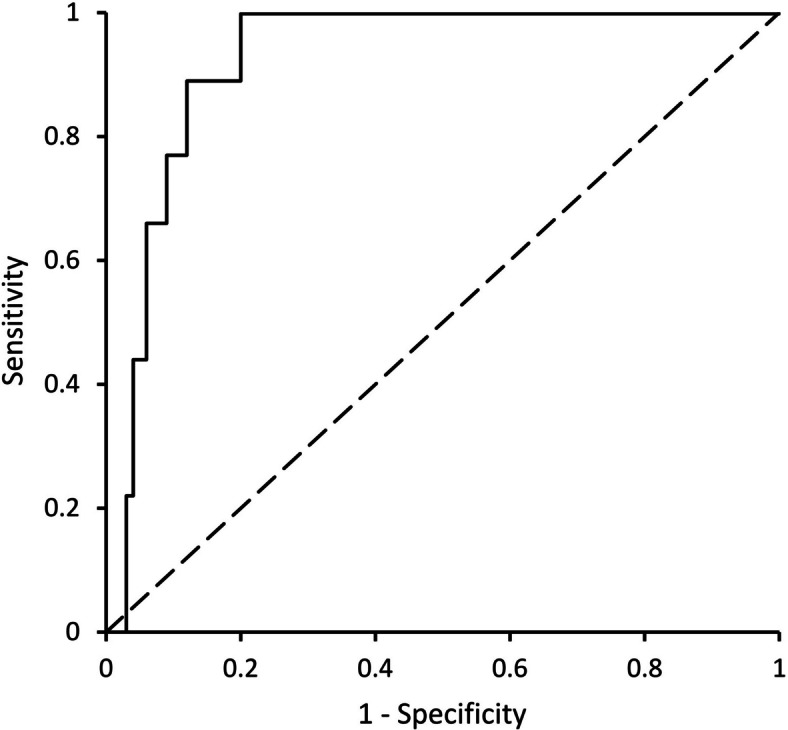
Receiver operating characteristic curve (ROC) for PromarkerD test score as a continuous variable in predicting incident chronic kidney disease or a decline in the estimated glomerular filtration rate of ≥ 30% over four years in individuals with type 1 diabetes
Discussion
The present data provide evidence that PromarkerD has strong clinical utility as a prognostic test of renal decline in type 1 diabetes. The very high ROC AUC of 0.93, and lower 95% CI of 0.87, compare favorably with values of ≤ 0.78 for several panels of protein biomarkers assessed using larger scale samples and data from the Scottish Diabetes Research Network Type 1 Bioresource and Finnish Diabetic Nephropathy Study [17]. The modest PPV at both risk cut-offs needs to be interpreted against the relatively small number of false positive results and the implication that intensive renal risk reduction management, if not needed for CKD, may have other benefits including for CVD risk [18]. The very high NPVs at moderate and high risk cut-offs mean that few people with type 1 diabetes will be burdened by unnecessary management strategies and misplaced concern regarding the future development of CKD.
Although CKD complicating type 1 and type 2 diabetes shares common pathophysiologic determinants, people with type 1 diabetes are generally younger and healthier at diagnosis and carry fewer co-morbidities than those with type 2 diabetes. Consequently, CKD in type 1 diabetes may be less affected by non-glycemic contributing factors including ageing, vascular disease, insulin resistance and obesity [19]. The higher ROC AUC in the present study than in studies of PromarkerD in participants with type 2 diabetes in the FDS2 (0.93 versus ≤ 0.88 [8, 9]), likely reflect this situation, with the PromarkerD biomarker panel less influenced by confounding variables in the present cohort. In addition, the predictive performance of PromarkerD is reassuringly greater than that in a variety of studies in type 1 diabetes, some with smaller sample sizes than the present FDS2 cohort, utilizing available clinical and laboratory data without biomarker concentrations to predict renal decline [20].
The present sample size was constrained by the recruitment strategy for FDS2 and was consequently small since type 1 diabetes constitutes < 10% of all diagnosed diabetes in Australia [12]. We had relatively few pediatric participants but would likely have captured most people in the catchment area with type 1 diabetes given the prominent peaks in incidence in adolescence and middle age [21]. The low numbers precluded analysis of whether PromarkerD had the same performance in sub-groups such as those defined by race or ethnicity. There is a clear need for validation of the present findings in independent cohorts of people with type 1 diabetes, ideally community-based, with sufficient baseline data (demographic and laboratory variables including age, serum HDL-cholesterol and eGFR as well as PromarkerD protein biomarker assays) and subsequent follow-up renal function measurements (over at least four years) to allow the same assessment of PromarkerD prognostic performance. Larger samples would be preferable, but there is the potential to combine the data from other small-scale studies with those of the present study to increase statistical power and to facilitate sub-group analyses. The strengths of the present study include its representative, community based sample [11] and the availability of detailed phenotypic data including rigorous diagnostic criteria for diabetes type [12].
Conclusion
Although further validation studies are needed, the present data suggest that PromarkerD has strong clinical utility in identifying people with type 1 diabetes at risk of future adverse renal outcomes.
Acknowledgements
We thank the FDS2 participants and staff for their involvement and PathWest Laboratory Medicine at Fremantle Hospital for laboratory tests.
Authors’ contributions
TMED, the principal investigator of FDS2, designed the study and produced the initial version of the manuscript. WAD, a co-investigator of FDS2, co-supervised data analysis and reviewed/edited the manuscript. DB, JKCL and TSCL contributed to method development, generated test results and reviewed/edited the manuscript. KEP co-supervised data analysis and reviewed/edited the manuscript. RJL supervised assay development and generation of tests results, and reviewed/edited the manuscript.
Funding
The FDS2 was funded by the National Health and Medical Research Council of Australia (project grants 513781 and 1042231). The present analyses were supported by Australian Centre for Accelerating Diabetes Innovations. TMED was supported by a Medical Research Future Fund Practitioner Fellowship.
Availability of data and materials
Data are available from the authors upon reasonable request.
Declarations
Ethics approval and consent to participate
The FDS2 protocol was approved by the Human Research Ethics Committee of the Southern Metropolitan Area Health Service (reference 07/397) and all participants provided written informed consent to study procedures.
Consent for publication
Not applicable.
Competing interests
Proteomics International and the University of Western Australia are beneficiaries of patent PCT/AU2011/001212 which relates to the biomarkers described in this manuscript. TMED, WAD, KEP and RJL are named as inventors of this patent. There are no other potential conflicts of interest relevant to this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Esposito P, Picciotto D, Cappadona F, Costigliolo F, Russo E, Maccio L, et al. Multifaceted relationship between diabetes and kidney diseases: Beyond diabetes. World J Diabetes. 2023;14(10):1450–62. 10.4239/wjd.v14.i10.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu H, Liu S, Bastacky SI, Wang X, Tian XJ, Zhou D. Diabetic kidney diseases revisited: A new perspective for a new era. Mol Metab. 2019;30:250–63. 10.1016/j.molmet.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. CJASN. 2017;12(12):2032–45. 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kristofi R, Bodegard J, Norhammar A, Thuresson M, Nathanson D, Nystrom T, et al. Cardiovascular and renal disease burden in type 1 compared with type 2 diabetes: a two-country nationwide observational study. Diabetes Care. 2021;44(5):1211–8. 10.2337/dc20-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YB, Han K, Kim B, Jun JE, Lee SE, Ahn J, et al. Risk of end-stage renal disease from chronic kidney disease defined by decreased glomerular filtration rate in type 1 diabetes: A comparison with type 2 diabetes and the effect of metabolic syndrome. Diabetes Metab Res Rev. 2019;35(8): e3197. 10.1002/dmrr.3197. [DOI] [PubMed] [Google Scholar]
- 6.Colhoun HM, Marcovecchio ML. Biomarkers of diabetic kidney disease. Diabetologia. 2018;61(5):996–1011. 10.1007/s00125-018-4567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rico-Fontalvo J, Aroca-Martinez G, Daza-Arnedo R, Cabrales J, Rodriguez-Yanez T, Cardona-Blanco M, et al. Novel biomarkers of diabetic kidney disease. Biomolecules. 2023;13(4):633. 10.3390/biom13040633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters KE, Davis WA, Ito J, Bringans SD, Lipscombe RJ, Davis TME. Validation of a protein biomarker test for predicting renal decline in type 2 diabetes: The Fremantle Diabetes Study Phase II. J Diabetes Complications. 2019;33(12): 107406. 10.1016/j.jdiacomp.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Peters KE, Davis WA, Ito J, Winfield K, Stoll T, Bringans SD, et al. Identification of novel circulating biomarkers predicting rapid decline in renal function in type 2 diabetes: The Fremantle Diabetes Study Phase II. Diabetes Care. 2017;40(11):1548–55. 10.2337/dc17-0911. [DOI] [PubMed] [Google Scholar]
- 10.Peters KE, Xu J, Bringans SD, Davis WA, Davis TME, Hansen MK, et al. PromarkerD predicts renal function decline in type 2 diabetes in the Canagliflozin Cardiovascular Assessment Study (CANVAS). J Clin Med. 2020;9(10):3212. 10.3390/jcm9103212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis TM, Bruce DG, Davis WA. Cohort profile: the Fremantle Diabetes Study. Int J Epidemiol. 2013;42(2):412–21. 10.1093/ije/dys065. [DOI] [PubMed] [Google Scholar]
- 12.Davis WA, Peters KE, Makepeace A, Griffiths S, Bundell C, Grant SFA, et al. Prevalence of diabetes in Australia: insights from the Fremantle Diabetes Study Phase II. Intern Med J. 2018;48(7):803–9. 10.1111/imj.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis TM, Zimmet P, Davis WA, Bruce DG, Fida S, Mackay IR. Autoantibodies to glutamic acid decarboxylase in diabetic patients from a multi-ethnic Australian community: the Fremantle Diabetes Study. Diabet Med. 2000;17(9):667–74. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bringans S, Peters K, Casey T, Ito J, Lipscombe R. The new and the old: platform cross-validation of immunoaffinity mass spectrometry versus ELISA for PromarkerD, a predictive test for diabetic kidney disease. Proteomes. 2020;8(4):31. 10.3390/proteomes8040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Power M, Fell G, Wright M. Principles for high-quality, high-value testing. Evid Based Med. 2013;18(1):5–10. 10.1136/eb-2012-100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo M, Valo E, McGurnaghan SJ, Sandholm N, Blackbourn LAK, Dalton RN, et al. Biomarker panels associated with progression of renal disease in type 1 diabetes. Diabetologia. 2019;62(9):1616–27. 10.1007/s00125-019-4915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovre D, Shah S, Sihota A, Fonseca VA. Managing diabetes and cardiovascular risk in chronic kidney disease patients. Endocrinol Metab Clin North Am. 2018;47(1):237–57. 10.1016/j.ecl.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, et al. Diabetic kidney disease. Nat Rev Dis Primers. 2015;1:15018. 10.1038/nrdp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radcliffe NJ, Seah JM, Clarke M, MacIsaac RJ, Jerums G, Ekinci EI. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig. 2017;8(1):6–18. 10.1111/jdi.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderniet JA, Jenkins AJ, Donaghue KC. Epidemiology of type 1 diabetes. Curr Cardiol Rep. 2022;24(10):1455–65. 10.1007/s11886-022-01762-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the authors upon reasonable request.


