Abstract
Dravet Syndrome (DS) is a pediatric-onset epilepsy with an elevated risk of Sudden Unexpected Death in Epilepsy (SUDEP). Most individuals with DS possess mutations in the voltage-gated sodium channel gene Scn1a, expressed in both the brain and heart. Previously, mutations in Scn1a have been linked to arrhythmia. We used a Scn1a−/+ DS mouse model to investigate changes to cardiac mitochondrial function that may underlie arrhythmias and SUDEP. We detected significant alterations in mitochondrial bioenergetics that were sex-specific. Mitochondria from male Scn1a−/+ hearts had deficits in maximal (p = 0.02) and Complex II-linked respiration (p = 0.03). Male Scn1a−/+ mice were also more susceptible to cardiac arrhythmias under increased workload. When isolated cardiomyocytes were subjected to diamide, cardiomyocytes from male Scn1a−/+ hearts were less resistant to thiol oxidation. They had decreased survivability compared to Scn1a+/+ (p = 0.02) despite no whole-heart differences. Lastly, there were no changes in mitochondrial ROS production between DS and wild-type mitochondria at basal conditions, but Scn1a−/+ mitochondria accumulated more ROS during hypoxia/reperfusion. This study determines novel sex-linked differences in mitochondrial and antioxidant function in Scn1a-linked DS. Importantly, we found that male Scn1a−/+ mice are more susceptible to cardiac arrhythmias than female Scn1a−/+ mice. When developing new therapeutics to address SUDEP risk in DS, sex should be considered.
Keywords: Dravet, SUDEP, Hypoxia, Glutathione, Arrhythmia, Sex differences
1. Introduction
Dravet syndrome (DS) is a severe pediatric-onset epileptic encephalopathy. Characteristics of DS include severe, pharmacoresistent seizures, alongside neuropsychiatric comorbidities, such as delayed psychomotor and cognitive development [[1], [2], [3]]. Individuals with DS are at especially high risk of premature mortality (≤17 %) due to Sudden Unexpected Death in Epilepsy (SUDEP), one of the most common causes of death in persons with epilepsy [[1], [2], [3]]. While the exact mechanisms underlying SUDEP remain to be elucidated, cardiac, pulmonary, and neurological factors have all been implicated in its pathogenesis. Common contributing factors to SUDEP are believed to include cardiac ventricular arrhythmias, pulmonary apnea or edema, autonomic dysfunction, and postictal suppression of cerebral and brainstem structures [[4], [5], [6], [7], [8], [9], [10], [11]]. DS is primarily caused by loss of function mutations in genes encoding subunits of voltage-gated sodium channels (VGSCs). Approximately 85 % of individuals with DS possess heterozygous loss-of-function mutations in the gene SCN1A, which encodes an α subunit of the VSGC type-1 (NaV1.1) [12]. The α subunit contains the voltage sensor and the ion-conducting pore of the VGSC channel. The SCN1A mutations seen in DS result in haploinsufficiency and consequentially, the loss of sufficient NaV1.1 function [[13], [14], [15], [16]].
Attenuation of NaV1.1 current may be particularly detrimental to cardiac electrophysiology, as NaV1.1 is a fundamental membrane protein in cardiac tissues, involved in the upstroke and propagation of the cardiac action potential [14,16]. Previous research in the Scn1a−/+ mouse model of DS shows Scn1a−/+ mice are vulnerable to developing cardiac arrhythmias. In vivo electrocardiogram recordings demonstrated that compared to age-matched wild-type animals, Scn1a−/+ mice possess a long QT phenotype and experience more episodes of ventricular arrhythmias, including potentially fatal dysrhythmias such as ventricular fibrillation [17]. In some cases, these extreme disturbances to cardiac rhythm were observed immediately preceding death. In addition to the whole heart, altered electrophysiological properties have been recorded at the cardiomyocyte level in Scn1a−/+ mouse models. Ventricular cardiomyocytes from Scn1a−/+ mice are hyperexcitable, possess elevated levels of arrhythmogenic early afterdepolarizations, and have increased transient and persistent Na+ current density (INa) [[17], [18], [19]]. Likewise, some of these electrical disturbances have also been observed in human patients with DS. Our previous work with induced pluripotent stem cell-derived cardiac myocytes from individuals with SCN1A-linked DS showed these cells possessed abnormal contractility and increases in transient current (INa) [18]. Furthermore, we have demonstrated that an imbalance in sodium currents may underlie arrhythmias induced under increased workload in a model of SCN8A epilepsy [20]. However, the exact molecular mechanisms underlying the vulnerability to arrhythmias in SCN1A-linked DS have yet to be determined.
One cellular component linked to long-term homeostasis in cardiomyocytes is mitochondria (for review see [21]) and mitochondrial diseases have been linked to cardiac arrhythmia [[22], [23], [24]]. Due to the heart's limited anaerobic capacity, it relies critically upon mitochondria to synthesize ATP. It has been estimated that under physiological conditions, at least 95 % of ATP generated in the heart is derived from the mitochondrial [25]. The vast majority is used to fuel the activity of ATP-dependent enzymes involved in cardiac contraction, particularly the sarcoplasmic reticulum Ca2+ ATPase [25]. Mitochondria also serve as cellular ion buffers, due to the presence of Na+ and Ca2+ transporters. For instance, Ca2+ has been implicated in matching ATP supply with demand in the heart [26]. As a result, in DS, increases in INa may negatively affect mitochondrial ion homeostasis as changes to Na+/Ca2+ exchange have been shown to occur. Targeting mitochondrial calcium uptake with teriflunomide has also been shown as potentially protective in the brain of DS [27], however in the heart it may have negative effects on cardiac outcomes [28]. Finally, mitochondria contribute significantly to ROS production within cells. Our previous work in the heart suggests that mitochondrial-derived ROS plays a role in pathological conditions [29,30] and imbalances between ROS production and scavenging negatively affect cardiac energetics and can lead to arrhythmia. Furthermore, our previous work demonstrates that ROS production is increased in Scn1b-linked DS [31].
The goal of this study was to determine if altered cardiac mitochondrial energetics and ROS production exist in the Scn1a−/+ mouse model of DS, predisposing them to cardiac arrhythmias and SUDEP events. Specifically, we examined whether Scn1a+/− mice exhibit differences in mitochondrial respiratory pathways, imbalances in ROS production and scavenging, and overall are more susceptible to cardiac arrhythmia development. Our results indicate male Scn1a−/+ mice possess unique deficits in mitochondrial bioenergetics and ROS buffering, with male Scn1a−/+ mice predominantly affected. These observations could render males with Scn1a-linked DS uniquely vulnerable to adverse cardiac events or SUDEP.
2. Materials and methods
2.1. Mice
All animal procedures were approved by the Institutional Animal Care and Usage Committee of East Tennessee State University and in conformity with the NIH Guide for the Care and Use of Laboratory Animals. Animals underwent euthanasia via CO2. 129-Scn1atm1Kea mice (Jackson Laboratory #037107-JAX) were maintained by breeding 129-Scn1a−/+ mice to 129S6/SvEvTac mice (Taconic). Experimental mice were generated as F1 hybrids by breeding 129-Scn1a−/+ mice to C57/Bl6J mice.
2.2. Mitochondrial isolation, respiration, and reactive oxygen species detection
To assess respiratory chain activity, mitochondria were isolated from P20 – P25 ventricular samples Scn1a−/+ and Scn1a+/+, and mitochondrial oxygen and H2O2 flux were concurrently measured using an O2k-FluoRespirometer (Oroboros) [31]. Mitochondrial protein content was assayed with a Bradford assay and equal amounts loaded per experiment. Different electron transport pathway states were assessed utilizing protocols designed to measure Complex I- and Complex II-linked activity using subsequent infusions of substrates as listed per figure. Respiration rate was standardized to background oxygen consumption via antimycin A and normalized to mg mitochondrial protein added. Furthermore, a series of experiments were conducted to test the effect of hypoxia/reperfusion on isolated mitochondria by energizing the mitochondria and allowing oxygen in the chamber to deplete. Reoxygenation occurred for 1 min and ROS production was calculated during the reoxygenation phase.
2.3. Isolated heart perfusion
Langendorff heart preparations were made from hearts isolated from P20 – P25 Scn1a−/+ and Scn1a+/+ mice, similarly to as previously described [20,32]. After a 15-min baseline recording, the perfusate was switched to a buffer containing 80 μM diamide for 30 min. Three blinded reviewers analyzed ECG traces for arrhythmia susceptibility and severity using previously established arrhythmia scoring systems [29].
2.4. Myocyte isolation and imaging
P20 – P25 ventricular myocytes were dissociated as previously described [32] and incubated with 500 nM 5-(6)-chloromethyl-2,7-dichlorodihydrofluorescein diacetate (CM-DCF; Invitrogen) [33] before being loaded on a coverslip. Images were acquired on an inverted microscope in 30-s intervals. After 10 min of stable baseline, 80 μM of diamide was added to the recording chamber and images were collected until cell death.
2.5. ECG recordings
Surface ECG recordings were performed on lightly anesthetized (1.0 % Isoflurane) mice at P25 using limb leads. At P30-P31 ECG was recorded in unrestrained conscious mice by placing them on the ECGenie Clinic (MouseSpecifics). For both recordings, a stable baseline recording (of at least 5 min) was obtained before injection of 2 mg/kg (i.p) norepinephrine (NE). 10 min after NE injection, 120 mg/kg caffeine (Caff) was injected (i.p.) and recorded for an additional 10 min. Analysis was performed in LabChart (AD Instruments) by three blinded reviewers.
2.6. Gene expression via qPCR
RT-qPCR was performed on left ventricular samples from P25 mice using TaqMan gene expression assays (ThermoFisher Scientific). Sod1 (Mm01344232_g1), Sod2 (Mm01313000_m1), Gpx (Mm00656761_g1), and Gsr (Mm00439154_m1), with Tbp (Mm01277042_m1) as a housekeeping gene.
2.7. Statistical analysis
All data are presented as mean ± SEM. A two-way ANOVA (sex x genotype) was first used to determine if sex differences existed. Comparison of respiration and H2O2 production was performed with a two-way ANOVA (genotype x substrate) with Fisher's LSD post-hoc. A student's t-test was used for all comparisons between the two groups. Between groups comparison for incidence of whole heart ventricular tachycardia/ventricular fibrillation (ex-vivo) or heart rate fluctuations (in-vivo) was determined using a χ2 test. Comparison of cellular survival (Kaplan-Meier curve) was analyzed using a log-rank test. P < 0.05 was considered statistically significant.
3. Results
3.1. Survival of Scn1a−/+ mice is decreased following seizure onset
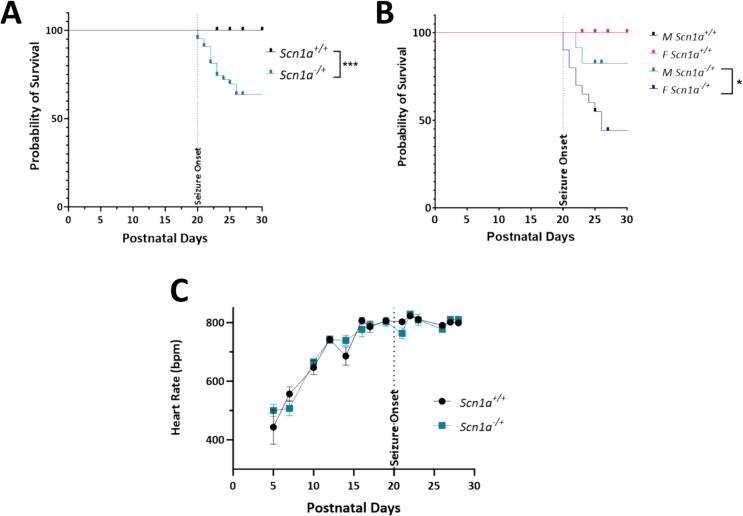
In the Scn1a−/+ model, seizures begin approximately 20 days after birth. From this time on, we observed a significant decrease in survival of Scn1a−/+ mice compared to Scn1a+/+ (Fig. 1A; p = 0.002). This effect was pronounced in the female Scn1a−/+ cohort, where lifespan was decreased significantly compared to both female Scn1a+/+ and the male Scn1a−/+ cohort (Fig. 1B; p = 0.04). Importantly, there were no developmental differences in the progression of heart rate increases in Scn1a−/+ mice (Fig. 1C; p = 0.87).
Fig. 1.
Survival of Scn1a−/+ mice is decreased compared to Scn1a+/+ following seizure onset. (A) Survival curve comparing lifespan of Scn1a−/+ (N = 43) and Scn1a+/+ mice (N = 37). Survivability of Scn1a−/+ is significantly reduced compared to wild-type (p = 0.002). (B) In Scn1a−/+ mice, survivability of female Scn1a−/+ (N = 20) mice is lower than their male (N = 23) counterparts (p = 0.038). (C) Heart rate of DS mice increases over time, but not significantly compared to Scn1a+/+ mice. Kaplan-Meier survival curve with Log-rank Mantel-Cox test (A-B) and two-way ANOVA (genotype x time) with Fisher's LSD post-hoc (C). *, P ≤ 0.05.
3.2. Mitochondrial respiratory chain function is decreased in Scn1a−/+ hearts
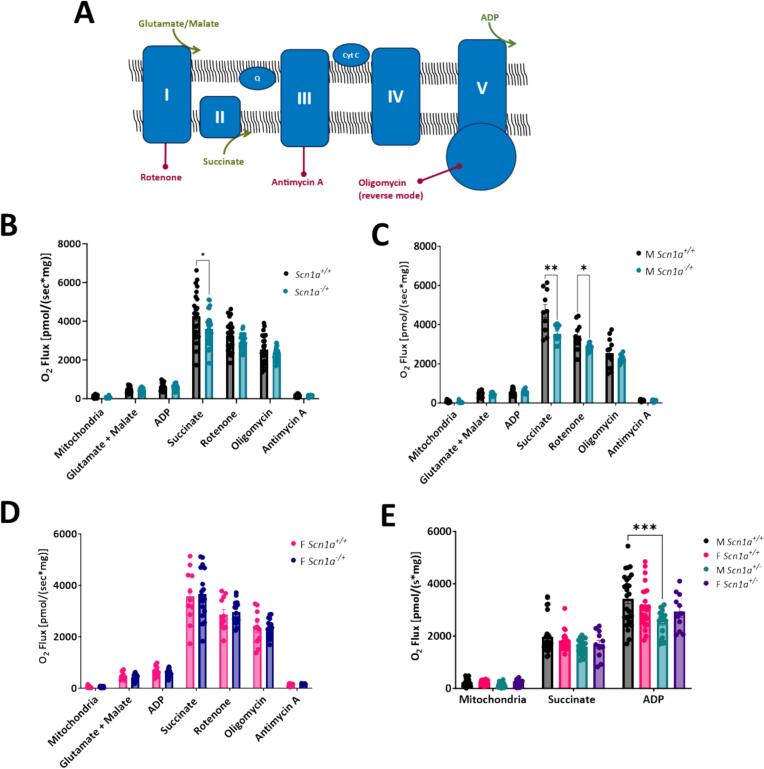
We investigated mitochondrial respiration chain activity under different electron transport states. Respiratory chain substrates and inhibitors were added to parallel chambers of an Oroboros Oxygraph to isolate the activity of Complex I- and Complex II-linked respiration in isolated mitochondria samples (Fig. 2A). First, the NADH-linked substrates glutamate and malate (G/M) were simultaneously added to isolated mitochondria samples to stimulate electron flow into Complex I. Upon the addition of G/M, no differences in oxygen consumption were detected between Scn1a+/+ and Scn1a−/+ samples (473.07 ± 30.72 in Scn1a+/+ vs. 427.27 ± 18.91 in Scn1a−/+; p = 0.19). Next, ADP was added to the energized mitochondria to stimulate oxidative phosphorylation. However, we detected no differences in oxygen consumption between Scn1a+/+ and Scn1a−/+ samples in the presence of G/M + ADP (625.94 ± 37.98 in Scn1a+/+ vs. 594.13 ± 23.95 in Scn1a−/+; p = 0.48), suggesting Complex-I linked ATP production is unchanged between Scn1a+/+ and Scn1a−/+ hearts. Therefore, to next assess Complex II-linked respiration, we added saturating levels of succinate, the endogenous substrate of Complex II, to isolated mitochondria. The addition of succinate after G/M and ADP leads to the simultaneous activation of both Complex I and II-linked substrate states, and is an estimate of physiologically relevant maximal respiratory capacity. Upon the titration of succinate, oxygen consumption in mitochondria isolated from Scn1a−/+ hearts were decreased significantly (4277.65 ± 265.34 in Scn1a+/+ vs. 3612.90 ± 144.92 in Scn1a−/+; p = 0.03) (Fig. 2B). Lastly, rotenone (an inhibitor of Complex I) was added to our isolated mitochondria samples. After rotenone addition, oxygen consumption was reduced similarly between Scn1a+/+ and Scn1a−/+ samples. Oxygen consumption under this state reflects rates of Complex II-linked respiratory activity was not different between Scn1a+/+ and Scn1a−/+ (3255.10 ± 161.25 in Scn1a+/+ vs. 2922.39 ± 66.08 in Scn1a−/+; p = 0.06). Next, oligomycin and antimycin A were added to isolated mitochondrial samples to inhibit reverse ATP synthase activity and measure background O2 consumption. No differences in oxygen consumption between DS and wild-type samples were observed after titration of these substrates (p values = 0.29–0.70).
Fig. 2.
Male Scn1a−/+ mice have deficits in Complex II-linked respiration. (A) Schematic of oxidative phosphorylation and substrates used in protocols. (B) N- and S-linked substrates were titrated to isolated mitochondria, and oxygen consumption measured using an O2k-FluoRespirometer. No differences in respiratory chain activity were detected between Scn1a−/+ (N = 9; n = 29) and Scn1a+/+ (N = 8; n = 23) samples (p = 0.06–0.48). (C) Deficits in electron transfer states supporting both Complex I- and Complex II-linked respiration (p = 0.02) as well as Complex II-linked respiration were observed in mitochondria isolated from male Scn1a−/+ (N = 4, n = 11) hearts compared to male Scn1a+/+ (N = 4, n = 12) hearts (p = 0.03). (D) No differences in Complex I- or Complex II-linked respiration were observed between female Scn1a−/+ (N = 5, n = 18) hearts compared to female Scn1a+/+ (N = 4, n = 11) hearts (p = 0.14–0.90). (E) To verify the deficits observed in Complex-II linked respiration in male Scn1a−/+ mice, S-linked substrates were added to isolated mitochondria in the absence of N-linked substrates. Upon titration of succinate + ADP, oxygen consumption was decreased in mitochondria from male Scn1a−/+ (N = 7, n = 26) hearts compared to male Scn1a+/+ (N = 7, n = 20) hearts (p = 0.0002). No differences were observed in female Scn1a−/+ (N = 5, n = 11) hearts compared to female Scn1a+/+ (N = 9, n = 23) hearts (p = 0.44). Two-way ANOVA (genotype x substrate) with Fisher's LSD post-hoc (B-E). *, P ≤ 0.05, **; P ≤ 0.01, ***; P ≤ 0.001.
3.3. Sex differences in mitochondrial respiration in male Scn1a−/+ hearts but not female
Initially, as described above, we detected a difference in oxygen consumption when Complex I- and Complex II-linked respiratory pathways were engaged, but no differences were found when these pathways were engaged separately in Scn1a−/+ cardiac mitochondria. Interestingly, upon the analysis of our respirometry data by sex, we uncovered mitochondria respiratory deficits in male Scn1a−/+ hearts, but not females. No differences in Complex I-linked ATP production were detected in male Scn1a−/+ hearts. Upon the addition of N-linked substrates G/M, and ADP, respiration was unaffected in mitochondria from male Scn1a−/+ hearts compared to Scn1a+/+ (585.24 ± 48.41 in Scn1a+/+ vs. 596.18 ± 33.08 in Scn1a−/+; p = 0.86). However, upon the simultaneous engagement of both Complex-I and Complex-II linked electron transfer pathways via the addition of G/M, ADP, and succinate to mitochondria, oxygen consumption in mitochondria from male Scn1a−/+ hearts were significantly reduced (4692.97 ± 342.12 in Scn1a+/+ vs. 3534.90 ± 126.11 in Scn1a−/+; p = 0.01). The subsequent addition of rotenone to block Complex I activity revealed that oxygen consumption under the S-pathway control state was significantly lower in mitochondria from male Scn1a−/+ hearts (3430.72 ± 219.64 in Scn1a+/+ vs. 2839.87 ± 54.47 in Scn1a−/+; p = 0.03) (Fig. 2C). These results indicated that a deficit in Complex II-linked respiration in our male cohort was likely responsible for this decreased oxygen consumption observed in Scn1a−/+ cardiac mitochondria during our assessment of mitochondrial respiratory capacity. No differences in mitochondrial respiration were detected between mitochondria isolated from female Scn1a+/+ and Scn1a−/+ hearts (p = 0.14–0.90) (Fig. 2D). To confirm these sex-specific deficits in Scn1a−/+ cardiac mitochondria, we analyzed Complex II-linked respiration through an alternative protocol, where succinate + ADP was added directly to isolated mitochondria, in the absence of NADH-linked substrates or rotenone. Upon the titration of these substrates, oxygen consumption was significantly decreased in mitochondria from male Scn1a−/+ hearts compared to Scn1a+/+ (3425.93 ± 191.92 in Scn1a+/+ vs. 2512.06 ± 111.85 in Scn1a−/+; p = 0.02) (Fig. 2E). As in the initial protocol, there were no differences in oxygen consumption in mitochondria from female mouse hearts (3148.82 ± 171.14 in Scn1a+/+ vs. 2936.78 ± 210.86 in Scn1a−/+; p = 0.44).
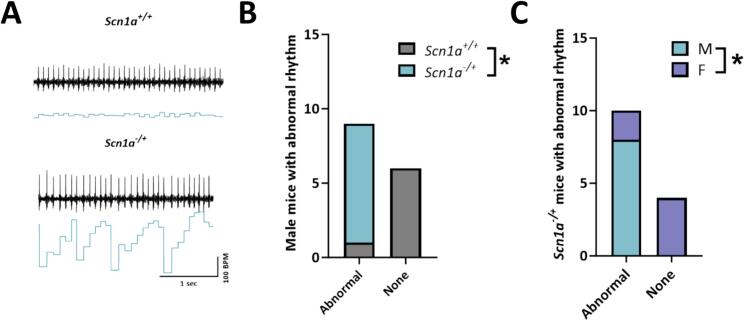
3.4. Increased susceptibility to spontaneous arrhythmogenic events under increased sympathetic activation in male Scn1a−/+ mice
Our previous data indicated contradictory results that mortality in female Scn1a−/+ mice is increased compared to males, but deficits in Complex II-linked respiration are exclusively in Scn1a−/+ males. Given that Complex II (succinate dehydrogenase) is responsive to increases in activity during times of increased workload, this observation raised the possibility that mortality in female Scn1a−/+ may proceed through non-SUDEP epileptic mechanisms (i.e. status epilepticus) whereas in males, death occurs more through cardiac arrhythmias and SUDEP. Therefore, we hypothesized that that under times of increased physiological stress or demand on the heart would reveal an underlying predisposition to cardiac arrhythmias in male but not female Scn1a−/+ mice. Furthermore, in another epileptic encephalopathy model, we have shown that arrhythmias may develop under conditions that simulate a sympathetic surge [20]. To test this, we subjected lightly anesthetized mice to a 2 mg/kg norepinephrine (NE) followed by 120 mg/kg caffeine (Caff) challenge. Following the injection of NE, we observed an increased probability of spontaneous abnormal rhythm events in male Scn1a−/+ mice (Fig. 3). Contrary to our observations in the Scn8a model [20], differences were primarily seen following NE injection, and we saw no further rhythm disturbances following Caff injection in Scn1a−/+ mice. Since anesthetized mice have a much slower heart rate than conscious mice, we next assessed heart rhythm under physiological conditions. After allowing mice to recover for 5 days we measured unrestrained conscious ECG recordings. In this setting, we observed the same abnormal rhythmic pattern in male Scn1a−/+ mice. Male Scn1a−/+ were much more likely to experience dysrhythmias than female Scn1a−/+ mice (p = 0.01) in conscious mice as well. Taken together these results suggest that abnormal cardiac rhythm is more likely to occur during period of high sympathetic levels across multiple experimental conditions.
Fig. 3.
Abnormal cardiac rhythm following norepinephrine (NE) injection in male Scn1a−/+ mice. (A) Representative traces from Scn1a+/+ and Scn1a−/+ mice. (B) Every male Scn1a−/+ mouse exhibited abnormal rhythm following NE injection. This observation was significantly more likely than in Scn1a+/+ male mice. (C) Comparison of male vs female Scn1a−/+ mice demonstrate that the abnormal rhythm was significantly more likely to occur in male vs. female mice. Fisher's exact test (B—C). *, P ≤ 0.05. (N = 7 Scn1a+/+ male; 8 Scn1a−/− male; and 6 Scn1a−/− female).
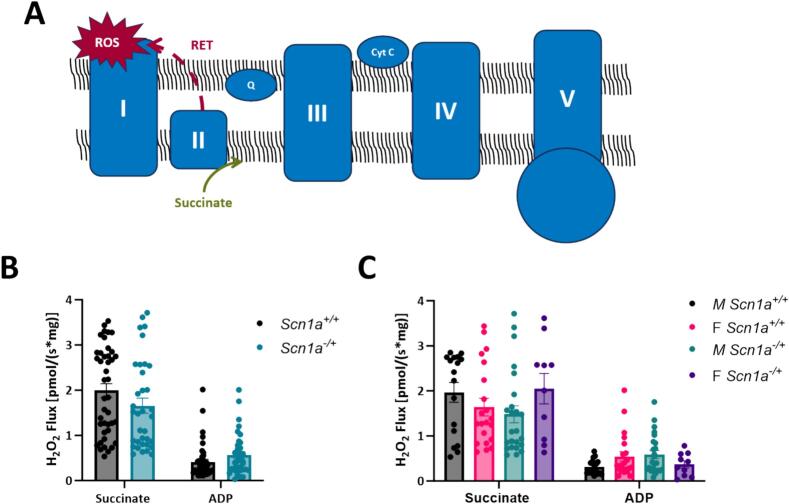
3.5. Mitochondrial reactive oxygen species production is unchanged between Scn1a−/+and Scn1a+/+ hearts, regardless of sex
Elevated levels of mitochondrial ROS production can lead to the accumulation of oxidative species and oxidative stress. Therefore, alongside our respirometry experiments, we also investigated if there are differences in ROS production in mitochondria isolated from Scn1a+/+ and Scn1a−/+ mouse hearts (Fig. 4). Specifically, we were interested in measuring mitochondrial ROS production under conditions of reverse electron transfer (RET) of electrons to Complex I. We chose to investigate mitochondrial ROS production under this state, as RET is the major pathway for pathological ROS generation within mitochondria. The large amounts of ROS produced during RET have been previously associated with cardiac injury [34,35]. To generate conditions supporting RET, saturating levels of succinate were added to isolated mitochondrial preparations. However, upon succinate titration, ROS production was not different between mitochondria isolated from Scn1a−/+ and Scn1a+/+ hearts (1.99 ± 0.15 in Scn1a+/+ vs. 1.66 ± 0.17 in Scn1a−/+; p = 0.052) (Fig. 4B). Contrary to our respirometry experiments, this observation held even after separating our data by sex, where mitochondrial ROS production between male ROS production was not greater in mitochondria isolated from male Scn1a−/+ animals compared to samples from Scn1a+/+ (1.97 ± 0.22 in Scn1a+/+ vs. 1.48 ± 0.19 in Scn1a−/+; p = 0.10) (Fig. 4C). In female hearts, mitochondria ROS production during RET was unchanged between Scn1a+/+ and Scn1a−/+ samples (1.64 ± 0.20 in Scn1a+/+ vs. 2.05 ± 0.31 in Scn1a−/+; p = 0.14). These results suggest respiratory chain deficits in male Scn1a−/+ hearts may exist independent of changes in mitochondrial ROS production.
Fig. 4.
No differences in mitochondrial reactive oxygen species production between Scn1a−/+ and Scn1a+/+ mice. (A) Schematic of reverse electron flow. (B) Isolated mitochondria were analyzed for reactive oxygen species production (ROS) under conditions of reverse electron transfer (addition of succinate). Although elevated, no significant differences in ROS production were observed between mitochondria from Scn1a−/+ (N = 12, n = 35) and Scn1a+/+ (N = 16, n = 42) hearts (p = 0.052). (C) When data was separated by sex, there were no differences in ROS production in male Scn1a−/+ (N = 7, n = 24) hearts compared to male Scn1a+/+ (N = 7, n = 17) hearts (p = 0.10), or between female Scn1a−/+ (N = 5, n = 10) hearts compared to female Scn1a+/+ (N = 9, n = 21) hearts (p = 0.14). Two-way ANOVA (genotype x substrate) with Fisher's LSD post-hoc (B—C).
3.6. Hypoxia-reoxygenation leads to increased reactive oxygen species production in Scn1a−/+ cardiac mitochondria
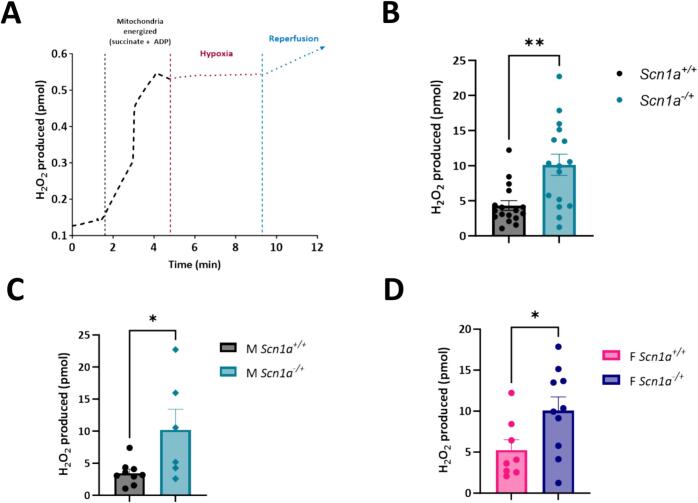
Next, as SUDEP is often a multisystem event, we wanted to test the effect of hypoxic conditions on our mitochondria by simulating a low-oxygen environment (Fig. 5). In addition, Dravet patients have been shown to undergo periods of oxygen desaturation either peri-ictally [36] or during sleep [38,41]. These events have been implicated in SUDEP related mortality in DS [36,39,42]. In addition, RNA-seq data from a mouse model of DS shows that hypoxia pathways are elevated [46]. However, if there is an effect of these hypoxic events on the heart has not been explored. Our previous work has shown that during similar events ROS production and collapses in mitochondrial membrane potential can occur during hypoxia/reoxygenation at the cellular level [37]. Isolated mitochondria from Scn1a+/+ and Scn1a−/+ hearts were loaded in parallel chambers of the O2k FluoRespirometer. After energizing mitochondria with succinate and ADP, the oxygen in the recording chamber was allowed to run out, and mitochondria preparations were held in hypoxic conditions for five minutes and then reoxygenated (Fig. 5A). Upon stabilization of the signal, the total ROS (H2O2) produced during reoxygenation was measured over 1 min. Compared to Scn1a+/+, ROS produced during hypoxia was increased significantly in mitochondria isolated from Scn1a−/+ hearts (4.32 ± 2.83 in Scn1a+/+ vs. 10.13 ± 6.07 in Scn1a−/+; p = 0.001) (Fig. 5B). This effect in DS samples was not sex-dependent; ROS production was increased significantly during reperfusion in mitochondria isolated from male Scn1a−/+ hearts, as well as (3.47 ± 0.61 in Scn1a+/+ vs. 10.22 ± 3.21 in Scn1a−/+; p = 0.03) female Scn1a−/+ hearts, ROS (5.27 ± 1.24 in Scn1a+/+ vs. 10.07 ± 1.65 in Scn1a−/+; p = 0.04) (Fig. 5C-D).
Fig. 5.
SUDEP in Scn1a-linked DS may proceed through a multisystem mechanism. (A) Example trace of a mitochondrial sample undergoing hypoxia/reperfusion. Isolated mitochondria from Scn1a−/+ and Scn1a+/+ hearts were energized with succinate + ADP, then subjected to a five-minute period of hypoxia in a O2k-FluoRespirometer, followed by one minute of reperfusion. (B) Mitochondrial reactive oxygen species production during reperfusion was significantly elevated in Scn1a−/+ (N = 9, n = 16) compared to Scn1a+/+ (N = 8, n = 17) (p = 0.001). Upon separating data by sex, ROS production was higher in both mitochondrial samples from male Scn1a−/+ hearts (N = 4; n = 6) (p = 0.03) (C) and female Scn1a−/+ hearts (N = 5; n = 10) (p = 0.04) (D) compared to their respective wild-type counterparts. t-test (B—D). *, P ≤ 0.05; **, P ≤ 0.01.
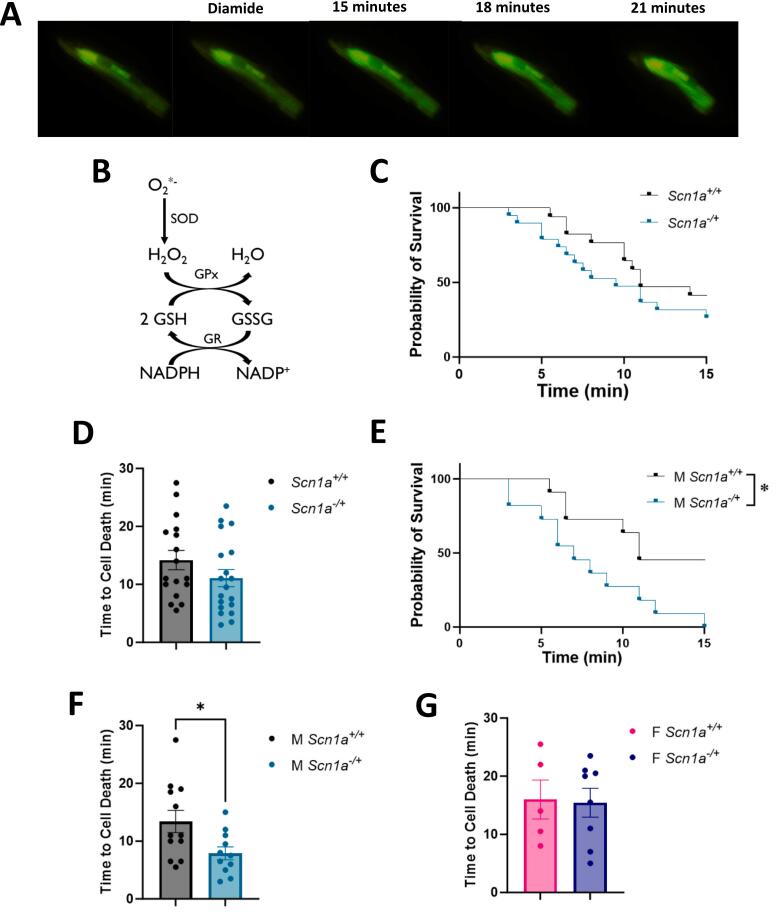
3.7. Cardiomyocytes from male Scn1a−/+ hearts have increased susceptibility to thiol oxidation
After our determination that there were no differences in mitochondrial ROS production under basal conditions, we next wanted to establish if there are differences in cellular ROS scavenging and/or accumulation in Scn1a−/+ cardiomyocytes under stress (Fig. 6). Similar to our previous work [33], we specifically tested the proficiency of the glutathione (GSH) redox buffer within cardiomyocytes. The GSH system is closely linked to mitochondrial function [40,43], and is one of the main antioxidant pathways within the heart [44]. We isolated primary ventricular cardiomyocytes from Scn1a+/+ and Scn1a−/+ hearts and loaded cells with CM-DCF, a fluorescent indicator of global ROS within cells. Fluorescent microscopy was used to assess changes in the DCF signal and detect cell death (Fig. 6A). After an initial recording period to ensure cell stability, we treated cardiomyocytes with 80uM of diamide. Diamide slowly oxidizes thiols, like GSH, and therefore presents a prolonged oxidative challenge to the cell and a test of the strength of its GSH system (Fig. 6B). There was no difference in survivability between DS and wild-type cells (p = 0.20) (Fig. 6C). In cardiomyocytes from Scn1a−/+ mice, we found that, while decreased, the average time to cell death was not significantly lower compared to those from Scn1a+/+ hearts (14.18 ± 1.65 in Scn1a+/+ vs. 11.08 ± 1.47 in Scn1a−/+; p = 0.17) (Fig. 6D). However, upon the separation of data by sex, we once again detected deficits in samples from male Scn1a−/+ mice (p = 0.01) (Fig. 6E). Cardiomyocytes isolated from male Scn1a−/+ hearts died on average 5.82 min sooner than cells isolated from Scn1a+/+ following diamide treatment (13.42 ± 1.93 in Scn1a+/+ vs. 7.91 ± 1.12 in Scn1a−/+; p = 0.02) (Fig. 6F). These results indicate that male Scn1a−/+ animals may possess a weakened GSH system or an increased susceptibility to thiol oxidation, and therefore a reduced ability to handle ROS accumulations and oxidative stress. No significant difference in cell survivability was observed between cardiomyocytes isolated from female Scn1a−/+ mice compared to Scn1a+/+ (16.00 ± 3.35 in Scn1a+/+ vs. 15.44 ± 2.47 in Scn1a−/+; p = 0.89) (Fig. 6G).
Fig. 6.
Cardiomyocytes from Scn1a−/+ mice are more susceptible to thiol oxidation. (A) Isolated primary ventricular cardiomyocytes from Scn1a−/+ and Scn1a+/+ hearts were given an 80 սM treatment of the thiol oxidant, glutathione, and analyzed using fluorescent microscopy and the ROS indicator, CM-DCF. (B) The glutathione (GSH) system is the primary thiol buffer in the heart that scavenges ROS and is linked to mitochondrial function. (C) Survival curve comparing lifespan of Scn1a−/+ (N = 8; n = 19) and Scn1a+/+ (N = 10; n = 17) cardiomyocytes following diamide treatment. Survivability of Scn1a−/+ cells is not significantly reduced compared to Scn1a+/+ (p = 0.20). (D) The average time to cell death between Scn1a−/+ and Scn1a+/+ cardiomyocytes is not significantly different (p = 0.17). (E) Survival of cells from male Scn1a−/+ (N = 5; n = 11) mice is significantly lower than those from male Scn1a+/+ (N = 5; n = 12) mice following diamide treatment, along with average time to cell death (p = 0.03) (F). (G) Average time to cell death was similar between cardiomyocytes isolated from female Scn1a−/+ (N = 3; n = 8) mice compared to cells isolated from wild-type (N = 5; n = 5) (p = 0.89). Kaplan-Meier Survival curve with Log-rank Mantel-Cox test (C, E), t-test (D, F, G). *, P ≤ 0.05.
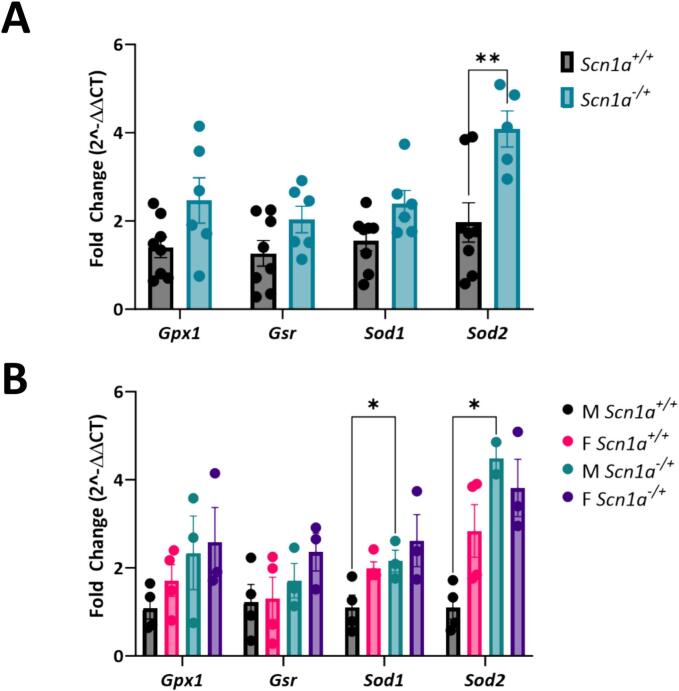
3.8. Expression of antioxidant genes is increased in male Scn1a−/+ hearts
Following our findings that male Scn1a−/+ cardiomyocytes have reduced capacity to handle thiol oxidation, we next wanted to determine any underlying differences in the expression of antioxidant enzymes (Fig. 7). We used RT-qPCR to quantify the expression of GSH system antioxidant enzymes, glutathione reductase (Gsr), and glutathione peroxidase (Gpx) as well as the expression of the cytosolic and mitochondrial isoforms of superoxide dismutase, Sod1 and Sod2. Upon comparing gene expression in Scn1a−/+ and Scn1a+/+ hearts, we found that expression of Sod2 was significantly increased in Scn1a−/+ samples (2.08-fold increase in Scn1a−/+; p = 0.005) (Fig. 7A). Upon separating our data by sex, we found that male Scn1a−/+ hearts were the main driver of this difference (4.09-fold increase in male Scn1a−/+; p = 0.01) (Fig. 7B). These analyses also showed that Sod1 expression is increased in male Scn1a−/+ hearts (4.09-fold increase in male Scn1a−/+; p = 0.04). No differences in the expression of genes were observed between female Scn1a−/+ and Scn1a+/+ hearts (p = 0.16–0.40).
Fig. 7.
Increased expression of antioxidant genes in male Scn1a−/+ hearts. (A) The expression of antioxidant genes Gpx, Gsr, Sod1, and Sod2 was evaluated in left ventricular samples from Scn1a−/+ (N = 6) and Scn1a+/+ (N = 8) hearts. Expression of Sod1 was significantly elevated in Scn1a−/+ samples (p = 0.005). (B) Evaluation of gene expression by sex revealed that the expression of Sod1 (p = 0.04) and Sod2 (p = 0.01) is decreased in male Scn1a−/+ hearts (N = 3) compared to Scn1a+/+ (N = 4). Two-way ANOVA (genotype x gene) (A) or (genotype + sex x gene) (B) with Fisher's LSD post-hoc. *, P ≤ 0.05; **, P ≤ 0.01.
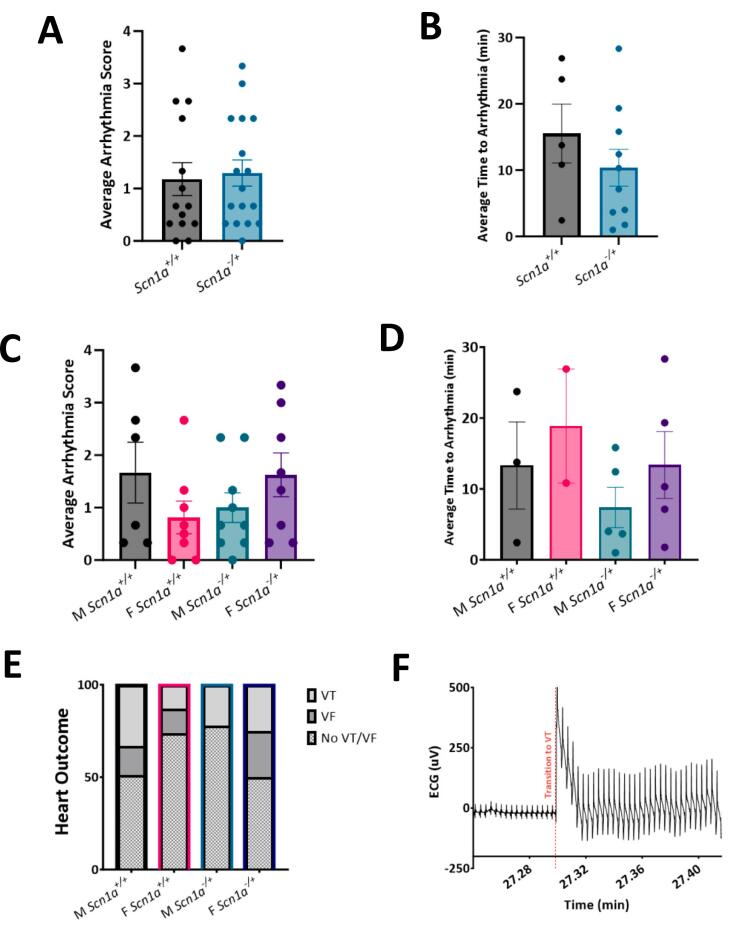
3.9. Susceptibility to arrhythmias under thiol oxidation is unchanged in hearts from Scn1a−/+ mice compared to Scn1a+/+
From the results of our cellular data, we hypothesized that the increased cell death in male Scn1a−/+ myocytes following diamide treatment may translate to an increased susceptibility to arrhythmia in whole hearts (Fig. 8). To test this theory, we excised hearts from DS mice and retrograde perfused hearts with 80 mM diamide for 30 min while collecting continuous ECG measurements. The resulting ECG signal was then scored similar to our previous work [45]. Our results indicated were no significant differences in arrhythmia occurrence between Scn1a−/+ and Scn1a+/+ mice following diamide treatment (average arrhythmia score 1.179 ± 0.31 in Scn1a+/+ vs. 1.29 ± 0.25 in Scn1a−/+; p = 0.77) (Fig. 8A). There was also a similar incidence of arrhythmia between sample groups. Of hearts that did experience VT or VF, the time between diamide administration and the first incidence of arrhythmia was also not significantly shorter in Scn1a−/+ mice (15.53 ± 4.43 in Scn1a+/+ vs. 10.38 ± 2.78 in Scn1a−/+; p = 0.70) (Fig. 8B). Once data was separated by sex, it appeared hearts from Scn1a−/+ male mice are not more susceptible to arrhythmia following oxidative stress than those from Scn1a+/+. Arrhythmia scores (average arrhythmia score 1.67 ± 0.58 in Scn1a+/+ vs. 1.00 ± 0.28 in Scn1a−/+; p = 0.25) (Fig. 8C) as well as average time to arrhythmia following diamide perfusion (Fig. 8D) was unchanged in Scn1a−/+ hearts. Similarly, average arrhythmia score was not different between female Scn1a−/+ and Scn1a+/+ hearts (0.81 ± 0.31 in Scn1a+/+ vs. 1.63 ± 0.42 in Scn1a−/+; p = 0.14). Contrary to our predictions, the female Scn1a−/+ mice were the main driver of arrhythmias in the DS cohort. 50 % of Scn1a−/+ female hearts experienced an episode of VT or VF during the recording period, compared to only 22 % of male Scn1a−/+ hearts (Fig. 8E). These observations are contrary to our mitochondria data, which suggested a more extreme phenotype in male Scn1a−/+ mice. These results indicate there may be distinct cardiac mechanisms leading to arrhythmia and SUDEP between male and female Scn1a−/+ animals.
Fig. 8.
No differences in susceptibility to thiol oxidation were observed in whole hearts. (A) ECG traces collected from isolated hearts perfused with diamide for 30 min were scored for arrhythmia incidence. No difference in this score was observed between Scn1a−/+ (N = 17) and Scn1a+/+ (N = 14) hearts (p = 0.77). (B) Average time to arrhythmia incidence after the onset of diamide perfusion is unchanged between Scn1a−/+ and Scn1a+/+ hearts (p = 0.70). (C) No sex-dependent differences in arrhythmia scores were detected between male Scn1a−/+ (N = 9) and Scn1a+/+ (N = 6) hearts (p = 0.27), or female Scn1a−/+ (N = 8) and Scn1a+/+ (N = 8) hearts (p = 0.14). (D) No sex-dependent differences in time to arrhythmia onset were detected between male Scn1a−/+ and Scn1a+/+ hearts (p = 0.40), or female Scn1a−/+ and Scn1a+/+ hearts (p = 0.50). (E) Incidence of potentially fatal arrhythmias, ventricular tachycardia (VT) or ventricular fibrillation (VF), between Scn1a−/+ and Scn1a+/+ cohorts. (F) ECG trace from a Scn1a−/+ heart showing an episode of VT. t-test (A-B), one-way ANOVA with Fisher's LSD post-hoc (C—D), and Fisher's exact test (E).
4. Discussion
In this study, we sought to determine if alterations in mitochondrial bioenergetics and reactive oxygen species are present in a mouse model of Scn1a-linked DS, as these pathways may contribute to the development of arrhythmias and SUDEP. Our main findings demonstrate that male Scn1a−/+ Dravet mice are more susceptible to deficits in mitochondrial ATP production, ROS balance, and cardiac arrhythmias. Our male mice showed a deficit in their ability to generate ATP through Complex II-mediated respiration, without a change in ROS production. We then showed that Scn1a−/+ mice have a decreased ability to buffer ROS accumulation, alongside altered expression of antioxidant enzymes. Lastly, spontaneous arrhythmogenic events were significantly increased under conditions of physiological stress in male, but not female Scn1a−/+ mice. Despite an earlier mortality rate in female mice, our data demonstrates that male Scn1a−/+ mice are more susceptible to cardiac arrhythmias. Given the complex nature of SUDEP and mortality in DS, we have shown that sex differences may play an important role in risk factors associated with early mortality in DS. This work further supports evidence that multiple physiological mechanisms may contribute to how cardiac arrhythmias have the potential to underly SUDEP in DS.
We initially detected a difference in respiratory capacity between Scn1a−/+ and Scn1a+/+ cardiac mitochondria when Complex I- and Complex II-linked electron transfer pathways were simultaneously engaged. Upon analyzing data by sex, we found this effect was driven by respiratory deficits in Complex II-linked respiration, specifically in the male Scn1a−/+ cohort. Overall, a decreased energy supply within the heart can be dangerous. Upon β-adrenergic stimulation of the heart, the cAMP-dependent protein kinase A (PKA) is activated. PKA phosphorylates multiple Ca2+ handling proteins within the excitation-contracting (EC) coupling process, which increases cellular Ca2+ flux. Elevated Ca2+ concentrations within the cell leads to increased sequestration of Ca2+ via the mitochondrial Ca2+ uniporter, the primary mitochondrial Ca2+ uptake pathway. Ca2+ then stimulates the activity of citric acid cycle enzymes, and consequently, electron transfer through Complex II [47,48]. Therefore, deficits in Complex II-linked respiration may lead to a mismatch between cardiac demand and output. Our data support this observation, as we showed male Scn1a−/+ mice develop abnormal rhythms under β-adrenergic stimulation with a decreased ability to increase heart rate. Deficits in this pathway could be especially detrimental to patients with DS, especially during epileptic episodes, which can activate autonomic control centers in the brain.
SUDEP is often believed to result from the culmination of postictal depression of neuronal reflex centers, along with cardiac and respiratory arrest [6]. The heart depends on oxidative phosphorylation as it possesses only a small ATP reserve and is thus exceptionally vulnerable to hypoxic conditions. In a cohort of patients with genetic epilepsies, hypercapnia occurring during seizures was found to lower the threshold for cardiac arrhythmias [49]. Therefore, in this study, we decided to perform experiments testing the effect of hypoxia on isolated cardiac mitochondria. Our results indicated that mitochondria from Scn1a−/+ hearts have a reduced ability to handle hypoxia/reperfusion injury than their wild-type counterparts, regardless of sex. The increased ROS production by Scn1a−/+ during hypoxia/reperfusion may highlight the importance of the multisystem nature of SUDEP in generating adverse cardiac events. Especially, as we observed no differences in ROS production during RET in mitochondria from Scn1a−/+ at steady normoxic conditions.
In many of our experiments, we observed sex differences in mitochondrial and cellular responses, specifically in our male Scn1a−/+ cohort. In male Scn1a−/+ hearts, respiration is compromised through Complex II-linked pathways. Furthermore, male cardiomyocytes had increased sensitivity to thiol oxidation, alongside elevated activation of antioxidant genes, suggesting an increased baseline of oxidative stress. The effect of sex chromosomes on epilepsy and SUDEP is not extensively studied. However, biological sex may be an important consideration in the development of new therapeutics to prevent seizures and SUDEP. Differences in response to antiepileptics have been found to occur due to patient sex [50]. In DS, one group has reported that there may be a slightly higher incidence of Scn1a-linked DS in males [51], although majorly, DS is believed to impact both sexes equally [[52], [53], [54]]. Furthermore, it has been reported that SUDEP susceptibility in Scn1a-linked DS is roughly equal among the sexes [55]. Contrarily, in the Scn1a−/+ mouse model, female mice were found to experience more severe seizures resulting in a greater SUDEP risk [56]. This latter observation is consistent with our data, where female Scn1a−/+ exhibited decreased survival following the onset of seizures, even when compared to male Scn1a−/+ animals. The increased death rate in female Scn1a−/+ mice may be due to an increased number of status epilepticus events, as opposed to a SUDEP mechanism. This is an important distinction for our findings, as the cause of death across our mice was not determined to be SUDEP (via cardiac arrhythmias or other causes), increased seizure burden, or other cause of death. However, our data suggest that male Scn1a−/+ mice may be more susceptible to cardiac arrhythmias following onset of seizures. These sex differences may have important implications for an increased need to examine cardiac abnormalities and treatments in the clinical population, especially in the males.
Biological sex may also exert structural and physiological changes in the brain that impact epilepsy etiology [57]. Human male and female genomes produce sex-specific signals that influence gene activation. Many of these sex-specific signals play significant roles in development and early life and therefore may be especially pertinent in juvenile epilepsies such as DS. Androgens, like testosterone, have been linked to increased seizure susceptibility due to their neuroexcitatory effects [[58], [59], [60]]. Sex hormones are also known to influence neuronal activity by affecting gene expression and chromatin packaging, modulating membrane excitability through binding membrane receptors [61,62]. These effects can also be temporal, as X- and Y-linked factors are differentially expressed among brain structures [52]. Diverging epileptic phenotypes between sexes may also impact the heart. The hippocampus is a structure often linked to epileptic networks due to its recurrent circuits and is also part of the limbic system of higher autonomic control of the heart. An increased number of cells in the hippocampus are generated during the perinatal period in male animals, eventually developing into mature neurons at juvenility [63,64]. In our study we often saw exacerbated effects in males; however, further research is needed to clarify the conflicting data on sex on SUDEP risk in Scn1a-linked DS.
Source of funding
This work was supported by a National Institute of Neurological Disorders and Stroke at the National Institutes of Health [R21NS116647] and a Research Development Committee Grant at East Tennessee State University to C.R.F.
CRediT authorship contribution statement
Jessa L. Aldridge: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Emily Davis Alexander: Formal analysis, Investigation, Resources, Writing – review & editing. Allison A. Franklin: Resources, Writing – review & editing. Elizabeth Harrington: Resources, Writing – review & editing. Farah Al-Ghzawi: Resources, Writing – review & editing. Chad R. Frasier: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors did not use generative AI or AI-assisted technologies in the development of this manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Chad R. Frasier reports financial support was provided by National Institute of Neurological Disorders and Stroke. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Genton P., Velizarova R., Dravet C. Dravet syndrome: the long-term outcome. Epilepsia. 2011;52(Suppl. 2):44–49. doi: 10.1111/j.1528-1167.2011.03001.x. [DOI] [PubMed] [Google Scholar]
- 2.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl. 2):3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- 3.Dravet C., Bureau M., Oguni H., Fukuyama Y., Cokar O. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol. 2005;95:71–102. [PubMed] [Google Scholar]
- 4.Swallow R.A., Hillier C.E.M., Smith P.E.M. Sudden unexplained death in epilepsy (SUDEP) following previous seizure-related pulmonary oedema: case report and review of possible preventative treatment. Seizure. 2002;11:446–448. doi: 10.1053/seiz.2002.0683. [DOI] [PubMed] [Google Scholar]
- 5.So E.L., Sam M.C., Lagerlund T.L. Postictal central apnea as a cause of SUDEP: evidence from near-SUDEP incident. Epilepsia. 2000;41:1494–1497. doi: 10.1111/j.1528-1157.2000.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 6.Devinsky O. Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr. 2004;4:43. doi: 10.1111/J.1535-7597.2004.42001.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Surges R., Adjei P., Kallis C., Erhuero J., Scott C.A., Bell G.S., et al. Pathologic cardiac repolarization in pharmacoresistant epilepsy and its potential role in sudden unexpected death in epilepsy: a case-control study. Epilepsia. 2010;51:233–242. doi: 10.1111/j.1528-1167.2009.02330.x. [DOI] [PubMed] [Google Scholar]
- 8.Schuele S.U., Widdess-Walsh P., Bermeo A., Lüders H.O. Sudden unexplained death in epilepsy: the role of the heart. Cleve Clin J Med. 2007;74(Suppl. 1):121–127. doi: 10.3949/CCJM.74.SUPPL_1.S121. [DOI] [PubMed] [Google Scholar]
- 9.Pathak SJ, Yousaf MIK, Shah VB. Sudden Unexpected Death in Epilepsy.
- 10.Ryvlin P., Nashef L., Lhatoo S.D., Bateman L.M., Bird J., Bleasel A., et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 11.Tomson T., Nashef L., Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol. 2008;7:1021–1031. doi: 10.1016/S1474-4422(08)70202-3. [DOI] [PubMed] [Google Scholar]
- 12.Marini C., Scheffer I.E., Nabbout R., Suls A., De Jonghe P., Zara F., et al. The genetics of Dravet syndrome. Epilepsia. 2011;52(Suppl. 2):24–29. doi: 10.1111/J.1528-1167.2011.02997.X. [DOI] [PubMed] [Google Scholar]
- 13.Brunklaus A., Ellis R., Reavey E., Semsarian C., Zuberi S.M. Genotype phenotype associations across the voltage-gated sodium channel family. J Med Genet. 2014;51:650–658. doi: 10.1136/JMEDGENET-2014-102608. [DOI] [PubMed] [Google Scholar]
- 14.Catterall W.A. Sodium channels, inherited epilepsy, and antiepileptic drugs. Annu Rev Pharmacol Toxicol. 2014;54:317–338. doi: 10.1146/ANNUREV-PHARMTOX-011112-140232. [DOI] [PubMed] [Google Scholar]
- 15.Isom L.L., Arbor A. 2001. Sodium channel β subunits: anything but auxiliary. doi:101177/107385840100700108 7: 42–54. [DOI] [PubMed] [Google Scholar]
- 16.Edokobi N., Isom L.L. Voltage-gated sodium channel β1/β1B subunits regulate cardiac physiology and pathophysiology. Front Physiol. 2018;9:351. doi: 10.3389/FPHYS.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auerbach D.S., Jones J., Clawson B.C., Offord J., Lenk G.M., Ogiwara I., et al. Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS One. 2013;8:77843. doi: 10.1371/JOURNAL.PONE.0077843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frasier C.R., Zhang H., Offord J., Dang L.T., Auerbach D.S., Shi H., et al. Channelopathy as a SUDEP biomarker in Dravet syndrome patient-derived cardiac myocytes. Stem Cell Rep. 2018;11:626. doi: 10.1016/J.STEMCR.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Santiago L.F., Meadows L.S., Ernst S.J., Chen C., Malhotra J.D., McEwen D.P., et al. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol. 2007;43:636. doi: 10.1016/J.YJMCC.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frasier C.R., Wagnon J.L., Bao Y.O., McVeigh L.G., Lopez-Santiago L.F., Meisler M.H., et al. Cardiac arrhythmia in a mouse model of sodium channel SCN8A epileptic encephalopathy. Proc Natl Acad Sci USA. 2016;113 doi: 10.1073/pnas.1612746113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng J., Jiang Y., Chen Z.B., Rhee J.W., Deng Y., Wang Z.V. Mitochondrial dysfunction in cardiac Arrhythmias. Cells. 2023;Vol 12, Page 679 12: 679, 2023 doi: 10.3390/CELLS12050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B., Daneshgar N., Lee H.C., Song L.S., Dai D.F. Mitochondrial oxidative stress mediates Bradyarrhythmia in Leigh syndrome mitochondrial disease mice. Antioxidants. 2023;12 doi: 10.3390/ANTIOX12051001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anan R., Nakagawa M., Miyata M., Higuchi I., Nakao S., Suehara M., et al. Cardiac involvement in mitochondrial diseases. Circulation. 1995;91:955–961. doi: 10.1161/01.CIR.91.4.955. [DOI] [PubMed] [Google Scholar]
- 24.Duran J., Martinez A., Adler E. Cardiovascular manifestations of mitochondrial disease. Biology (Basel) 2019;8 doi: 10.3390/BIOLOGY8020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doenst T., Nguyen T.D., Abel E.D. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113:709–724. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y., Rasmussen T.P., Koval O.M., Joiner M.A., Hall D.D., Chen B., et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun. 2015;6:6081. doi: 10.1038/ncomms7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Styr B., Gonen N., Zarhin D., Ruggiero A., Atsmon R., Gazit N., et al. Mitochondrial regulation of the hippocampal firing rate set point and seizure susceptibility. Neuron. 2019;102:1009. doi: 10.1016/J.NEURON.2019.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander E.D., Aldridge J.L., Burleson T.S., Frasier C.R. Teriflunomide treatment exacerbates cardiac ischemia reperfusion injury in isolated rat hearts. Cardiovasc Drugs Ther. 2023;37:1021–1026. doi: 10.1007/S10557-022-07341-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frasier C.R., Moukdar F., Patel H.D., Sloan R.C., Stewart L.M., Alleman R.J., et al. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc Res. 2013;98:47–55. doi: 10.1093/cvr/cvt009. [DOI] [PubMed] [Google Scholar]
- 30.Brown D.A., Hale S.L., Baines C.P., del Rio C.L., Hamlin R.L., Yueyama Y., et al. Reduction of early reperfusion injury with the mitochondria-targeting peptide bendavia. J Cardiovasc Pharmacol Ther. 2014;19:121–132. doi: 10.1177/1074248413508003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldridge JL, Alexander ED, Franklin AA, Frasier CR. Altered cardiac energetics in mice lacking Scn1b. [DOI] [PMC free article] [PubMed]
- 32.Brown D.A., Aon M.A., Frasier C.R., Sloan R.C., Maloney A.H., Anderson E.J., et al. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol. 2010;48:673–679. doi: 10.1016/j.yjmcc.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frasier C.R., Sloan R.C., Bostian P.A., Gonzon M.D., Kurowicki J., Lo Presto S.J., et al. Short-term exercise preserves myocardial glutathione and decreases arrhythmias after thiol oxidation and ischemia in isolated rat hearts. J Appl Physiol. 2011;111:1751–1759. doi: 10.1152/JAPPLPHYSIOL.01214.2010/ASSET/IMAGES/LARGE/ZDG0121198610009.JPEG. [DOI] [PubMed] [Google Scholar]
- 34.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431. doi: 10.1038/NATURE13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robb E.L., Hall A.R., Prime T.A., Eaton S., Szibor M., Viscomi C., et al. Control of mitochondrial superoxide production by reverse electron transport at complex I. J Biol Chem. 2018;293:9869. doi: 10.1074/JBC.RA118.003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y., Bravo E., Thirnbeck C.K., Smith-Mellecker L.A., Kim S.H., Gehlbach B.K., et al. Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest. 2018;128:1141. doi: 10.1172/JCI94999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kloner R.A., Hale S.L., Dai W., Gorman R.C., Shuto T., Koomalsingh K.J., et al. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective peptide. J Am Heart Assoc. 2012;1 doi: 10.1161/JAHA.112.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Licheni S.H., Mcmahon J.M., Schneider A.L., Davey M.J., Scheffer I.E. Sleep problems in Dravet syndrome: a modifiable comorbidity. Dev Med Child Neurol. 2018;60:192–198. doi: 10.1111/DMCN.13601. [DOI] [PubMed] [Google Scholar]
- 39.Ryvlin P., Nashef L., Lhatoo S.D., Bateman L.M., Bird J., Bleasel A., et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 40.Brown D.A., Aon M.A., Frasier C.R., Sloan R.C., Maloney A.H., Anderson E.J., et al. Cardiac arrhythmias induced by glutathione oxidation can be inhibited by preventing mitochondrial depolarization. J Mol Cell Cardiol. 2010;48:673–679. doi: 10.1016/J.YJMCC.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuberi S.M. Sleep, oxygen saturation, and seizures in Dravet syndrome. Dev Med Child Neurol. 2018;60:118. doi: 10.1111/DMCN.13644. [DOI] [PubMed] [Google Scholar]
- 42.Devinsky O., Hesdorffer D.C., Thurman D.J., Lhatoo S., Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15:1075–1088. doi: 10.1016/S1474-4422(16)30158-2. [DOI] [PubMed] [Google Scholar]
- 43.Aon M.A., Cortassa S., Maack C., O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889. doi: 10.1074/JBC.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 45.Frasier C.R., Sloan R.C., Bostian P.A., Gonzon M.D., Kurowicki J., Lo Presto S.J., et al. Short-term exercise preserves myocardial glutathione and decreases arrhythmias after thiol oxidation and ischemia in isolated rat hearts. J Appl Physiol. 2011;111 doi: 10.1152/japplphysiol.01214.2010. [DOI] [PubMed] [Google Scholar]
- 46.Valassina N., Brusco S., Salamone A., Serra L., Luoni M., Giannelli S., et al. Scn1a gene reactivation after symptom onset rescues pathological phenotypes in a mouse model of Dravet syndrome. Nat Commun. 2022;13:1–18. doi: 10.1038/s41467-021-27837-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y., Rasmussen T.P., Koval O.M., Joiner M.L.A., Hall D.D., Chen B., et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat Commun. 2015;6:1 6: 1–13, 2015 doi: 10.1038/ncomms7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernández-Sada E., Silva-Platas C., Villegas C.A., Rivero S.L., Willis B.C., García N., et al. Cardiac responses to β-adrenoceptor stimulation is partly dependent on mitochondrial calcium uniporter activity. Br J Pharmacol. 2014;171:4207–4221. doi: 10.1111/BPH.12684/SUPPINFO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiely D.G., Cargill R.I., Lipworth B.J. Effects of hypercapnia on hemodynamic, inotropic, lusitropic, and electrophysiologic indices in humans. Chest. 1996;109:1215–1221. doi: 10.1378/chest.109.5.1215. [DOI] [PubMed] [Google Scholar]
- 50.Kanner A.M., Bicchi M.M. Antiseizure medications for adults with epilepsy: a review. JAMA. 2022;327:1269–1281. doi: 10.1001/jama.2022.3880. [DOI] [PubMed] [Google Scholar]
- 51.Brunklaus A., Ellis R., Reavey E., Forbes G.H., Zuberi S.M. Prognostic, clinical and demographic features in SCN1A mutation-positive Dravet syndrome. Brain. 2012;135:2329–2336. doi: 10.1093/brain/aws151. [DOI] [PubMed] [Google Scholar]
- 52.Gupta G., Dang L.T., O’Brien L.M., Shellhaas R.A. Parent-reported sleep profile of children with early-life epilepsies. Pediatr Neurol. 2022;128:9–15. doi: 10.1016/j.pediatrneurol.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cetica V., Chiari S., Mei D., Parrini E., Grisotto L., Marini C., et al. Clinical and genetic factors predicting Dravet syndrome in infants with SCN1A mutations. Neurology. 2017;88:1037–1044. doi: 10.1212/WNL.0000000000003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho N.T., Kroner B., Grinspan Z., Fureman B., Farrell K., Zhang J., et al. Comorbidities of rare epilepsies: results from the rare epilepsy network. J Pediatr. 2018;203:249–258.e5. doi: 10.1016/j.jpeds.2018.07.055. [DOI] [PubMed] [Google Scholar]
- 55.Skluzacek J.V., Watts K.P., Parsy O., Wical B., Camfield P. Dravet syndrome and parent associations: the IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52:95–101. doi: 10.1111/j.1528-1167.2011.03012.x. [DOI] [PubMed] [Google Scholar]
- 56.Gerbatin R.R., Augusto J., Boutouil H., Reschke C.R., Henshall D.C. Life-span characterization of epilepsy and comorbidities in Dravet syndrome mice carrying a targeted deletion of exon 1 of the Scn1a gene. Exp Neurol. 2022;354 doi: 10.1016/j.expneurol.2022.114090. [DOI] [PubMed] [Google Scholar]
- 57.Oguz K.K., Tezer I., Sanverdi E., Has A.C., Bilginer B., Dolgun A., et al. Effect of patient sex on white matter alterations in unilateral medial temporal lobe epilepsy with hippocampal sclerosis assessed by diffusion tensor imaging. AJNR Am J Neuroradiol. 2013;34:1010–1015. doi: 10.3174/ajnr.A3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frye C.A., Rhodes M.E. edited by Schwartzkroin PABT-E of BER. Academic Press; Oxford: 2009. HORMONES AND GENDER | male sex steroids and neuronal excitability; pp. 507–513. [Google Scholar]
- 59.Mejias-Aponte C.A., Jiménez-Rivera C.A., Segarra A.C. Sex differences in models of temporal lobe epilepsy: role of testosterone. Brain Res. 2002;944:210–218. doi: 10.1016/S0006-8993(02)02691-4. [DOI] [PubMed] [Google Scholar]
- 60.Frye C.A. Effects and mechanisms of progestogens and androgens in ictal activity. Epilepsia. 2010;51:135–140. doi: 10.1111/j.1528-1167.2010.02628.x. [DOI] [PubMed] [Google Scholar]
- 61.Velíšková J., Desantis K.A. Sex and hormonal influences on seizures and epilepsy. Horm Behav. 2013;63:267–277. doi: 10.1016/j.yhbeh.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai H.-W., Grant P.A., Rissman E.F. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J.-M., Konkle A.T.M., Zup S.L., McCarthy M.M. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCarthy M.M., Arnold A.P. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]