Abstract
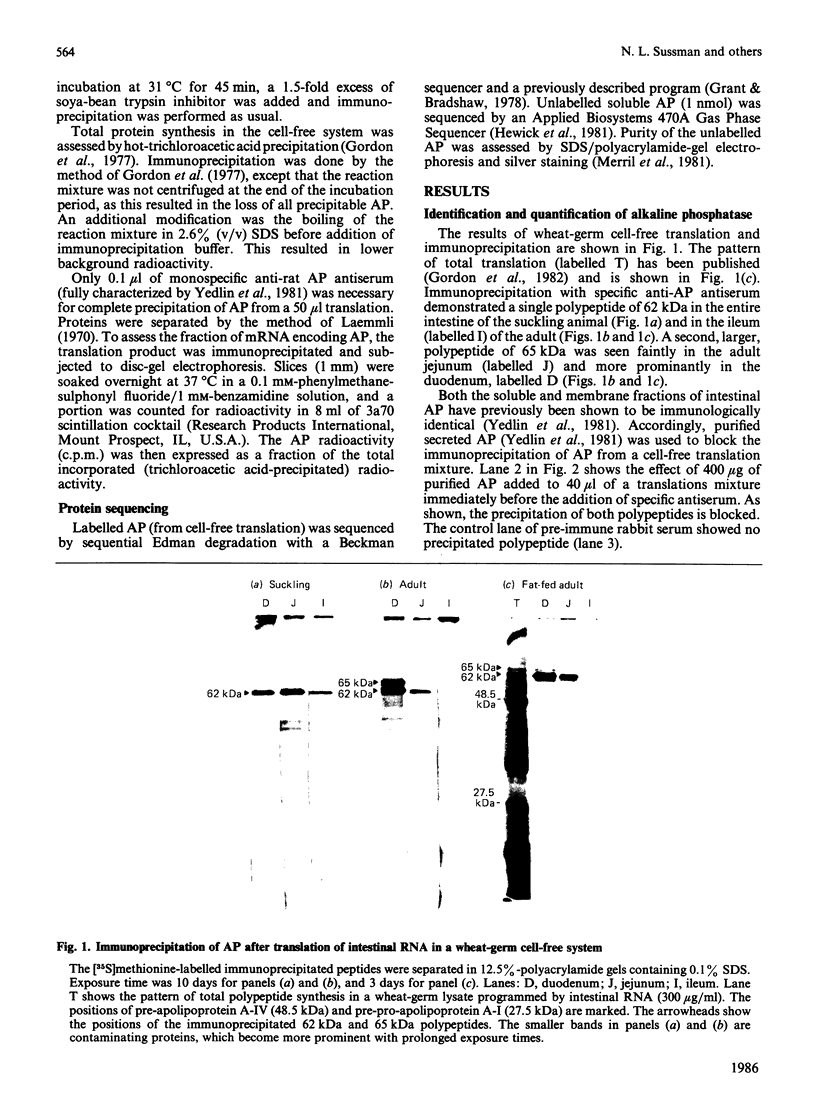
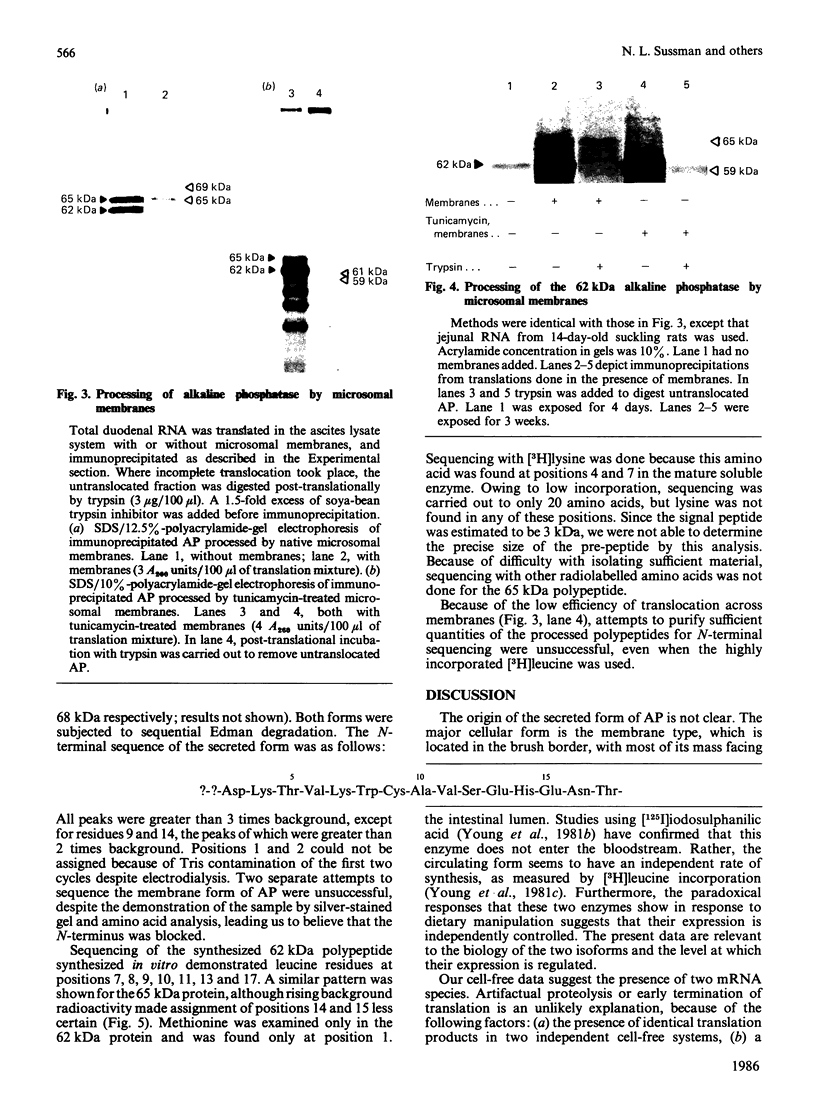
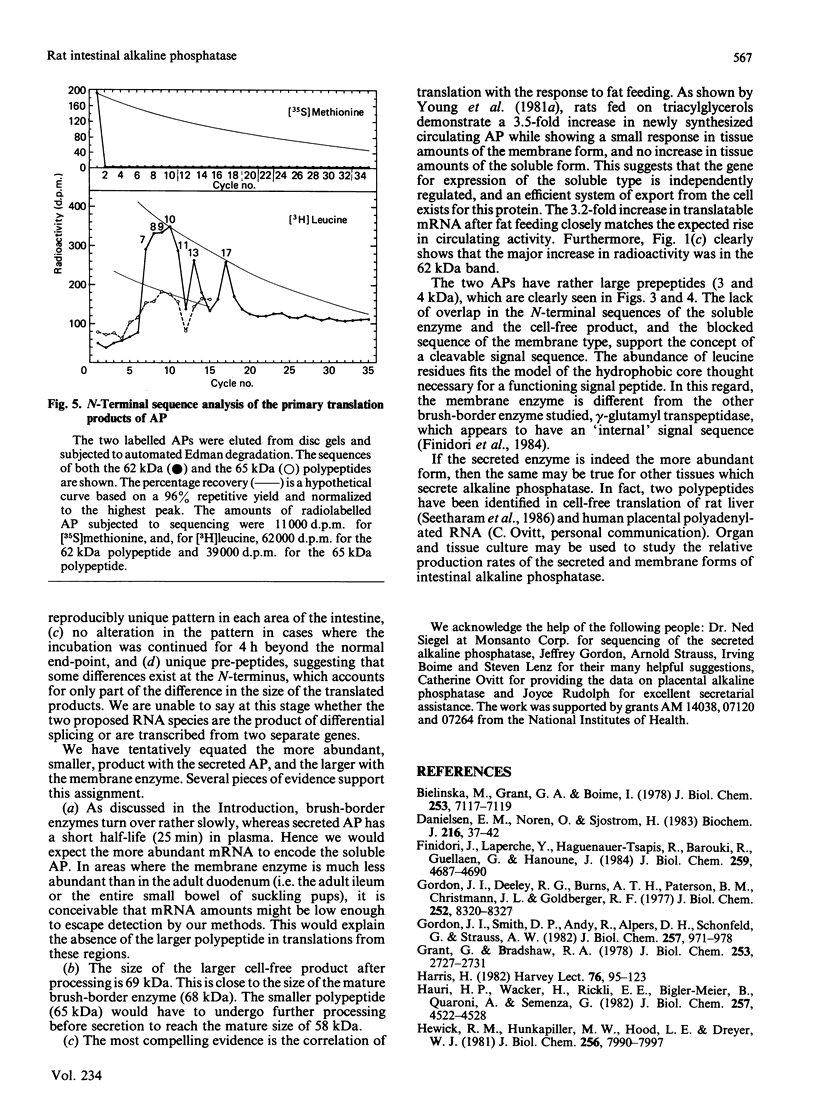
After translation of total rat intestinal RNA, immunoprecipitation using monospecific antiserum against rat intestinal alkaline phosphatase yielded two polypeptides in the adult duodenum and jejunum (molecular masses 62 and 65 kDa). Immunoprecipitation of both bands was blocked by a single purified alkaline phosphatase. In the adult ileum and in the entire small intestine of suckling pups, only the 62 kDa translation product was found. After fat feeding, translated alkaline phosphatase increased by an amount proportionate to the increase in enzyme activity previously seen in the serum. A small fraction of nascent alkaline phosphatase was translocated into microsomal vesicles, producing peptides of 65 and 69 kDa. Tunicamycin-treated membranes demonstrated a different signal peptide for each translation product. N-Terminal sequencing of the translation products showed leucine residues at similar positions, but overlap with the mature protein sequence was not demonstrated. On the basis of these data, we propose the presence of two mRNAs encoding alkaline phosphatase in the rat intestine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bielinska M., Grant G. A., Boime I. Processing of placental peptide hormones synthesized in lysates containing membranes derived from tunicamycin-treated ascites tumor cells. J Biol Chem. 1978 Oct 25;253(20):7117–7119. [PubMed] [Google Scholar]
- Danielsen E. M., Cowell G. M., Poulsen S. S. Biosynthesis of intestinal microvillar proteins. Role of the Golgi complex and microtubules. Biochem J. 1983 Oct 15;216(1):37–42. doi: 10.1042/bj2160037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finidori J., Laperche Y., Haguenauer-Tsapis R., Barouki R., Guellaen G., Hanoune J. In vitro biosynthesis and membrane insertion of gamma-glutamyl transpeptidase. J Biol Chem. 1984 Apr 25;259(8):4687–4690. [PubMed] [Google Scholar]
- Gordon J. I., Deeley R. G., Burns A. T., Paterson B. M., Christmann J. L., Goldberger R. F. In vitro translation of avian vitellogenin messenger RNA. J Biol Chem. 1977 Nov 25;252(22):8320–8327. [PubMed] [Google Scholar]
- Gordon J. I., Smith D. P., Andy R., Alpers D. H., Schonfeld G., Strauss A. W. The primary translation product of rat intestinal apolipoprotein A-I mRNA is an unusual preproprotein. J Biol Chem. 1982 Jan 25;257(2):971–978. [PubMed] [Google Scholar]
- Grant G. A., Bradshaw R. A. D-3-Phosphoglycerate dehydrogenase from chicken liver. II. Chemical and physical properties. J Biol Chem. 1978 Apr 25;253(8):2727–2731. [PubMed] [Google Scholar]
- Harris H. Multilocus enzyme systems and the evolution of gene expression: the alkaline phosphatases as a model example. Harvey Lect. 1980;76:95–124. [PubMed] [Google Scholar]
- Hauri H. P., Wacker H., Rickli E. E., Bigler-Meier B., Quaroni A., Semenza G. Biosynthesis of sucrase-isomaltase. Purification and NH2-terminal amino acid sequence of the rat sucrase-isomaltase precursor (pro-sucrase-isomaltase) from fetal intestinal transplants. J Biol Chem. 1982 Apr 25;257(8):4522–4528. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hortin G., Boime I. Transport of an uncleaved preprotein into the endoplasmic reticulum of rat pituitary cells. J Biol Chem. 1981 Feb 25;256(4):1491–1494. [PubMed] [Google Scholar]
- James W. P., Alpers D. H., Gerber J. E., Isselbacher K. J. The turnover of disaccharidases and brush border proteins in rat intestine. Biochim Biophys Acta. 1971 Feb 23;230(2):194–203. doi: 10.1016/0304-4165(71)90204-2. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehmann F. G. Human alkaline phosphatases. Evidence of three isoenzymes (placental, intestinal and liver-bone-kidney-type) by lectin-binding affinity and immunological specificity. Biochim Biophys Acta. 1980 Nov 6;616(1):41–59. doi: 10.1016/0005-2744(80)90262-4. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- McKenna M. J., Hamilton T. A., Sussman H. H. Comparison of human alkaline phosphatase isoenzymes. Structural evidence for three protein classes. Biochem J. 1979 Jul 1;181(1):67–73. doi: 10.1042/bj1810067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G., Gruenebaum J., Boime I. Reconstitution of a tandem Co- and post-translational processing pathway with rat liver subcellular fractions. J Biol Chem. 1982 Apr 25;257(8):4179–4186. [PubMed] [Google Scholar]
- Sabban E. L., Greene L. A., Goldstein M. Mechanism of biosynthesis of soluble and membrane-bound forms of dopamine beta-hydroxylase in PC12 pheochromocytoma cells. J Biol Chem. 1983 Jun 25;258(12):7812–7818. [PubMed] [Google Scholar]
- Seargeant L. E., Stinson R. A. Evidence that three structural genes code for human alkaline phosphatases. Nature. 1979 Sep 13;281(5727):152–154. doi: 10.1038/281152a0. [DOI] [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker H., Jaussi R., Sonderegger P., Dokow M., Ghersa P., Hauri H. P., Christen P., Semenza G. Cell-free synthesis of the one-chain precursor of a major intrinsic protein complex of the small-intestinal brush border membrane (pro-sucrase-isomaltase). FEBS Lett. 1981 Dec 28;136(2):329–332. doi: 10.1016/0014-5793(81)80647-3. [DOI] [PubMed] [Google Scholar]
- Yedlin S. T., Young G. P., Seetharam B., Seetharam S., Alpers D. H. Characterization and comparison of soluble and membranous forms of intestinal alkaline phosphatase from the suckling rat. J Biol Chem. 1981 Jun 10;256(11):5620–5626. [PubMed] [Google Scholar]
- Young G. P., Friedman S., Yedlin S. T., Allers D. H. Effect of fat feeding on intestinal alkaline phosphatase activity in tissue and serum. Am J Physiol. 1981 Dec;241(6):G461–G468. doi: 10.1152/ajpgi.1981.241.6.G461. [DOI] [PubMed] [Google Scholar]
- Young G. P., Yedlin S. T., Alpers D. H. Distribution of soluble and membranous forms of alkaline phosphatase in the small intestine of the rat. Biochim Biophys Acta. 1981 Aug 17;676(2):257–265. doi: 10.1016/0304-4165(81)90194-x. [DOI] [PubMed] [Google Scholar]
- Young G. P., Yedlin S. T., Alpers D. H. Independent biosynthesis of soluble and membrane-bound alkaline phosphatases in the suckling rat ileum. Biochem J. 1981 Dec 15;200(3):645–654. doi: 10.1042/bj2000645. [DOI] [PMC free article] [PubMed] [Google Scholar]