Abstract
The pathogenicity of individual rabies virus strains appears to correlate inversely with the extent of apoptotic cell death they induce and with the expression of rabies virus glycoprotein, a major inducer of an antiviral immune response. To determine whether the induction of apoptosis by rabies virus contributes to a decreased pathogenicity by stimulating antiviral immunity, we have analyzed these parameters in tissue cultures and in mice infected with a recombinant rabies virus construct that expresses the proapoptotic protein cytochrome c. The extent of apoptosis was strongly increased in primary neuron cultures infected with the recombinant virus carrying the active cytochrome c gene [SPBN-Cyto c(+)], compared with cells infected with the recombinant virus containing the inactive cytochrome c gene [SPBN-Cyto c(−)]. Mortality in mice infected intranasally with SPBN-Cyto c(+) was substantially lower than in SPBN-Cyto c(−)-infected mice. Furthermore, virus-neutralizing antibody (VNA) titers were significantly higher in mice immunized with SPBN-Cyto c(+) at the same dose. The VNA titers induced by these recombinant viruses paralleled their protective activities against a lethal rabies virus challenge infection, with SPBN-Cyto c(+) revealing an effective dose 20 times lower than that of SPBN-Cyto c(−). The strong increase in immunogenicity, coupled with the marked reduction in pathogenicity, identifies the SPBN-Cyto c(+) construct as a candidate for a live rabies virus vaccine.
Tissue culture-adapted laboratory and wild rabies virus strains differ greatly in their ability to cause a lethal rabies virus encephalitis (12). The pathogenicity of individual rabies virus strains for immunocompetent adult mice appears to correlate inversely with their capacity to induce cell death (14). For example, we have found that CVS-N2c, a highly pathogenic variant derived from the mouse-adapted CVS-24 rabies virus strain, induced significantly less apoptosis in primary hippocampal neuron cultures than did the less pathogenic variant CVS-B2c (14). Moreover, the extent of apoptosis correlated inversely with their levels of expression of rabies virus glycoprotein (G protein) (14), the major inducer of rabies virus-neutralizing antibodies. Highly pathogenic rabies viruses fail to elicit a protective immune response, whereas weakly pathogenic, tissue culture-adapted rabies viruses induce a strong antiviral response, in particular rabies virus-specific cytotoxic T cells (29–31) and G protein-specific virus-neutralizing antibody (VNA) (27), which are considered to be the major effectors in the immune defense against a lethal rabies virus infection (6). Together, these observations raise the possibility of a causal association between enhanced virus-induced cell death and increased antiviral immune responses. In this context, it has been suggested that virus-induced apoptosis may play a physiological role in protecting the central nervous system (CNS) from progression of infection by allowing contact between virus and immune system components (7). The findings that cell injury induces the release of endogenous adjuvants that stimulate cytotoxic T-cell responses (24) and that apoptotic cells induce maturation of dendritic cells and stimulate their presentation of antigen to both class I- and class II-restricted T cells (5, 16, 17) support the notion that the enhanced cell death induced by less pathogenic rabies viruses contributes to their immunogenicity. To test this hypothesis, we exploited recent advances in the use of rabies virus as an expression vector (22, 23) to construct a recombinant virus expressing cytochrome c, a proapoptotic protein that is essential for the proteolytic activity of Apaf-1 and for activation of caspases (8) and that induces accelerated apoptotic cell death when overexpressed (3). Cytochrome c is also highly conserved among species, such that any effect on the immunogenicity of a rabies virus strain in mice should be applicable to other target species. We demonstrate here that the expression of cytochrome c by a recombinant rabies virus is associated with accelerated cell death in vitro and enhanced immunogenicity and attenuated pathogenicity in vivo.
MATERIALS AND METHODS
Viruses and cells.
CVS-N2c and CVS-B2c are highly pathogenic and less pathogenic subclones, respectively, of the mouse-adapted CVS-24 rabies virus (13). The recombinant rabies virus SPBN was generated from a SAD B19 cDNA clone as described elsewhere (15, 22, 23). Neuroblastoma NA cells of A/J mouse origin were grown at 37°C in RPMI 1640 medium supplemented with 10% fetal bovine serum. BSR cells, a cloned cell line derived from BHK-21 cells were grown at 37°C in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Primary neuron cultures were prepared from the hippocampi of prenatal Swiss Webster mice as described previously (14).
Construction of recombinant rabies virus cDNA clones.
Total RNA was isolated from HeLa cells by the RNAzol B method (Biotex Laboratories, Inc., Houston, Tex.). The extracted RNA was reverse transcribed into cDNA by using avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.) as described previously (13). Human cytochrome c cDNA was amplified using Eppendorf Taq DNA polymerase (Fisher Scientific, Pittsburgh, Pa.) and primers Cyt 5 (5′-AAACGTACGAATATGGGTGATGTTGAGAA-3′ [BsiWI site underlined]) and Cyt 3 (5′-GAAGCTAGCTTACTCATTAGTAGCTTTTTTGAG-3′ [NheI site underlined]) to introduce BsiWI and NheI recognition sites before and after the cytochrome c coding region. The PCR product was digested with BsiWI and NheI (New England Biolabs) and ligated into rabies virus vector pSPBN, which had been digested with BsiWI and NheI (22). The resulting plasmid was designated pSPBN-Cyto c(+) (Fig. 1). To inactivate the cytochrome c gene, a stop codon was introduced into the coding sequence 70 bp after the start codon by amplifying a cytochrome c fragment with Vent polymerase (New England Biolabs) and primers Cyt 5 and Cyt stop 3 (5′-GTGGCACTGGGATCACTTCATAAT-3′). A second fragment was amplified with Vent polymerase using primer Cyt 3 and complementary primer Cyt stop 5 (5′-ATTATGAAGTGATCCCAGTGCCAC-3′). Both fragments were annealed and amplified by Vent polymerase using primers Cyt 5 and Cyt 3. The PCR product was digested and ligated into pSPBN as described above for pSPBN-Cyto c(+). The resulting plasmid was designated pSPBN-Cyto c(−) (Fig. 1). The sequences of both cytochrome c genes were confirmed by DNA sequencing.
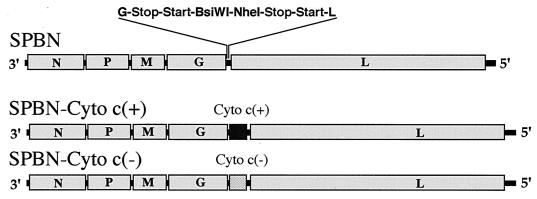
FIG. 1.
Schematic diagram of cytochrome c recombinant rabies viruses. The pSPBN vector was derived from SPBN-10 by removing the ψ gene and introducing BsiWI and NheI sites between the G and L genes. Human cytochrome c cDNA was amplified by PCR and, after introduction of BsiWI and NheI sites, ligated into pSPBN, resulting in pSPBN-Cyto c(+). To construct pSPBN-Cyto c(−), a stop codon was introduced into the cytochrome c gene 70 bp after the start codon.
Recovery of recombinant viruses.
Recombinant viruses were rescued as described previously (13, 14). Briefly, BSR-T7 cells were transfected using a calcium phosphate transfection kit (Stratagene, La Jolla, Calif.) with 5.0 μg of pSPBN-Cyto c(+) or pSPBN-Cyto c(−), 5.0 μg of pTIT-N, 2.5 μg of pTIT-P, 2.5 μg of pTIT-L, and 2.0 μg of pTIT-G. After a 3-day incubation, supernatants were transferred onto BSR cells and incubation was continued for 3 days at 37°C. The cells were examined for the presence of rescued virus by immunostaining with fluorescein isothiocyanate-labeled anti-rabies virus N protein antibody (Centocor, Malvern, Pa.). The correct nucleotide sequences of the inserted genes were confirmed by reverse transcription-PCR and DNA sequencing.
Virus infectivity assay.
Infectivity assays were performed at 34 or 37°C on monolayers of NA cells in 96-well plates as described previously (32). All titer determinations were carried out in triplicate.
Immunoprecipitation analysis.
BSR cells were infected with SPBN-Cyto c(−) or SPBN-Cyto c(+) at a multiplicity of infection (MOI) of 5 and 24 h later were incubated with 50 μCi of [35S]methionine/ml for 2 h at 37°C. A mixture of polyclonal antisera against rabies virus N and G protein and a polyclonal antiserum against cytochrome c was used for immunoprecipitation. The labeled immunocomplexes were adsorbed to protein A-Sepharose beads (rProtein A Sepharose TM Fast Flow; Amersham Pharmacia Biotech, Piscataway, N.J.) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15% polyacrylamide) as described previously (14). The gel was dried and exposed to X-ray film.
Determination of VNA.
Retro-orbital bleeding of mice was performed under isoflurane inhalation anesthesia. Not more than 100 μl of blood was collected from each mouse. Mouse sera were tested for the presence of VNA using the rapid fluorescent inhibition test (RFFIT) as described previously (32). The neutralization titers, defined as the inverse of the highest serum dilution that neutralizes 50% of the challenge virus, were normalized to international units (IU) using the World Health Organization (WHO) anti-rabies virus antibody standard. Geometric mean titers were calculated from individual titers in sera from 10 mice that received identical concentrations of the same vaccine virus. VNA GMT values obtained with the different vaccine dilutions were compared between vaccination groups in a paired-sample t test.
Immunofluorescence staining and in situ terminal end labeling of rabies virus-infected primary neuron cultures.
Primary neuron cultures prepared from the hippocampus of prenatal mice (14) were infected with SPBN-Cyto c(+) and SPBN-Cyto c(−) at a MOI of 5 and incubated at 37°C. For immunofluorescence analysis, infected neurons were fixed in 80% acetone at 24 h postinfection (p.i.) and stained with fluorescein isothiocyanate-labeled anti-rabies virus N protein-specific monoclonal antibody as described previously (14). To detect DNA strand breaks indicative of apoptotic cell death, the infected neurons were fixed with 4% paraformaldehyde at 24 h p.i. and subjected to the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay as described previously (14).
Pathogenicity studies in mice.
Groups of 8 to 10-week-old female C3H or Rag 2 mice (Taconic Farms) were infected intranasally (i.n.) with 25 μl containing 5 × 105 recombinant virus infectious particles. After infection, the mice were observed daily for 4 weeks for the appearance of clinical signs of rabies.
Immunohistochemical analysis.
At 10 days p.i, mice infected with SPBN, SPBN-Cyto c(+), or SPBN-Cyto c(−) (five mice per group) were anesthetized by intramuscular (i.m.) injection of 100 μl of ketamine-xylazine (1:1) and perfused transcardially with phosphate-buffered saline containing 20,000 IU of heparin per liter followed by Bouin-Holland solution as described previously (9). Brains were removed and postfixed for 24 h in the same fixative. After dehydration in a graded series of ethanol, the brains were embedded in paraffin, cut into 7-μm-thick coronal sections, and analyzed immunohistologically for rabies virus N protein as described previously (9).
Immunization and virus challenge.
Groups of 10 8- to 10-week-old female Swiss Webster mice (Taconic Farms) were inoculated i.m. with 100 μl of serial 10-fold dilutions of live recombinant rabies viruses. After 10 days, blood was collected from each mouse and the animals were infected intracranially (i.c.) under isoflurane anesthesia with 10 μl containing 100 50% lethal doses (LD50) of CVS-N2c. The mice were observed for 4 weeks for the development of clinical signs of rabies. Mice that showed definitive clinical signs of rabies such as paralysis, tremors, and spasms were euthanized by CO2 intoxication. Survivorship rates obtained with the different vaccine dilutions were compared between the different vaccination groups using a paired-sample t test. The 50% effective dose (ED50) was calculated as described previously (33). In another experiment, mice were immunized orally with 25 μl containing 106 FFU of recombinant virus by instillation into the buccal cavity (10 mice per group). Blood samples were obtained 2 weeks later, and VNA titers were determined.
RESULTS
Phenotypic characterization of recombinant viruses in vitro.
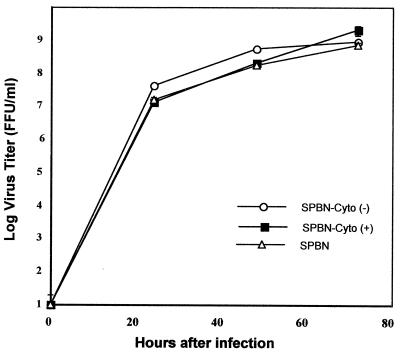
Comparison of the time course of parental and recombinant virus production in BSR cells (Fig. 2) revealed no substantial differences between SPBN-Cyto c(+), SPBN-Cyto c(−), and the parental strain SPBN, indicating that the insertion of foreign genes did not affect virus replication. Immunoprecipitation analysis of cytochrome c expression in infected BSR cells indicated markedly higher expression levels in SPBN-Cyto c(+)-infected cells than in SPBN-Cyto c(−)-infected cells, whereas no differences in N and G protein expression were detected (Fig. 3).
FIG. 2.
Rate of production of recombinant and parental rabies virus strains in BSR cells. Cells were infected with SPBN, SPBN-Cyto c(+), and SPBN-Cyto c(−) at a MOI of 5 and incubated at 37°C. Viruses were harvested on days 1, 2, and 3 after infection and subjected to titer determination by a fluorescence staining method. Data are given as the mean and standard error of six virus titer determinations.
FIG. 3.
Immunoprecipitation analysis of the rabies virus N protein and cytochrome c in mouse neuroblastoma cells infected with SPBN-Cyto c(−) (lane a) or SPBN-Cyto c(+) (lane b). Infected cells were labeled with 50 μCi of [35S]methionine per ml for 2 h at 37°C and then lysed, and 100 μl of lysate was subjected to immunoprecipitation with either polyclonal anti-rabies N protein (A) or anti-cytochrome c (B) antiserum. Immune complexes were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide). The gel was dried and exposed to X-ray film. MW, molecular weight (in thousands).
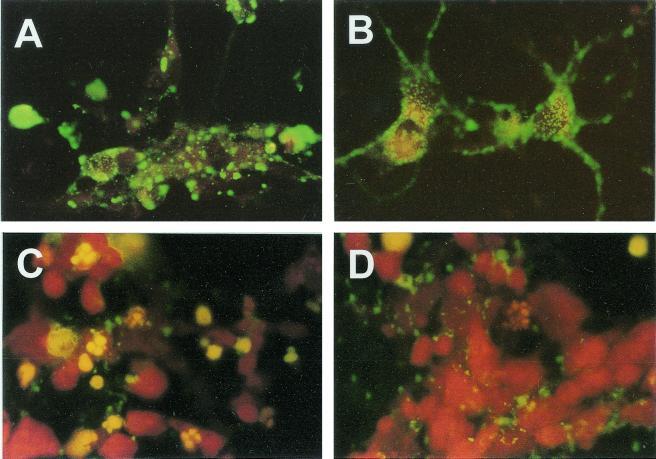
Immunofluorescence analysis of N protein expression in SPBN-Cyto c(+)-infected primary hippocampal neuron cultures at 24 h p.i. revealed large N protein-positive inclusion bodies in the cell body cytoplasm and almost no N protein-specific staining in neuronal processes (Fig. 4A). In contrast, SPBN-Cyto c(−)-infected neurons showed a more fine-granular N protein staining pattern which extended into the neuronal processes (Fig. 4B). TUNEL staining revealed a large number of TUNEL-positive nuclei in SPBN-Cyto c(+)-infected neurons at 24 h p.i. (Fig. 4C), whereas only a few TUNEL-positive nuclei were detected in SPBN-Cyto c(−)-infected neurons at that time (Fig. 4D).
FIG. 4.
Immunofluorescence analysis of the rabies virus N protein (A and B) and TUNEL analysis (C and D) of primary neuron cultures infected with SPBN-Cyto c(−) (B and D) or SPBN-Cyto c(+) (A and C) and examined 24 h p.i.
Effect of cytochrome c overexpression on pathogenicity.
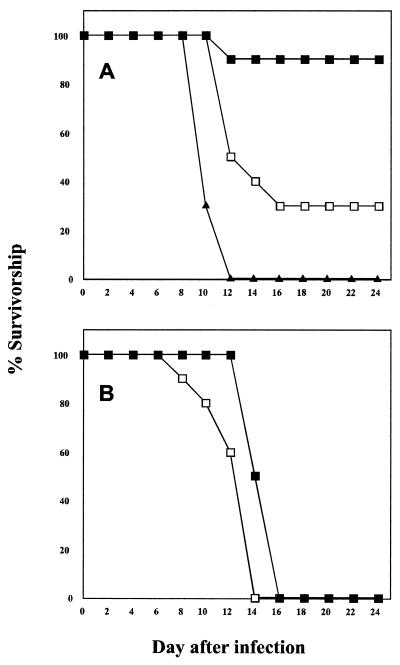
All rabies viruses used in this study were nonpathogenic for C3H mice when inoculated i.m. and were not detected in brain tissue by RT-PCR analysis when this route of infection was used (data not shown). On the other hand, comparison of the SPBN virus and the recombinant viruses for their ability to cause lethal rabies virus encephalitis in immunocompetent adult C3H mice after i.n. infection showed that 100 and 70% of mice infected with 106 FFU of SPBN or SPBN-Cyto c(−), respectively, succumbed to rabies virus infection whereas only 10% of mice infected i.n. with the same dose of SPBN-Cyto c(+) died from rabies encephalitis (Fig. 5A). In contrast to C3H mice, 100% of the Rag2 mice infected i.n. with either SPBN-Cyto c(−) or SPBN-Cyto c(+) succumbed to rabies virus infection (Fig. 5B), suggesting that an enhanced immune response is, at least in part, responsible for the reduced mortality rate caused by SPBN-Cyto c(+) in C3H mice following i.n. infection.
FIG. 5.
Survivorship of C3H mice (A) and Rag 2 mice (B) infected i.n. with 106 FFU of SPBN (triangles), SPBN-Cyto c(−) (open squares), or SPBN-Cyto c(+) (solid squares). Groups of 10 mice were infected with each virus and observed for 4 weeks for clinical signs of rabies.
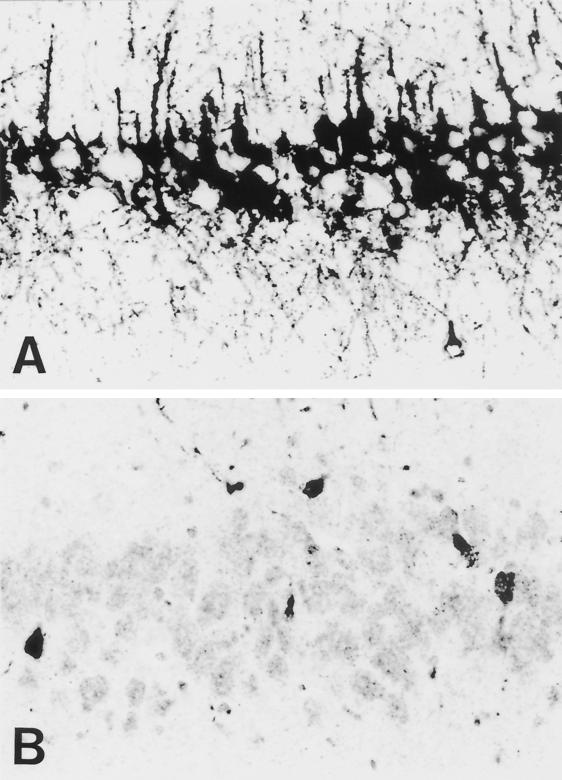
Immunohistological analysis was performed to determine whether the decrease in mortality seen after i.n. infection of immunocompetent C3H mice with SPBN-Cyto c(+) correlated with a change in the extent of virus spread within the brain. Three brains were examined for each mouse strain and recombinant virus strain on day 10 after i.n. infection. This analysis revealed only minor variations in immunostaining of mouse brain sections from three mice of a particular group, and the immunohistological analyses shown in Fig. 6 are representative of this group. Large numbers of N protein-positive neurons were present in almost all areas of C3H mouse brains infected with the SPBN virus (Fig. 6A). Although fewer infected neurons were detected in SPBN-Cyto c(−)-infected C3H mouse brains (Fig. 6B), the smallest number of infected neurons was observed in C3H mouse brains infected with SPBN-Cyto c(+) (Fig. 6C), as expected if a more potent immune response was active. High magnification of immunostained sections through the CA1 region of the hippocampus of SPBN-Cyto c(−)-infected C3H mouse brains showed intense N protein-specific immunoreactivity in both cell bodies and neuronal processes (Fig. 7A). In contrast, corresponding SPBN-Cyto c(+)-infected C3H mouse brain sections showed considerable weaker N protein staining of cell bodies, with no distinct axons or dentrites being visible (Fig. 7B).
FIG. 6.
Immunohistochemical analysis for rabies virus N protein expression in coronal sections through the hippocampus of C3H mice (A to C) and Rag 2 mice (D and E) infected i.n. with SPBN (A), SPBN-Cyto c(−) (B and D), or SPBN-Cyto c(+) (C and E). At 10 days p.i., brain sections were prepared and stained with a polyclonal antibody specific for rabies virus N protein (see Materials and Methods).
FIG. 7.
High magnification of N protein-immunostained sections through the CA1 region of the hippocampus of SPBN-Cyto c (−)-infected mice (A) and SPBN-Cyto c (+)-infected C3H mice (B) at 10 days p.i.
Immunohistological analysis of brains from B- and T-cell-deficient Rag 2 mice at 10 days p.i. revealed no differences in the extent of virus spread between the two recombinant viruses in the absence of an adaptive immune response (Fig. 6D and E). These data demonstrate that the different mortality rates resulting from infection of C3H mice with the parental and recombinant viruses correlate with their ability to spread within the brain following i.n. infection, which is, in turn, associated with their immunogenicity.
Effect of cytochrome c overexpression on immunity.
Analysis of the VNA responses in mice inoculated i.m. with serial dilutions of SPBN-Cyto c(+) or SPBN-Cyto c(−) indicated higher (on average 3.5-fold) geometric mean VNA titers induced by SPBN-Cyto(+) (Table 1; Fig. 8A). Paired sample t-test analysis of the VNA titers shown in Fig. 8A indicated that the differences in VNA titers were highly significant (P = 0.016). In mice vaccinated with serial dilutions of SPBN-Cyto c(+) or SPBN-Cyto c(−) and challenged i.c. with 100 LD50 of CVS-24 virus (Fig. 8B), survivorship was significantly higher (on average, 1.7-fold; P = 0.009) in the SPBN-Cyto c(+)-immunized mice (Fig. 8B). The ED50 calculated from the mortality rates in the different vaccine dilution groups (Fig. 8C) indicated a 20-fold higher efficacy of SPBN-Cyto c(+) than of SPBN-Cyto c(−), clearly demonstrating that overexpression of cytochrome c by a recombinant rabies virus strongly enhances protective immunity against rabies. The enhanced immune response to SPBN-Cyto c(+) compared with SPBN-Cyto c(−) was further demonstrated after oral and i.n. immunization of C3H mice. While 10 days after oral immunization the geometric mean VNA titer induced by SPBN-Cyto c(+) was 10.4 IU (range, 2 to 18 IU), the same amount of SPBN-Cyto c(−) resulted in a geometric mean VNA titer of only 1.25 IU (range, 0.2 to 4 IU) (Fig. 9). The geometric mean titers on day 7 after i.n. immunization were 1.4 IU (range, 0.4 to 6.0 IU) for SPBN-Cyto c(+)-immunized mice and 0.3 IU (range, 0.1 to 1.3 IU) for the mice that received SPBN-Cyto c(−).
TABLE 1.
VNA response to immunization with recombinant rabies virus
| Virus concna (log FFU × 3) | Mean VNA GMT (range) (IU)b
|
|
|---|---|---|
| SPBN-Cyto c(+) | SPBN-Cyto c(−) | |
| 2 | 64.9 (36–324) | 33.9 (12–36) |
| 3 | 56.5 (36–108) | 24.6 (12–36) |
| 4 | 29.0 (12–36) | 6.6 (0.4–12) |
| 5 | 18.2 (12–36) | 2.6 (0–12) |
| 6 | 6.7 (0.4–12) | 2.2 (0–12) |
Mice were immunized i.m., and blood was obtained 10 days later.
Serum VNA titers were determined using RFFIT (32).
FIG. 8.
Immunogenicity of SPBN-Cyto c(+) and SPBN-Cyto c(−) after i.m. immunization of mice. (A) Groups of 10 mice were injected with serial 10-fold dilutions of the recombinant rabies viruses. After 10 days, blood samples were obtained and VNA titers of mice immunized with SPBN-Cyto c(+) (solid squares) or SPBN-Cyto c(−) (open squares) were determined using RFFIT (31) and CVS-B2c as challenge virus. Titers were normalized to IU using the WHO standard and are given as geometric mean titers. (B) Two weeks after immunization, the mice were infected i.c. with 100 LD50 of CVS-N2c and observed for 4 weeks, and survivorship in mice immunized with SPBN Cyto c(+) (solid bars) and SPBN-Cyto c(−) (shaded bars) were recorded. (C) The ED50 were calculated from the survivorship rates in the two vaccination groups as described previously (32).
FIG. 9.
Induction of VNA in C3H mice immunized orally with SPBN-Cyto c(+) or SPBN-Cyto c(−). A 25-μl volume containing 106 FFU of each virus was instilled into the buccal cavity of groups of 10 mice. After 2 weeks, blood samples were obtained and VNA titers were determined using RFFIT (32). VNA titers were normalized to IU using the WHO standard and are presented as geometric mean titers.
DISCUSSION
This study is based on the almost counterintuitive concept that rabies viruses inducing greater cell death are actually less pathogenic for the infected animal because of increased immunogenicity. To determine whether acceleration of the apoptotic process enhances antiviral immune responses and attenuates the pathogenicity of a rabies virus, we inserted the gene encoding the proapoptotic cytochrome c protein into the rabies virus genome. Expression of cytochrome c was markedly higher in cells infected with the recombinant virus carrying the active cytochrome c gene [SPBN-Cyto c(+)] than in cells infected with the inactive cytochrome c gene construct [SPBN-Cyto c(−)]. Furthermore, the level of apoptosis was strongly increased in primary neuron cultures infected with SPBN-Cyto c(+) compared with that in cultures infected with SPBN-Cyto c(−), consistent with previous observations indicating that overexpression of cytochrome c results in accelerated apoptosis (3). Because the two constructs had similar replication rates, the enhanced apoptosis in SPBN-Cyto c(+)-infected neurons is most probably a direct consequence of cytochrome c overexpression as opposed to the heightened expression of a potentially proapoptotic viral product such as G protein. Morphological differences in the distribution of rabies virus N protein were also evident in cells infected with the two recombinant viruses, with SPBN-Cyto c(−)-infected neurons showing a constant fine granular staining pattern that extended into the neuronal processes while SPBN-Cyto c(+)-infected neurons showed large N protein-positive inclusion bodies and almost no N protein staining in neuronal processes. The failure to translocate N protein to the periphery of SPBN-Cyto c(+)-infected neuronal cells may be linked to the apoptotic process, which results in the depolymerization of the actin filaments, which are required for intracellular transport of the N protein (4, 14).
Consistent with a previous comparison of CVS-24 variants (14), the more cytopathic virus, SPBN-Cyto c(+), caused substantially less mortality following i.n. infection than did SPBN-Cyto c(−), even though the latter was somewhat less pathogenic than the SPBN vector. These results point to a causal relationship between the induction of apoptosis and the marked reduction in pathogenicity associated with SPBN-Cyto c(+). Since the low mortality caused by SPBN-Cyto c(+) is paralleled by a strong reduction in the capacity to invade the CNS, the increased induction of apoptosis probably interferes with the progression of the infection into the CNS. Given the comparable replicative abilities of SPBN-Cyto c(+) and SPBN-Cyto c(−), the limited spread of infection observed in SPBN-Cyto c(+) infection might be due to apoptosis of infected neurons before the virus can spread to adjoining noninfected neurons or might be due to an apoptosis-driven enhanced immune response that clears the virus before it spreads in the CNS.
Mice immunized i.m. with SPBN-Cyto c(+) developed VNA titers that were, on average, threefold higher than those in mice immunized with the same concentration of SPBN-Cyto c(−), regardless of the route of administration or the quantity of infectious recombinant virus particles used for immunization. The higher VNA titers in i.m. SPBN-Cyto c(+)-immunized mice conferred greater protection against a lethal i.c. challenge infection with the highly pathogenic rabies virus strain CVS-N2c. Survivorship was significantly higher in mice immunized with SPBN-Cyto c(+) than in mice that received SPBN-Cyto c(−), with the ED50 being 20 times higher in the SPBN-Cyto c(+)-immunized group. Thus, the immunogenicity of a live rabies vaccine virus can be significantly enhanced by increasing its capacity to induce apoptosis, without modifying the production of viral proteins. The protective immune response to rabies virus involves both G protein-specific VNA and cellular immune mechanisms. While other studies have focused largely on the effects of apoptosis on cellular aspects of immunity (5, 16, 17, 24), our results show that antibody responses are also enhanced by the apoptosis of infected cells. Since neither SPBN-Cyto c(−) nor SPBN-Cyto c(+) invades the brains of immunocompetent mice after i.m. inoculation, we must conclude that these viruses replicate in peripheral tissues such as muscle cell or neuronal cells of the dorsal root ganglia, where contact with professional antigen-presenting cells (APCs) is possible. Because apoptotic bodies have an exceptional ability to deliver immunogens to APCs (21), the enhanced apoptogenic property of SPBN-Cyto c(+) most probably accounts for its increased immunogenicity. In this case, virus replication in the CNS, where professional APCs are not resident, is probably not directly relevant to the initiation of a strong antiviral immune response.
The strong increase in immunogenicity, coupled with the marked reduction in pathogenicity, makes SPBN-Cyto c(+) a candidate for a live rabies virus vaccine. To be suitable for vaccination of wildlife, a rabies virus vaccine must be effective and readily administrable. Increasing the immunogenicity of a modified live rabies virus vaccine could have great significance in reducing the amount of virus required to successfully immunize wildlife and stray dogs. Rabies is a major zoonotic disease and remains an important public health concern, causing approximately 60,000 annual deaths worldwide (10). In most developing countries, dogs represent the major reservoir of rabies virus (11). However, the situation in the Americas is much more complex, since large reservoirs of rabies viruses exist in many wild-animal species (20). Oral immunization of wildlife with live vaccines such as the modified live rabies virus vaccines SAD B19, SAG-1, and SAG-2 or the vaccinia virus-rabies virus glycoprotein recombinant virus vaccine VRG is the most effective method of controlling and eventually eradicating rabies in terrestrial wildlife (28). Vaccination with modified live rabies virus vaccines has resulted in almost complete eradication of vulpine rabies in Western Europe (1, 2, 27). On the other hand, while these vaccines induce protective immunity in foxes, neither SAD- nor ERA-based modified live rabies vaccines nor recombinant vaccinia viruses work well in skunks (19, 25, 26) or dogs (18). Administration of more than 109 infectious virus particles is required for minimum effect in dogs in the laboratory (18), and the widespread use of this amount of material is currently beyond feasible commercial production capacities. Furthermore, current modified live rabies virus vaccines may actually cause disease (19). Our results demonstrate that a live rabies virus vaccine strain, modified using reverse genetics technology to express the proapoptotic protein cytochrome c, is considerably more immunogenic without changing antigenically and spreads less readily to the CNS. These findings not only help to explain why rabies viruses that are potent inducers of apoptosis in infected neuron cultures are less pathogenic in animals but also provide the foundation for the development of safer and more effective wildlife rabies vaccines.
ACKNOWLEDGMENTS
We thank Suchita Santosh Hodawadekar, Elke-Rodenberg-Frank, Petra Sack, Marion Zibuschka, and Heidemarie Schneider for excellent technical help.
This work was supported by Public Health Service grant AI45097. E.W. was supported by SFB 297 and the Volkswagen-Stiftung.
REFERENCES
- 1.Aubert M F A, Masson E, Artois M, Barrat J. Oral wildlife rabies vaccination field trials in Europe with recent emphasis on France. In: Rupprecht C E, Dietzschold B, Koprowski H, editors. Lyssaviruses. Berlin, Germany: Springer-Verlag KG; 1994. pp. 219–243. [DOI] [PubMed] [Google Scholar]
- 2.Blancou J, Meslin F-X. Modified live virus vaccines for oral immunization of carnivores. In: Meslin F-X, Kaplan M M, Koprowski H, editors. Laboratory techniques in rabies. Geneva, Switzerland: World Health Organization; 1991. pp. 324–337. [Google Scholar]
- 3.Bradham C A, Quian T, Streetz K, Trautwein C, Brenner D A, Lemasters J J. The mitochondrial permeability transition is required for tumor necrosis factor alpha-mediated apoptosis and cytochrome c release. Mol Cell Biol. 1998;18:6353–6364. doi: 10.1128/mcb.18.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceccaldi P-E, Valtorta F, Braud S, Hellio R, Tsiang H. Alteration of the actin-based cytoskeleton by rabies virus. J Gen Virol. 1997;78:2831–2835. doi: 10.1099/0022-1317-78-11-2831. [DOI] [PubMed] [Google Scholar]
- 5.Chattergoon M A, Kim J J, Yang J S, Robinson T M, Lee D J, Dentchev T, Wilson D M, Ayyavoo V, Weiner D B. Targeted antigen delivery to antigen-presenting cells including dendritic cells by engineered Fas-mediated apoptosis. Nat Biotechnol. 2000;18:974–979. doi: 10.1038/79470. [DOI] [PubMed] [Google Scholar]
- 6.Cox J H, Dietzschold B, Schneider L G. Rabies virus glycoprotein II. Biological and serological characterization. Infect Immun. 1977;16:754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galelli A, Baloul L, Lafon M. Abortive rabies virus central nervous infection is controlled by T lymphocyte local recruitment and induction of apoptosis. J Neurovirol. 2000;6:359–372. doi: 10.3109/13550280009018300. [DOI] [PubMed] [Google Scholar]
- 8.Harvey N L, Kumar S. The role of caspases in apoptosis. Adv Biochem Eng Biotechnol. 1998;62:107–128. doi: 10.1007/BFb0102307. [DOI] [PubMed] [Google Scholar]
- 9.Hooper D C, Morimoto M, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998;72:3711–3719. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez L. Global infectious disease surveillance. Int J Infect Dis. 2000;4:222–228. doi: 10.1016/s1201-9712(00)90114-0. [DOI] [PubMed] [Google Scholar]
- 11.Meslin F-X, Fishbein D B, Matter H C. Rationale and prospects for rabies elimination in developing countries. In: Rupprecht C E, Dietzschold B, Koprowski H, editors. Lyssaviruses. Berlin, Germany: Springer-Verlag KG; 1994. pp. 1–26. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto K, Foley H D, McGettigan J P, Schnell M J, Dietzschold B. Reinvestigation of the role of the rabies virus glycoprotein in viral pathogenesis using a reverse genetics approach. J Neurovirol. 2000;6:373–381. doi: 10.3109/13550280009018301. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto K, Hooper D C, Carbaugh H, Fu Z F, Koprowski H, Dietzschold B. Rabies virus quasispecies: implications for pathogenesis. Proc Natl Acad Sci USA. 1998;95:3152–3156. doi: 10.1073/pnas.95.6.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morimoto K, Hooper D C, Spitsin S, Koprowski H, Dietzschold B. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J Virol. 1999;73:510–517. doi: 10.1128/jvi.73.1.510-518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morimoto K, McGettigan J P, Foley H D, Hooper D C, Dietzschold B, Schnell M J. Genetic engineering of live rabies vaccines. Vaccine. 2001;19:3543–3551. doi: 10.1016/s0264-410x(01)00064-0. [DOI] [PubMed] [Google Scholar]
- 16.Restifo N P. Building better vaccines: how apoptotic cell death can induce inflammation and active innate and adaptive immunity. Curr Opin Immunol. 2000;12:597–603. doi: 10.1016/s0952-7915(00)00148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rovere P, Vallinoto C, Bodanza A, Crosti M C, Rescigno M, Castagnoli P R, Rugarli C, Manfredi A A. Cutting edge: bystander apoptosis triggers dentritic cell maturation and antigen-presenting function. J Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- 18.Rupprecht C E, Kieny M-P. Development of a vaccinia-rabies glycoprotein recombinant virus vaccine. In: Campbell J B, Charlton K M, editors. Rabies. Boston, Mass: Kluwer Academic Publishers; 1988. pp. 335–364. [Google Scholar]
- 19.Rupprecht C E, Charlton K M, Artois M, Casey G A, Webster W A, Campbell J B, Lawson K F, Schneider L G. Ineffectiveness and comparative pathogenicity of attenuated rabies virus vaccines for the striped skunk (Mephitis mephitis) J Wildl Dis. 1990;26:99–102. doi: 10.7589/0090-3558-26.1.99. [DOI] [PubMed] [Google Scholar]
- 20.Rupprecht C E, Smith J S, Fekadu M, Childs J E. The ascension of wildlife rabies: a cause for public health concern or intervention? Emerg Infect Dis. 1995;1:107–114. doi: 10.3201/eid0104.950401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki S, Amara R R, Oran A E, Smith J M, Robinson H L. Apotosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat Biotechnol. 2001;19:543–547. doi: 10.1038/89289. [DOI] [PubMed] [Google Scholar]
- 22.Schnell M J, Foley H D, Siler C A, McGettigan J P, Dietzschold B, Pomerantz R J. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc Natl Acad Sci USA. 2000;97:3544–3549. doi: 10.1073/pnas.050589197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnell M J, Mebatsion T, Conzelmann K-K. Infectious rabies viruses from cloned cDNA. EMBO J. 1994;13:4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Zheng W, Rock K L. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cells. Proc Natl Acad Sci USA. 2000;97:14590–14595. doi: 10.1073/pnas.260497597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolson N D, Charlton K M, Lawson K F, Campbell J B, Wiktor T J. Immune response in skunks to a vaccinia virus recombinant expressing the rabies virus glycoprotein. Can J Vet Res. 1987;51:363–366. [PMC free article] [PubMed] [Google Scholar]
- 26.Tolson N D, Charlton K M, Lawson K F, Campbell J B, Stewart R B. Studies of ERA/BHK-21 rabies vaccine in skunks and mice. Can J Vet Res. 1988;52:58–62. [PMC free article] [PubMed] [Google Scholar]
- 27.Tolson N D, Charlton K M, Stewart R B, Casay G A, Webster W A, MacKenzie K, Campbell J B, Lawson K F. Mutants of rabies viruses in skunks: immune response and pathogenicity. Can J Vet Res. 1990;54:178–183. [PMC free article] [PubMed] [Google Scholar]
- 28.Wandeler A I, Capt S, Kappeler A, Hauser R. Oral immunization of wildlife against rabies: practical experiences and field experiments. Rev Infect Dis. 1988;10(Suppl. 4):649–653. doi: 10.1093/clinids/10.supplement_4.s649. [DOI] [PubMed] [Google Scholar]
- 29.Wiktor T J. Cell-mediated immunity and postexposure protection from rabies by inactivated vaccines of tissue culture origin. Dev Biol Stand. 1978;40:255–264. [PubMed] [Google Scholar]
- 30.Wiktor T J, Doherty P C, Koprowski H. In vitro evidence of cell-mediated immunity after exposure of mice to both live and inactivated rabies virus. Proc Natl Acad Sci USA. 1977;74:334–338. doi: 10.1073/pnas.74.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiktor T J, Doherty P C, Koprowski H. Suppression of cell-mediated immunity by street rabies virus. J Exp Med. 1977;145:1617–1622. doi: 10.1084/jem.145.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiktor T J, MacFarlan R I, Foggin C M, Koprowski H. Antigenic analysis of rabies and Mokola virus from Zimbabwe using monoclonal antibodies. Dev Biol Stand. 1984;57:199–221. [PubMed] [Google Scholar]
- 33.Wilbur L A, Aubert M F A. The NIH test for potency. In: Meslin F-X, Kaplan M M, Koprowski H, editors. Laboratory techniques in rabies. Geneva, Switzerland: World Health Organization; 1996. pp. 360–368. [Google Scholar]