Abstract
Mast cells are critical components of innate and adaptive immunity that differentiate in tissues in situ from circulating committed progenitor cells. We now demonstrate that human cord blood-derived mast cell progenitors are susceptible to infection with macrophagetropic (M-tropic) and dualtropic human immunodeficiency virus type 1 (HIV-1) isolates but not with T-cell-tropic (T-tropic) strains. Mast cell progenitors (c-kit+ CD13+ cells with chloroacetate esterase activity) were purified from 4-week-old cultures of cord blood mononuclear cells maintained in stem cell factor, interleukin-6 (IL-6), and IL-10 using a CD14 depletion column. These progenitors expressed CCR3, CCR5, and CXCR4, as well as low levels of CD4. When infected in vitro with viruses pseudotyped with different HIV and simian immunodeficiency virus envelope glycoproteins, only M-tropic and dualtropic, but not T-tropic, viruses were able to enter mast cell progenitors. Both the CCR5-specific monoclonal antibody 2D7 and TAK-779, a nonpeptide inhibitor of CCR5-mediated viral entry, blocked HIV-1 strain ADA infection by >80%. Cultures infected with replication-competent virus produced progressively increasing amounts of virus for 21 days as indicated by p24 antigen detection. Mast cell progenitors that were exposed to an M-tropic, green fluorescent protein-expressing HIV-1 strain exhibited fluorescence indicative of viral entry and replication on a single-cell level and retained virus production during differentiation. The trafficking of mast cell progenitors to multiple tissues, combined with the long life span of mature mast cells, suggests that they could provide a widespread and persistent HIV reservoir in AIDS.
Mast cells (MC) are immune effector cells residing in perivascular connective tissues and mucosal surfaces (22). They arise in situ by a stem cell factor (SCF)-dependent mechanism from a population of bone marrow-derived circulating committed progenitors (PrMC) expressing c-kit and the membrane-associated aminopeptidase CD13 (17) and lacking the monocyte-associated lipopolysaccharide receptor subunit, CD14 (1). In mice, PrMC reside constitutively in the intestine, permitting the rapid development of an MC hyperplasia driven by T-cell-derived factors elaborated in response to infections with helminthic parasites (32). This MC response is required for the normal elimination of adult helminthic worms (19). Other experimental evidence supports an essential role for resident MC in innate immune responses to gram-negative bacteria (22). Thus, MC participate in both innate and adaptive protective responses, in addition to their established role in inflammation and allergy (5).
Information about PrMC homing determinants is limited due to their small numbers in blood in vivo (17). The culture of human cord blood mononuclear cells (CBMNC) in the presence of the triad of recombinant soluble SCF, interleukin-6 (IL-6), and IL-10 (the latter for inhibition of monocyte/macrophage growth) gave rise to cultures primarily composed of human (h)PrMC, characterized by a c-kit+ CD13+ cytofluorographic profile, by a requirement for SCF to achieve maximal thymidine incorporation, and by a lack of responses to macrophage colony-stimulating factor granulocyte (M-CSF), G-CSF, and IL-2 (26). The hPrMC exhibited cytoplasmic staining for chloroacetate esterase (CAE), a marker of the myeloid and MC lineages that does not stain monocytes, macrophages, or basophils. By 9 weeks, these hPrMC differentiated into mature, fully functional mature hMC containing strongly CAE-positive secretory granules. hPrMC expressed four functional chemokine receptors: CXCR2, CCR3, CXCR4, and CCR5. Both CCR3 and CCR5 can serve as coreceptors for macrophage-tropic (M-tropic) and dualtropic human immunodeficiency virus (HIV) strains on CD4+ cells (6), such as macrophages and dendritic cells; CXCR4 serves as a coreceptor with CD4 for T-cell-tropic (T-tropic) HIV strains (8). Since hPrMC also express CD4 (26), these findings led us to investigate whether HIV strains might enter hPrMC and replicate.
In this study, we demonstrate that hPrMC derived in vitro can be infected by M-tropic HIV strains via a CCR5-dependent mechanism but not by CXCR4-utilizing T-tropic strains. Furthermore, infected hPrMC support M-tropic HIV-1 replication over sustained periods. Because hPrMC traffic to multiple tissues and abound in tissues where HIV and simian immunodeficiency virus (SIV) infections are initiated (13, 23, 32), the findings may have implications for the pathophysiology of the resultant diseases.
MATERIALS AND METHODS
Isolation and culture of hPrMC and hMC.
Cord blood obtained from human placentas after routine cesarean section was sedimented with dextran, and the mononuclear cell (CBMNC) fraction was isolated by centrifugation through a cushion of Ficoll-Paque (1.77 g/ml) at 350 × g for 30 min. CBMNC were cultured at 106/ml in RPMI 1640 medium (Gibco BRL, Gaithersburg, Md.) containing 10% fetal bovine serum, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 100 U of penicillin per ml, 100 mg of streptomycin per ml, 2 μg of gentamicin per ml (all from Sigma, St. Louis, Mo.), 0.2 μM 2-mercaptoethanol (Gibco BRL), 100 ng of SCF (Amgen, Thousand Oaks, Calif.) per ml, 50 ng of IL-6 (R & D Systems, Minneapolis, Minn.) per ml, and 10 ng of IL-10 (Endogen, Cambridge, Mass.) per ml, hPrMC were harvested at 4 weeks due to their expression of CAE, c-kit, and CD13, and lack of proliferative responses to IL-2, G-CSF, and M-CSF at this time point, as previously reported (26). For experiments performed with mature hMC, the cultures were carried to 9 weeks, by which time more than 98% of the cells were toluidine blue and tryptase positive.
To eliminate contaminating monocytes/macrophages and hMC (29), the crude 4-week hPrMC preparations were depleted of CD14+ cells with magnetic cell separation microbeads (Miltenyi Biotec, Sunnyvale, Calif.). Flow cytometry confirmed the virtually complete lack of the CD14 marker after the column purification. The CD14− cells were stained for CAE, for metachromasia with toluidine blue (26), and Astra blue (9) prior to studies involving infection to ensure relative uniformity. In some experiments, the CD14+ cells were similarly assessed after their recovery from the column.
Reagents and antibodies.
Anti-CCR5 antibody (Ab) 2D7 (immunoglobulin G2a [IgG2a]), anti-CXCR4 Ab 12G5 (IgG2a), anti-CD13 (IgG1), anti-CD4 (IgG1 from PharMingen), anti-CD14 (IgG2a), and recombinant SDF1α were purchased from PharMingen (San Diego, Calif.). The CCR3-specific Ab 7B11 was obtained from the AIDS Research and Reagents Program (National Institutes of Health Bethesda, Md.). Anti-c-kit (K69, IgG1) was purchased from Biosource International (Camarillo, Calif.). Anti-CD13 (IgG1) and anti-CD14 (IgG2a) were purchased from PharMingen. TAK-779 was provided by Takeda Chemical Industries (Osaka, Japan). Azidothymidine (AZT) was purchased from Sigma. The concentrations of the inhibitors used were chosen based on doses previously shown to reduce HIV entry (2, 4, 12, 31).
Flow cytometry and staining of hPrMC.
Flow cytometry was carried out in the presence of cold Hanks' balanced salt solution containing 2% fetal bovine serum, 0.1% human serum, and 0.01% sodium azide (fluorescence-activated cell sorter [FACS] buffer). After fixation for 5 min in FACS buffer containing 3% paraformaldehyde and exposure to monoclonal Abs, the cells were stained with fluorescein isothiocyanate-conjugated sheep anti-mouse IgG (Calbiochem, La Jolla, Calif.) and then analyzed using FACSort (Becton Dickinson, Oxnard, Calif.). The results were analyzed as overlaid histograms. Low-level expression of c-kit and CD13 and lack of CD14 were used to identify hPrMC, as described previously (26), while mature hMC were identified based on a high level of c-kit staining, continued expression of CD13, positive CD14 expression, and a higher relative level of side-angle light scatter (SSC).
HIV-1 replication assay.
Replication-competent recombinant NL4-3-ADA and NL4-3-HXBc2 were generated in HeLa cells as described previously (18). hPrMC (4 × 106) were incubated with 105 cpm of reverse transcriptase (RT) activity for 12 h, washed three times with phosphate-buffered saline (PBS), and cultured in 2.5 ml of complete medium with cytokines. Samples (0.5 ml) were removed every third day and replaced by an equal volume of fresh medium with cytokines, and the nonadherent cells were transferred to a new culture vessel. Virus production was determined by measurement of p24gag in the medium with a commercial antigen capture assay kit (NEN Life Science Products, Boston, Mass.).
Env complementation assay.
HIV-1 proviral DNA lacking a functional env gene and containing the chloramphenicol acetyltransferase (CAT) gene in place of the nef reading frame (pHXBH10ΔenvCAT) was cotransfected into HeLa cells together with plasmids encoding HIV-1 or SIVmac envelope glycoproteins (6). Supernatants containing recombinant viruses were added to 4 × 106 cells at a concentration of 105 cpm of RT activity/ml for 12 h at 37°C. The infected cells were washed three times with PBS and cultured in 3 ml of regular cytokine-supplemented medium. Five days after infection, nonadherent cells were lysed and CAT activity was determined (6).
Recombinant viruses containing the green fluorescent protein (GFP) gene were produced by cotransfection of HEK 293T cells with a Δgag-pol/Δenv vector pHIvec2.GFP, the packaging vector pCMVΔP1ΔenvpA, psrev, and plasmids encoding HIV-1 envelope glycoproteins, as described previously (14). A total of 4 × 106 hPrMC were infected with 400,000 RT units of virus overnight. At 5 to 14 days after infection, infected cells (0.3 to 2.5% of the total cells 2 weeks after infection; n = 5 donors) were separated by FACS with the MoFlo sorter (Cytomation, Fort Collins, Colo.) using the GFP expression as marker for infection. Sorted cells were stained on slides for CAE activity and for immunoreactivity for tryptase as described previously (26).
RESULTS
Purity of 4-week-old hPrMC and expression of CD4 and HIV coreceptors.
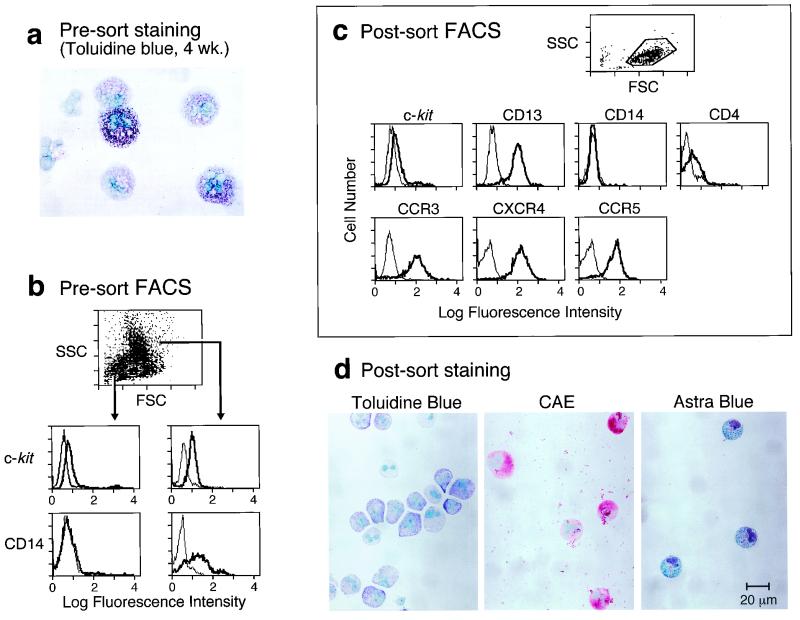
Cultures of CBMNC that were 4 weeks old were analyzed for their identity and purity. The crude 4-week-old preparations contained between 20 and 40% toluidine blue-positive hMC (as shown for one donor in Fig. 1a). Cytofluorographic analysis of the crude preparations revealed two populations differing in SSC as previously reported (as shown for one donor [Fig. 1b]) (26). The high-SSC population (mature hMC) was c-kit+, was also CD13+, expressed CCR3 and some CXCR4 but not CCR5 or CD4 (data not shown), and was CD14+ (Fig. 1b). The lower SSC population was more weakly but uniformly c-kit+ and was also CD13+; more than 95% of this cell population was CD14− (Fig. 1b). This low-SSC population of hPrMC expressed CD4, CXCR4, and CCR5, as well as CCR3 (data not shown).
FIG. 1.
Characteristics of 4-week-old cultured hPrMC before (a and b) and after (c and d) CD14 depletion. (a) Toluidine blue staining reveals both fully granulated hMC and nongranulated cells. (b) FACS analysis revealing low-SSC (left) and high-SSC (right) populations in the scatter plot, indicative of differences in granularity, both with c-kit expression. Only the high-SSC group is CD14+. FSC, forward scatter. (c) Following column purification, only the low-SSC population remains on the scatter plot. Cytofluorographic tracings reveal lack of CD14 and expression of c-kit, CD13, CD4, CCR3, CXCR4, and CCR5. (d) Purified hPrMC stained with toluidine blue for metachromasia (left), for CAE activity (center), and for Astra blue-positive granules (right). The results are representative of three experiments each.
To obtain a purified hPrMC population, the 4-week-old cultured cells were separated on an immunomagnetic CD14 depletion column. Only the low-SSC population lacking CD14 remained after the column purification procedure (Fig. 1c). The purified hPrMC remained c-kit+ (low), CD13+, CD4+, CXCR4+, CCR5+, and CCR3+ (n = 3, as shown for one donor [Fig. 1c]). Less than 5% of the CD14− cells had toluidine blue-positive granules (Fig. 1d, left). Virtually all (>98%) were positive for CAE (n = 3, as shown for one donor [Fig. 1d, center]), and most (∼60%) contained Astra blue-positive granules, a marker restricted to MC and basophils (9) (Fig. 1d, right), suggesting the presence of nascent secretory granules in hPrMC despite their lack of avidity for toluidine blue dye. The eluted CD14+ fraction contained mostly mature hMC based on toluidine blue staining, with the remaining cells having morphologic features consistent with macrophages or monocytes (data not shown).
M-tropic but not T-tropic HIV strains can replicate in hPrMC.
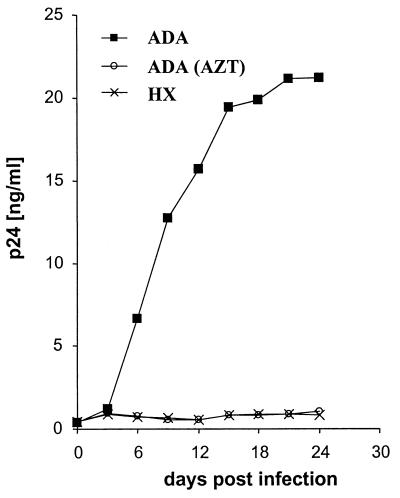
The expression of CD4 and HIV-1 coreceptors suggested that hPrMC might be susceptible to infection by M-tropic HIV-1 strains (which utilize CCR5 and, to a lesser extent, CCR3) and T-tropic strains (which utilize CXCR4). In the background of the NL4-3 strain, the replication-competent recombinant ADA (M-tropic) virus but not the HXBc2 (T-tropic) virus replicated in the crude 4-week-old preparations, as determined by monitoring p24gag levels in the supernatant (n = 2 [a representative experiment is shown in Fig. 2]). Progressive increases in p24gag levels were evident up to day 21 postinfection. The replication was completely inhibited in the presence of 50 μM AZT. No cytopathic effects were observed with the HIV-1 ADA strain. Both the ADA and HXBc2 strains replicated in human peripheral blood mononuclear cells (data not shown).
FIG. 2.
Replication of viruses in hPrMC. Crude 4-week-old hPrMC were infected with the M-tropic HIV-1 strain NL4-3-ADA and the T-tropic strain NL4-3-HXBc2. The RT inhibitor AZT was used on ADA-infected hPrMC at a concentration of 50 μM. Culture supernatants were harvested on the days indicated and assayed for p24gag concentration. Similar results were obtained in two independent experiments; the results for one experiment are shown.
Identity of infected CD14-negative cells as hPrMC and retention of HIV with maturation.
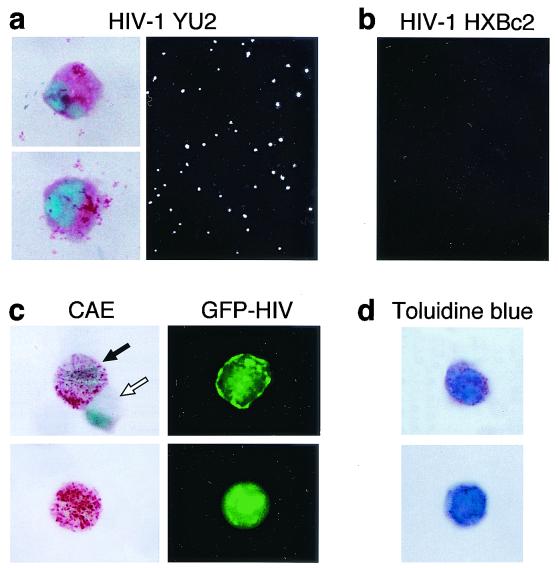
To demonstrate that the infected subset of cells was not a minor contaminating population, purified 4-week-old hPrMC were infected with recombinant single-round viruses containing the GFP gene and pseudotyped with HIV-1 YU2 and HXBc2 envelope glycoproteins. The YU2 envelope glycoproteins, like those of the ADA strain of HIV-1, utilize CCR5 and CCR3 as coreceptors (6). Five days following incubation of cells and virus, GFP-positive cells (0.2 to 1% of the total cells) were present in cultures infected with HIV-1 YU2 pseudotyped viruses (n = 3, as shown for one experiment [Fig. 3a, right]) but not in cultures infected with HIV-1 HXBc2 pseudotypes (Fig. 3b). When GFP-positive HIV-1 YU2 infected cells were separated by using FACSort, all of the GFP-positive cells exhibited diffuse cytoplasmic CAE staining (Fig. 3a, left).
FIG. 3.
In situ detection of HIV in hPrMC and hMC. Purified hPrMC (4 weeks old) were infected with GFP-encoding viruses carrying envelope glycoproteins from the M-tropic HIV-1 YU2 (a) or the T-tropic HIV-1 HXBc2 (b). GFP-positive cells were then visualized by fluorescence microscopy. Up to 1% of the cells infected with the HIV-1 YU2 single-round virus showed green fluorescence (as depicted in panel a). No green cells resulted from infection with a similar virus carrying the envelope glycoprotein of HIV-1 HXBc2 (b). The GFP-HIV-positive cells sorted 5 days after exposure to HIV-1 YU2 were positive for CAE (a, left). Results are representative of three experiments performed. (c) When sorting was carried out 2 weeks after infection, the GFP-HIV-positive cells had strongly CAE-positive secretory granules (left, black arrow) and the few GFP-negative cells were also CAE negative (left, white arrow). (d) The granules of the sorted GFP-HIV-positive cells were also toluidine blue positive.
To determine whether mature hMC could retain HIV, hPrMC exposed to the GFP-bearing HIV-1 YU2 were cultured for an additional 2 weeks with SCF, IL-6, and IL-10. The infected cells (0.3 to 2.5% of the total cells 2 weeks after infection; n = 5 donors) were separated by using FACsort and immobilized to glass slides. In each experiment, >90% of the sorted cells were again CAE positive, with the staining being localized to cytoplasmic granules (Fig. 3c). All of these mature, granulated hMC were GFP positive (as shown for individual cells in Fig. 3c). The few contaminating GFP-negative cells were CAE negative as well (as shown for an individual cell in Fig. 3c). The granules of >80% of the GFP-positive sorted hMC were toluidine blue positive (n = 3 experiments [one displayed in Fig. 3d]), and a similar proportion were also tryptase positive (n = 1 [data not shown]). Thus, hMC remained persistently HIV+ following their exposure to the virus as hPrMC.
Chemokine receptors function as HIV coreceptors on hPrMC.
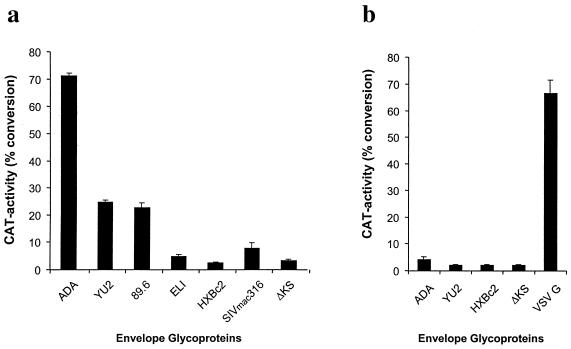
To determine the ability of M- and T-tropic HIV-1 viruses and an SIV strain (SIVmac316) (14) to infect hPrMC and hMC, we performed single-round assays using recombinant CAT-reporter viruses pseudotyped with HIV-1 and SIV envelope glycoproteins. Viruses bearing CCR5-utilizing envelope glycoproteins (ADA, YU2, and 89.6 strains of HIV-1 and SIVmac316) entered hPrMC. The highest infectivity level was detected with viruses carrying the ADA envelope glycoproteins, and the lowest was detected with the SIVmac316 envelope glycoproteins; the latter was only slightly above the background measured with the nonfunctional ΔKS envelope glycoprotein (16) (Fig. 4a). Pseudotypes containing the envelope glycoproteins of the primary T-tropic HIV-1 isolate ELI and the T-cell-line-adapted HIV-1 strain HXBc2 did not infect hPrMC. Thus, the replication block of HXBc2 in hPrMC is due to restricted or very inefficient entry, despite considerable CXCR4 and CD4 expression (Fig. 1). Infections performed with purified hPrMC gave consistently higher levels of CAT activity than did experiments performed with crude hPrMC cultures. Replicate CD14+ cells recovered after the column purification (consisting mostly of mature hMC) yielded negligible levels of CAT activity (n = 2 [data not shown]). The 9-week-old cord blood-derived hMC were not susceptible to new infection by viruses pseudotyped with ADA, YU2, and HXBc2 envelopes. However, viruses containing the vesicular stomatitis virus (VSV) G envelope gave a strong reporter signal in these assays, showing that events in the HIV-1 life cycle following entry, including reverse transcription and long terminal repeat-driven transcription, were not restricted (Fig. 4b).
FIG. 4.
Infection of hPrMC and mature hMC with pseudotyped CAT reporter viruses. (a) Purified hPrMC (4 weeks old) were infected with pseudotyped single-round viruses carrying HIV-1 (ADA, YU2, HXBc2, 89.6, ELI, and ΔKS) and SIV mac316 envelope glycoproteins. (b) hMC (9 weeks old) were infected with the same viruses (except for 89.6, SIVmac316, and ELI) and with VSV G. The reporter gene activity (CAT) in lysates of infected cells is shown 5 days after infection. ΔKS is a nonfunctional HIV-1 envelope and was used to determine nonspecific entry. Results are the means and standard deviations of triplicates from an experiment representative of the four experiments performed.
CCR5 is the dominant receptor used by M-tropic strains for entry into hPrMC.
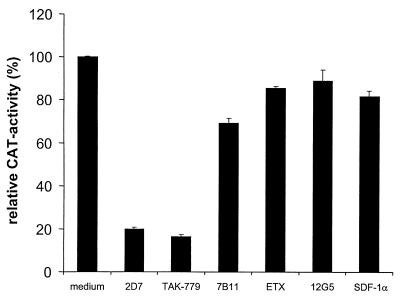
To determine the coreceptor used by M-tropic HIV-1 strains to infect hPrMC, the virus showing the highest infectivity (HIV-1 ADA) was used in experiments with specific CCR5, CCR3, and CXCR4 inhibitors to block entry. The CCR5-specific Ab 2D7 and TAK-779, a nonpeptide compound able to inhibit entry mediated by CCR5 and CCR2b (4), reduced the infection by more than 80% (Fig. 5). CCR2b is not expressed by hPrMC (26) and is not used by ADA or most other M-tropic HIV-1 strains. Thus, CCR5 is the major coreceptor for M-tropic HIV-1 isolates for entry into hPrMC. The CCR3-specific Ab 7B11, but not eotaxin, slightly decreased entry as well (Fig. 5). The CXCR4-specific Ab 12G5 and recombinant human SDF-1α, the natural ligand for CXCR4, did not affect entry.
FIG. 5.
Entry inhibition of reporter virus pseudotyped with the envelope of the M-tropic HIV-1 strain ADA. hPrMC were preincubated with medium alone, TAK-779 (100 nM), ETX (1 μg/ml), SDF-1α (1 μg/ml), or Ab to CCR5 (2D7), CCR3 (7B11), or CXCR4 (12G5) at 20 μg/ml for 30 min at 37°C and were then infected with pseudotyped virus in the presence of the inhibitor. After 12 h, the cells were washed three times with PBS and cultured for 5 days in regular medium before being lysed and assayed for CAT activity. Data are shown as the percentage of CAT activity obtained in the absence of a potential inhibitor (medium), expressed as the means and standard deviations of triplicates from an experiment representative of the five experiments performed.
DISCUSSION
This study reveals that hPrMC derived in vitro can be infected by M-tropic and dualtropic, but not T-tropic, HIV strains. The importance of hMC in both innate and adaptive immunity, the ability of hPrMC to home to multiple tissues, their abundance at sites of HIV introduction, and the tendency of tissue hMC to survive for long periods suggest that HIV-infected hPrMC might not only lead to the viral spreading but might also provide a long-lived sanctuary for the virus.
We suspected the potential for HIV infection of hPrMC based on their repertoire of surface coreceptors, CD4, CCR3, CXCR4, and CCR5 (Fig. 1). Crude 4-week-old cultured hPrMC were productively infected with replication-competent viruses containing the envelope genes of an M-tropic HIV-1 isolate (NL4-3-ADA) but not a T-tropic isolate (NL4-3-HXBc2). New virus was produced over a relatively sustained period, peaking at 21 to 24 days (Fig. 2). The infection of hPrMC was confirmed by the fact that FACSort-separated infected GFP-positive cells stained positively for CAE (Fig. 3) after infection with the HIV YU2 construct containing the GFP reporter.
The replication of the M-tropic but not the T-tropic HIV-1 strain led us to further explore the profiles of viruses able to infect hPrMC using Env complementation assays. Although hPrMC express abundant functional CXCR4 as determined by SDF-α-mediated calcium flux (26), CXCR4-utilizing HIV-1 HXBc2 and ELI strains did not infect hPrMC in these assays, while all of the M-tropic and dualtropic HIV-1 strains tested entered hPrMC. Crude and purified hPrMC populations yielded similar results. Indeed, the levels of HIV-1 ADA entry were higher in the purified hPrMC than in the crude hPrMC that contained some CD14+ cells consisting largely of mature hMC, while the CD14+ cells showed negligible HIV-1 entry and 9-week-old hMC were not susceptible to infection by any of the HIV strains tested (Fig. 4B), probably due to their lack of CD4 and CCR5 (26). Nonetheless, postentry events were functional in hMC, since infection with VSV G-pseudotyped viruses was successful. Moreover, mature hMC clearly continued to produce HIV as they developed from infected hPrMC, as indicated by the staining characteristics of the FACSort-separated cells 2 weeks after infection with the HIV YU2 construct containing the GFP reporter (Fig. 3c and d). These observations are consistent with the recent report of HIV-bearing, metachromatic, tryptase- and chymase-positive hMC-like cells in the blood of patients with AIDS (21).
There are several possible explanations for the lack of CXCR4 utilization by HIV1 in hPrMC. A replication block is reported for several other cells including macrophages, microglia, G0 stem cells, and a subset of a hematopoietic cell line (2, 25, 30), which all express CXCR4 along with CD4 but are resistant to productive infection. The pattern of posttranslational modifications of the CXCR4 extracellular domains or the ability to form a signaling-competent transient gp120-CD4-CXCR4 complex (3, 7, 20) may also dictate the strain specificity of HIV-1 entry into hPrMC. Additional postentry restrictions, although not evident in our study, were reported previously for human macrophages (31).
Coreceptor utilization on hPrMC by M-tropic HIV-1 strains was determined with specific CCR5, CCR3, and CXCR4 inhibitors to block entry of the ADA pseudotype in single-round infections. Both the CCR5-specific Ab 2D7 and the nonpeptide compound TAK-779 (4) reduced the infection of hPrMC by more than 80% (Fig. 5), strongly indicating that CCR5 is the major coreceptor used by M-tropic HIV-1 isolates to enter hPrMC. While the CCR3 ligand eotaxin did not inhibit virus infection in this assay, the CCR3-specific Ab 7B11 slightly (∼20%) inhibited entry. Thus, CCR3 might also function as a coreceptor on these cells. Similar results were obtained with human microglia (2, 12). Neither the CXCR4-specific Ab 12G5 nor recombinant human SDF-1α affected entry. Thus, as with dendritic cells (11, 28), hPrMC could serve to initiate the spread of HIV after becoming infected with the M-tropic strains that are consistently isolated during the initial stages of disease.
Our findings show that hPrMC can be productively infected by M-tropic HIV-1 strains in vitro. These observations carry potential implications for the pathophysiology of HIV. hPrMC in the intestinal mucosa could support initial infection with M-tropic HIV strains that are critical to the initiation of HIV disease. The migration of infected hPrMC or hMC to secondary lymphoid organs could facilitate the transfer of HIV to CD4-positive lymphocytes; indeed, hMC infiltrate local lymph nodes in certain inflammatory circumstances (34) and cervical lymph nodes from patients with AIDS contain increased numbers of hMC compared with normal controls (27). Additionally, hMC is one of the few immune cell types found in the normal central nervous system and brain (33). If hPrMC become infected during the viremic phase of HIV, they could potentially carry M-tropic HIV across the blood-brain barrier for transfer to microglia and astrocytes, which, like hPrMC, are susceptible to infection via CCR5 and CCR3 (12). Since hMC are long-lived in vivo and shed active virus for sustained periods (Fig. 2), infected hMC could provide a sustained source of viral production at mucosal and cutaneous sites. Indeed, the numbers of hMC observed in the submucosa and lamina propria of the intestines of HIV-infected patients are normal, even though the numbers of hMC in the adjacent epithelium are diminished, probably reflecting the requirement of this anatomic subset of hMC for normal T-cell function (15). Finally, it is possible that HIV infection could alter the function of hMC or their threshold for activation, since both urticaria (10) and intractable pruritus (24) are associated with HIV infection.
ACKNOWLEDGMENTS
N. Bannert and J. A. Boyce contributed equally to this work.
This work was supported by National Institutes of Health grants AI-01305, AI-31599, AI-22531, and HL-36110 and by a grant from the Hyde and Watson Foundation. N.B. was supported by a fellowship from the Deutsche Forschungsgemeinschaft (DFG).
We acknowledge Nancy Kedersha for providing her expertise in the use of fluorescence microscopy.
REFERENCES
- 1.Agis H, Willheim M, Sperr W R, Wilfing A, Kromer E, Kabrna E, Spanblochl E, Strobl H, Geissler K, Spittler A, Boltz-Nitulescu G, Majdic O, Lechner K, Valent P. Monocytes do not make mast cells when cultured in the presence of SCF. Characterization of the circulating mast cell progenitor as a c-kit+, CD34+, Ly−, CD14−, CD17−, colony-forming cell. J Immunol. 1993;151:4221–4227. [PubMed] [Google Scholar]
- 2.Albright A V, Shieh J T, Itoh T, Lee B, Pleasure D, O'Connor M J, Doms R W, Gonzales-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthos J, Rubbert A, Rabin R L, Cicala C, Machado E, Wild K, Hanbach M, Steenbeke T D, Swofford R, Farber J M, Fauci A S. CCR5 signal transduction in macrophages by human immunodeficiency virus and simian immunodeficiency virus envelopes. J Virol. 2000;74:6418–6424. doi: 10.1128/jvi.74.14.6418-6424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, Meguro K, Fujino M. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casale T B, Wood D, Richerson H B, Zehr B, Zavala D, Hunninghake G W. Direct evidence of a role for mast cells in the pathogenesis of antigen-induced bronchoconstriction. J Clin Investig. 1987;80:1507–1511. doi: 10.1172/JCI113234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Dimitrov D S, Norwood D, Stantchev T S, Feng Y, Xiao X, Broder C C. A mechanism of resistance to HIV-1 entry: inefficient interactions of CXCR4 with CD4 and gp120 in macrophages. Virology. 1999;259:1–6. doi: 10.1006/viro.1999.9747. [DOI] [PubMed] [Google Scholar]
- 8.Doranz B, Rucker J J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 9.Duffy J P, Smith P J, Crocker J, Matthews H R. Combined staining method for the demonstration of tissue eosinophils and mast cells. J Histochem Cytochem. 1993;16:143–144. [Google Scholar]
- 10.Duvic M. Human immunodeficiency virus and the skin: selected controversies. J Investig Dermatol. 1995;105:117S–121S. doi: 10.1111/1523-1747.ep12316647. [DOI] [PubMed] [Google Scholar]
- 11.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O'Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are coreceptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 13.Heise C, Vogel P, Lackner C J, Dandekar S. Distribution of SIV infection in the gastrointestinal tract of rhesus macaques at early and terminal stages of AIDS. J Med Primatol. 1993;22:187–193. [PubMed] [Google Scholar]
- 14.Hofmann W, Schubert D, LaBonte J, Munson L, Gibson S, Scammell J, Ferrigno P, Sodroski J. Species-specific, postentry barriers to primate immunodeficiency virus infection. J Virol. 1999;73:10020–10028. doi: 10.1128/jvi.73.12.10020-10028.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irani A A, Craig S S, DeBlois G, Elson C O, Schechter N M, Schwartz L B. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol. 1987;138:4381–4386. [PubMed] [Google Scholar]
- 16.Karlsson G B, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard N P, Marcon L, Margolin D, Fanton J, Axthelm M K, Letvin N L, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirshenbaum A S, Goff J P, Semere T, Foster B, Scott L M, Metcalfe D D. Demonstration that mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–2342. [PubMed] [Google Scholar]
- 18.Kolchinsky P, Mirzabekov T, Farzan M, Kiprilov E, Cayabyab M, Mooney L J, Choe H, Sodroski J. Adaptation of a CCR5-using, primary human immunodeficiency virus type 1 isolate for CD4-independent replication. J Virol. 1999;73:8120–8126. doi: 10.1128/jvi.73.10.8120-8126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lantz C S, Boesiger J, Song C H, Mach N, Kobayashi T, Mulligan R C, Nawa Y, Dranoff G, Galli S J. Role for interleukin-3 in mast cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 20.Lapham C K, Zaitseva M B, Lee S, Romanstseva T, Golding H. Fusion of monocytes and macrophages with HIV-1 correlates with biochemical properties of CXCR4 and CCR5. Nat Med. 1999;5:303–308. doi: 10.1038/6523. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Li L, Wadley R, Reddel S W, Qi J C, Archis C, Collins A, Clark E, Cooley M, Kouts S, Naif H M, Alali M, Cunningham A, Wong G W, Stevens R L, Krilis S A. Mast cells/basophils in the peripheral blood of allergic individuals who are HIV-1 susceptible due to their surface expression of CD4 and the chemokine receptors CCR3, CCR5, and CXCR4. Blood. 2001;97:3484–3490. doi: 10.1182/blood.v97.11.3484. [DOI] [PubMed] [Google Scholar]
- 22.Malaviya R, Ikeda T, Ross E, Abraham S N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 23.McNeil H, Austen K F. Biology of the mast cell. In: Frank M M, Austen K F, Claman H N, Unanue E R, editors. Samter's immunologic diseases. 5th ed. Boston, Mass: Little, Brown; 1995. pp. 185–201. [Google Scholar]
- 24.Milazzo F, Piconi S, Trabattoni D, Magni C, Coen M, Capetti A, Fusi M L, Parravicini C, Clerici M. Intractable pruritus in HIV infection: immunologic characterization. Allergy. 1999;54:266–272. doi: 10.1034/j.1398-9995.1999.00885.x. [DOI] [PubMed] [Google Scholar]
- 25.Moriuchi H, Moriuchi M, Fauci A S. Differentiation of promonocytic U937 subclones into macrophage-like phenotypes regulates a cellular factor(s) which modulates fusion/entry of macrophage tropic human immunodeficiency virus type 1. J Virol. 1998;72:3394–3400. doi: 10.1128/jvi.72.4.3394-3400.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochi H, Hirani W M, Yuan Q, Friend D, Austen K F, Boyce J A. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190:267–280. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paiva D D, Morais J C, Pilotto J, Veloso V, Duarte F, Lenzi H L. Spectrum of morphologic changes of lymph nodes in HIV infection. Mem Inst Oswaldo Cruz. 1996;91:371–379. doi: 10.1590/s0074-02761996000300023. [DOI] [PubMed] [Google Scholar]
- 28.Reece J C, Handley A J, Anstee E J, Morrison W A, Crowe S M, Cameron P U. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, Iikura Y, Awaji T, Tsujimoto G, Yanagida M, Uzumaki H, Takahashi G, Tsuji K, Nakahata T. Selective growth of human mast cells induced by steel factor, interleukin 6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- 30.Schen H, Cheng T, Preffer F I, Dombkowski D, Tomasson M H, Golan D E, Yang O, Hofmann W, Sodroski J G, Luster A D, Scadden D T. Intrinsic human immunodeficiency virus type 1 resistance of hematopoietic stem cells despite coreceptor expression. J Virol. 1999;73:728–737. doi: 10.1128/jvi.73.1.728-737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrader J W, Scollay R, Battye F. Intramucosal lymphocytes of the gut: Lyt-2 and Thy-1 phenotype of the granulated cells and evidence for the presence of both T cells and mast cell precursors. J Immunol. 1983;130:558–564. [PubMed] [Google Scholar]
- 33.Silver R, Silverman A J, Vitkovic L, Lederhendler I I. Mast cells in the brain: evidence and functional significance. Trends Neurosci. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- 34.Wang H-W, Tedia N, Lloyd A R, Wakefield D, McNeil H P. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J Clin Investig. 1998;102:1617–1626. doi: 10.1172/JCI3704. [DOI] [PMC free article] [PubMed] [Google Scholar]