Abstract
Background:
Adolescent depression is associated with both dysfunction in neural reward processing and peripheral inflammatory markers (PIMs), such as interleukin-6 (IL-6), C-reactive-protein (CRP), and tumor-necrosis factor alpha (TNFα). Few adolescent studies have examined neural-inflammatory marker associations and associated behavioral correlates, which would contribute to a better understanding of developmental processes linked to depression.
Methods:
36 adolescents at high risk of depression completed an fMRI reward task (during anticipation and outcome), blood draw for PIMs (IL-6, CRP, and TNFα), and a behavioral task assessing motivation to expend effort. Analyses examined associations of task-dependent functional connectivity (FC; ventral striatum to frontal and default mode network brain regions), and if the interaction of PIMs and task-dependent FC predicted motivation to expend effort.
Results:
For anticipation contrast, TNFα was associated with increased task-dependent FC between the LVS and PCC/vmPFC. In moderation analyses, for anticipation contrasts, the combination of higher IL-6 and stronger FC (LVS-precuneus/PCC) was associated with lower motivation to expend effort, while for outcome contrasts, the combination of higher IL-6 and stronger FC (VS-precuneus/PCC) was associated with greater motivation to expend effort.
Conclusions:
Our findings in adolescents during an important developmental time period suggest that PIMs are directly linked to greater FC between the VS and DMN brain regions during win anticipation, consistent with prior studies. Effects of PIMs on motivation to expend effort may vary the strength/type of neural reward processing (anticipation or outcome), which could guide better understanding how inflammatory markers and neural reward substrates contribute to development of depression in high-risk adolescents.
Keywords: reward circuitry, inflammation, adolescent, outcome, anticipation, depression
Introduction
Adolescent depression is characterized by significant functional impairment and distress and is associated with increased incidence of psychiatric disorders and deficits in psychosocial functioning in adulthood (Birmaher et al., 2002). Though existing studies have examined correlates and consequences of adolescent depression, studies are needed to concordantly examine multiple biological risk factors in youth at high risk to develop depression (e.g., youth who have family history of depression). Studying biological risk factors in high-risk adolescents could reveal mechanisms of heterogeneity in clinical outcomes in this population and enable earlier interventions to prevent development of depression in this age group characterized by a peak in incidence of depressive disorders (Kieling et al., 2019). A large literature base discusses the link between reward processing and depression risk in adolescence, and suggests that altered reward processes (e.g., blunted neural response to reward) are related to risk for depression (Luking et al., 2016; O’Callaghan and Stringaris, 2019). These studies suggest that particularly striatal response to reward across a variety of different reward task types (e.g., monetary, social) may be associated with depressive symptoms. Furthermore, a growing literature base suggests neural reward responsivity (both in anticipation and outcome of rewards) in adolescence predicts worsening of depressive symptoms in adolescence (Stringaris et al., 2015). Mechanistically, dysfunction of reward processing may relate to failure of stress buffering in adolescence (a timeperiod characterized by normative elevations of stress) or may be an early neural marker that may portend development of depressive symptoms, though the exact mechanism remains unclear.
Specifically, studies indicate that dysfunctional reward circuitry, specifically decreased connectivity between the ventral striatum(VS) and brain regions including the prefrontal cortex (PFC) and default mode network (DMN), may herald depressive symptoms in adolescence, but the mechanism by which this occurs is unclear (Hwang et al., 2016; Morgan et al., 2019). In recent years, increasing evidence has suggested that peripheral inflammatory markers (PIMs), particularly C-reactive protein (CRP), interleukin-6 (IL-6) and tumor-necrosis factor alpha (TNFα), are associated with this dysfunction in reward neural circuitry (Felger et al., 2016; Rengasamy et al., 2022a). These PIMs are thought to disrupt neuronal functioning and/or alter neurotransmitter activity to decrease dopaminergic signaling in the VS (Felger and Treadway, 2017). Thus, dysregulated PIM activity and dysfunctional reward-related neural circuitry might contribute to behavioral processes related to anhedonia or depression in adolescents (D’Acunto et al., 2019; Rengasamy et al., 2021a).
In studies of depressed youth and adults, dysfunction in neural circuitry is linked to dysregulated reward processing and behaviors related to reward processing, both in regard to reward anticipation (i.e., when the potential for the reward is realized) and reward consumption (i.e., when the reward is received or omitted) (Oldham et al., 2018). Existing theories suggest that reward anticipation and consumption involve inter-related but disparate neurobiological substrates and neurotransmitters (e.g. dopamine for anticipation and endogenous opioids for consumption) (Berridge and Robinson, 2016). Reward anticipation and reward consumption are both associated with greater VS activity, but reward consumption is linked to greater activity of DMN components (e.g., posterior cingulate cortex [PCC], ventromedial prefrontal cortex [vmPFC]) as compared to reward anticipation (Borsini et al., 2020; Oldham et al., 2018). However, studies have not examined how PIMs might associate or interact with these different temporal aspects of neural reward processes, which might explain heterogeneity found in results of studies attempting to link neural reward circuitry to behavior or clinical outcomes.
Although the literature in neural reward circuitry has focused on task-related differences in neural activity, to move beyond these more simplistic models of brain region activation and in recognition of the inter-related nature of different brain regions during reward processing, researchers have turned to task-dependent functional connectivity (FC) analyses (also termed generalized psychophysiological interactions; gPPI). gPPI allows examination of differences in functional connectivity during different reward conditions, thereby unveiling pathways of circuit-level connectivity that may not be apparent on more standard task-related activations. For instance, preliminary literature indicates that anhedonia may be associated with increased FC between the VS and mPFC/DMN regions (Healey et al., 2014; Quevedo et al., 2017). On separating reward processing into temporal components however, the literature suggests that functional connectivity between the VS and DMN regions might be decreased during reward anticipation (Schreiter et al., 2016) but increased during reward consumption (Dobryakova and Smith, 2021), emphasizing that dissecting such processes into these temporal phases may provide a more nuanced understanding of these distinct processes at the individual level.
Just as direct differences in functional connectivity exist between reward anticipation and consumption, dysregulation in PIM activity may be more strongly associated with dysfunction in processes related to reward anticipation (compared with reward outcome) based on conceptual models and prior studies (Treadway et al., 2019). For instance, as a part of the adaptive evolutionary strategy of “sickness behavior”, individuals with infectious illnesses and elevated PIMs exhibit decreases in motivation for external rewards, decreases in social interaction, and decreased engagement in activities with high energy burden, in order to preserve energy to adequately heal from illness. Thus, individuals with dysregulation in PIMs and dysfunction in neural connectivity during reward anticipation specifically may display behavioral effects of maladaptive reward processing (i.e., decreased motivation to expend effort), which may ultimately contribute to depressive symptomology (Stringaris et al., 2015; Treadway et al., 2019). Understanding potential links between PIMs and dysfunctional frontostriatal reward circuitry (such as prior studies finding negative correlations between PIMs and frontostriatal connectivity (Felger et al., 2016; Rengasamy et al., 2022a)) is particularly relevant in adolescence. Specifically, in adolescence, frontostriatal reward circuitry is in a period of active neurodevelopment that is vulnerable to external insults such as stress (Sinclair et al., 2014), with studies indicating that disruption to normative neurodevelopment may result in both an increased risk of depression in adolescence (Stringaris et al., 2015) and persistence of dysfunction in reward circuitry across the lifespan (Forbes and Dahl, 2012; Stringaris et al., 2015). Furthermore, adolescence is a period of important immunodevelopment (e.g., maturation of microglia) and maladaptive sensitization between the immune system and neural circuitry during this stage could lead to dysfunctional neuroimmune interactions that persist into adulthood . Based on these conceptualizations, PIMs may even moderate relationships between reward circuitry and behavioral outcomes.
Yet, few studies have examined whether how relationships of PIMs and task-dependent functional connectivity in reward circuitry vary between anticipation and consumption of reward in youth at high risk for depression, particularly as related to FC between the VS and PFC/DMN, which are reward regions thought to be more strongly affected by PIMs (Marsland et al., 2017). Furthermore, no studies to our knowledge have explored if PIMs moderate associations between task-dependent FC and behavioral outcomes such as motivation to expend effort. Such findings could elucidate potential mechanisms of how PIMs might be associated with reward-related neural circuitry and reward-related behavior. To address these existing gaps in the literature and given the prior evidence base, we sought to identify associations of PIMs with task-dependent functional connectivity (FC) of the VS with PFC and DMN regions (precuneus, vmPFC, and PCC) during a reward task (for both reward anticipation and reward outcome) in 36 youth at high risk for depression. We hypothesized that PIMs would be associated with decreased task-dependent FC (for both anticipation and consumption) between VS and the dmPFC/DMN regions given prior literature. Second, we hypothesized that moderation effects would exist such that greater PIM levels and greater task-dependent FC might predict lower motivation to expend effort on a behavioral effort task.
Methods
2.1. Participants
Participants included 36 healthy nondepressed adolescents who had a first-degree relative with lifetime history of Major Depressive Disorder (recurrent) or dysthymia, based on the Structured Clinical Interview for DSM-IV. Exclusion criteria included personal history of specific psychiatric conditions/diagnoses (depression, bipolar disorder, psychosis, substance use disorder), daily nicotine use, psychotropic medication use, MRI contraindications (e.g., pregnancy or presence of ferromagnetic metal in the body), or chronic inflammatory conditions. In terms of both diagnoses at time of study assessment and historical psychiatric diagnoses (assessed via the Kiddie Schedule for Affective Disorders and Schizophrenia; K-SADS), only one adolescent had a diagnosis of Post-traumatic Stress Disorder, with no adolescents having other psychiatric disorders that were assessed via the K-SADS (including MDD, dysthymic disorder, Bipolar I and II disorder, Generalized Anxiety Disorder, Separation Anxiety Disorder, and Oppositional Defiant Disorder). Informed consent was provided by parent/guardian of participants and assent was provided by participants. Of 46 participants initially recruited who completed the fMRI reward task, participants were excluded from the analyses below for not providing blood samples (n = 2), having PIM levels greater than 3 SDs above average PIM levels (n = 1), not completing all portions of the fMRI reward task (n=1), or having inadequate coverage (i.e., <65%) of the VS (n = 6), leading to ultimately 36 participants. For analyses examining effortful motivation, we did not include participants who completed less than 75% of behavioral effort task trials (n = 5) or did not complete the effort task (n = 1). All study procedures were approved by the University of Pittsburgh Institutional Review Board.
2.2. Cytokine Measurement
Serum samples were collected from a 20 mL blood draw. Samples were then centrifuged and stored in a deep freezer at −80º C. Enzyme linked immune sorbent (ELISA) assays (R&D Systems for TNFα/IL-6 and ALPCO for CRP) were used on a Bioteck Epoch plate reader, with all samples run in duplicate. The lower limits of detection are 0.049 pg/mL for TNFα, 1 ng/mL for CRP, and 0.2 pg/mL for IL-6. Manufacturer-reported intra-assay CVs were 3.6 – 4.7% for IL-6, 1.9 – 2.2% for TNF-α, and 6.7 – 6.8% for CRP.
2.3. BOLD fMRI Task
Participants completed one block of a well-validated 8-minute card-guessing monetary reward fMRI task, with the block consisting of 48 trials (20 seconds per trial) (Baranger et al., 2021; Eckstrand et al., 2019a; Eckstrand et al., 2019b). For each trial, participants were initially presented a card on a screen and asked to guess whether a number on a card (ranging from 1–9, described to participants as random card from a deck of cards though the actual card order was fixed) would be greater or lower than 5 (“guess” phase; 4 s). After participants indicated their guess with a button press, participants were presented with an expectancy cue (e.g., win, loss, or no change) where there was expectation of the given outcome; “anticipation” phase; jittered 2 – 6 s). Then, an outcome cue (e.g., number on the card and outcome on the card – gain money, lose money, or no change; “outcome” phase; 1 second) was presented. The reward task contained an equal amount of trials (n = 12) for 4 different trial types (48 total trials), with equal proportions of outcome for each trial type: win/no-win trials, loss/no-loss trials, mixed win/loss trials, and neutral trials. The win/no-win trial contained the anticipation of a win followed by a win or no-change outcome. The loss/no-loss trial contained the anticipation of a loss followed by a loss or no-change outcome. The mixed trials contained the anticipation of a win or loss, followed by a win (6 trials) or loss (6 trials) outcome. The neutral trials contained the anticipation of no win or loss, followed by a no win or loss outcome. In the present study, we examined each reward phase (i.e., anticipation or outcome phase) independently given the stronger literature base for examination of reward phases individually in relation to both inflammatory markers (Burrows et al., 2021) and behavioral measures of effort (Chat et al., 2021). Win outcome trials were defined as gaining money, while loss outcome trials were defined as losing money during the outcome phase. Analyses focused on contrasts of win anticipation > loss anticipation (WA > LA) and win outcome > loss outcome (WO > LO). Trial order was pseudorandom with outcomes being predetermined. All participants received a fixed amount of $10 after completion of the task and were debriefed about deception.
2.4. BOLD fMRI Data Acquisition and Analysis
Participants completed fMRI scans on a Siemens 3T Trio scanner. Functional blood oxygen level dependent (BOLD) responses were acquired with a multi-band gradient echo EPI (328 slices, three factor multiband; 2.3 mm isotropic voxels; TR = 1500 ms, TE = 3.17 ms; field of view = 220 × 220 mm; matrix 96 × 96; flip angle 58°, bandwidth 2004 Hz Px). Structural 3D axial MPRAGE images (TR = 1500 ms, TE = 3.17 ms; flip angle 8° FOV = 256 × 256 mm; 1 mm isotropic voxels; 176 continuous slices) were acquired as well during the scan. For all participants, the neuroimaging software Statistical Parametric Mapping (version 12) was used. Standard preprocessing steps were applied, including slice time correction, co-registration, spatial distortion correction, realignment, warping to standard MNI/ICBM 152 template, co-registration to anatomical image, realignment, and smoothing. The Artifact Detection Toolbox (ART; http://www.nitrc.org/projects/artifact_detect/) was utilized and censored volumes with movement of >3 standard deviations from participants mean, >.5-mm scan-to-scan translation, or >.01° of scan-to-scan rotation.
First-level single subject analysis involved a GLM fit with all task regressors (convolving stimulus onsets of each task phase with a canonical hemodynamic response function (HRF)) and nuisance covariates, including ART regressors and motion parameters/derivatives. In SPM, to compute functional connectivity, we used the built-in PPI toolbox. We extracted the generated seed region BOLD time series for the seed regions (left and right ventral striatum), which were then deconvolved using the standard deconvolution algorithm in SPM to estimate the neuronal time series for the given volume of interest (e.g., ventral striatum, VS). The neuronal time series was then multiplied by the psychological variable (task contrast, either WA>LA or WO>LO) to generate an interaction term which was then convolved with the HRF to generate an gPPI interaction term. The physiological variable (original BOLD time series of the volume of interest), psychological variable (stimulus onsets convolved with the HRF) and gPPI interaction term were then included into the PPI design matrix.
Masks for seed regions and ROIs were derived from the Neurosynth meta-analytic database (using thresholded versions of the association test map, thresholded at 50%)(Liu et al., 2020). Seed regions included the left and right ventral striatum, and ROIs, based on previous findings identifying PIM associations with the dmPFC and DMN(Marsland et al., 2017), included the dorsal medial prefrontal cortex (dmPFC), ventromedial prefrontal cortex (vmPFC), bilateral precuneus, left and right posterior cingulate cortex (PCC). Beta weights for the gPPI interaction term were then extracted from each ROI for analysis for use in regression analyses in the statistical software R.
2.5. Clinical Measures
To measure effort-based motivation, we used the behavioral Effort-Expenditure for Rewards Task (EEfRT) task, which was done on a computer outside the scanner, with one run of the fifteen-minute task done in identical fashion to the prior study which had validated the EEfRT task. In brief (with further description of the task detailed in prior studies), participants were presented with a brief fixation point, followed by screen presenting the probability of “winning” (i.e., 88%, 50% or 12%), and then had the option to select either an easy task for a lower reward amount or a hard task for a higher reward amount (Treadway et al., 2009). Participants were then required to press a button 100 times with the non-dominant little finger for the hard task in 30 seconds or 30 times with the dominant index finger for 7 seconds for the easy task. A screen then presented the result (“Success” or “Failure”) for the trial followed by the dollar amount for the reward.
For our primary analytic measure of motivation to expend effort, we used the proportion of hard trial choices at high probability of winning (88% odds of winning), given that this metric has been associated with anhedonic symptoms in prior studies (Treadway et al., 2009). The mean proportion of hard trial choices (Motivation to Expend Effort) was 57.8% (SD 24.3%) in this population of adolescents at high-risk for depression (see Supplement S3 for further descriptive statistics), which is concordant with studies identifying that depressed participants generally choose between 50% - 60% of hard trial choices using the same paradigm (Treadway et al., 2012). These findings are furthermore consistent with a growing literature suggesting reward and motivational processes in adolescents both at high-risk for depression and with depression have similar features (e.g., decreased response to reward and decreased effortful motivation) (Luking et al., 2016; Rzepa et al., 2017).
For sensitivity measures, depression severity was assessed through the Center for Epidemiologic Studies Depression Scale (CESD; a 20-item questionnaire with scores ranging from 0 to 60 with greater scores indicating worse depression severity) (Eaton et al., 2004) and anhedonia severity was measured through the Snaith-Hamilton Pleasure Scale (SHAPS; a 14-item questionnaire with scores ranging from 14 to 56 with greater scores indicating higher levels of anhedonia).
2.6. Statistical Analysis
Statistical analyses were conducted using the statistical software R (version 4.0.05). Across all analyses, we winsorized PIM values to reduce effects of any other outliers (rescaling extreme values less than the 5% percentile of values or greater than the 95% percentile of values, which included <15% of all values). In our statistical analysis examining direct associations of PIMs with task-dependent FC (for 2 task contrasts, WA>LA and WO>LO), we used multiple linear regression models with each ROI beta weight (from seed-based connectivity maps) as the dependent variable and each PIM as the independent variable, statistically adjusting for age, gender, and BMI consistent with prior studies (e.g., ROI Beta Weight ~ PIM + age + gender + BMI; see Supplement S5 for a detailed description of all analytic models used) (Rengasamy et al., 2021a). In moderation analyses, we similarly used linear regression to examine if PIMs interacted with the ROI Beta Weight to predict proportion of hard trials. Thus, we included the interaction terms (including main effects) for the ROI beta weight and PIM in predicting proportion of hard trials completed. To examine moderation effects in more detail, we utilized the Johnson-Neyman technique (using the johnson_neyman function from R package interactions), which identifies regions of significance, which reflect the values of the moderator (PIM) above and below which a statistically significant association exists between the independent (ROI beta weight) and dependent variable (proportion of hard trials). For each PIM, we conducted a False Discovery Rate (FDR) multiple-comparisons correction (q<0.05) for 20 tests, given 2 task contrasts (WA>LA and WO>LO), 2 seed regions (LVS and RVS), and 5 ROIs (bilateral precuneus, vmPFC, right PCC, left PCC, and dmPFC) for each outcome measure.
In sensitivity analysis, we also included potential confounders individually (which included race [defined as White/non-White], depression severity, or anhedonia severity) in linear regression models as done in prior studies(Rengasamy et al., 2022b; Rengasamy et al., 2021a). No appreciable differences in findings were noted on these sensitivity analyses (see Supplement S4). For interested readers, descriptive neuroimaging findings (e.g., group-level contrasts for WA>LA and WO>LO and whole-brain gPPI findings) along with associations of task-dependent FC with demographic variables are presented in the Supplement (see Supplement S2).
Given our sample size, presentation of reliability estimates of different parameters is important. For the behavioral EEfRT task, studies examining test-retest reliability have found fair to good reliability of the task (Reddy et al., 2015). Although prior studies do not directly assess immediate test-retest reliability of our fMRI reward task (which is a needed area of research), similar paradigms show fair reliability (Baranger et al., 2021; Waltmann et al., 2022) and similar versions of the task have been shown to reliably activate the ventral striatum (Corral-Frías et al., 2015; Delgado et al., 2000; Eckstrand et al., 2019a).
Results
3.1. Participant Characteristics
Participants included 36 healthy nondepressed adolescents who had a first-degree relative with lifetime history of MDD or dysthymia. Adolescents were 15.3 years old, 55.6% male, and identified as different races/ethnicities (69.4% Caucasian, 13.9% African American, 5.6% biracial and 11.1% not identifying a particular race/ethnicity). See Table 1 for baseline clinical characteristics (and Supplemental Table S1 for a full correlation matrix of variables). Average peripheral inflammatory marker levels (IL-6 – 1.1 pg/mL, TNFα – 1.2 pg/mL, CRP – 2.4 mg/L) fell uniformly within PIM values reported by prior studies for healthy adolescents and adults .
Table 1.
Sample Characteristics. For race/ethnicity, a more detailed breakdown of the race/ethnicity is provided.
| n | Mean or Percentage | Standard Deviation | Minimum | Maximum | ||
|---|---|---|---|---|---|---|
| Age | 36 | 15.333 | 1.707 | 13 | 19 | |
| BMI | 36 | 23.716 | 5.586 | 16.421 | 44.811 | |
| Gender (male) | 36 | 55.6% | - | - | - | |
| Race (Non-White) | 36 | 30.6% | - | - | - | |
| Caucasian | 25 | 69.4% | - | - | - | |
| Black | 5 | 13.9% | - | - | - | |
| More than 1 race/ethnicity | 2 | 5.6% | - | - | - | |
| Did not wish to provide | 4 | 11.1% | - | - | - | |
| CRP (mg/L) | 35 | 2.377 | 3.579 | 0.1 | 13.78 | |
| IL-6 (pg/mL) | 36 | 1.115 | 0.699 | 0.35 | 3.86 | |
| TNFα (pg/mL) | 35 | 1.224 | 0.416 | 0.39 | 2.24 | |
| CESD Scores | 35 | 9.886 | 5.764 | 0 | 24 | |
| SHAPS Scores | 34 | 24.059 | 5.444 | 14 | 35 | |
| Proportion of Hard Trial Choices (Motivation to Expend Effort) | 35 | 0.577 | 0.243 | 0.111 | 1 | |
3.2. Association of PIMs with gPPI
For the WA>LA contrast, we found that TNFα was positively associated with task-dependent functional connectivity between the LVS and the right PCC (B = 0.71, unadjusted p = 0.003, adjusted p = 0.026, n = 35), left PCC (B = 0.7, unadjusted p = 0.003, adjusted p = 0.026, n=35), and vmPFC (B = 0.71, unadjusted p = 0.004, adjusted p = 0.026, n = 35). No other direct associations between PIMs and task-dependent functional connectivity emerged (see Supplement S6).
3.3. Anticipation Contrast: Moderation Analysis of PIMs and gPPI in predicting behavioral effort
We then examined if individuals with both elevations in PIMs and increases in task-dependent connectivity (during anticipation and outcome phases) exhibited different patterns of effort-based motivation (defined by proportion of high-probability hard-trial choices), see Table: Moderator Findings. For the WA>LA contrast, we found that individuals with greater IL-6 and greater task-dependent functional connectivity for the LVS (to ROIs of precuneus, left PCC and right PCC;) and RVS (to ROIs of the precuneus and left PCC) exhibited less motivation to expend effort (B’s = −0.74 to −0.79, adjusted p’s < 0.05, n = 30). See Figure 1 and Table 2.
Figure 1.
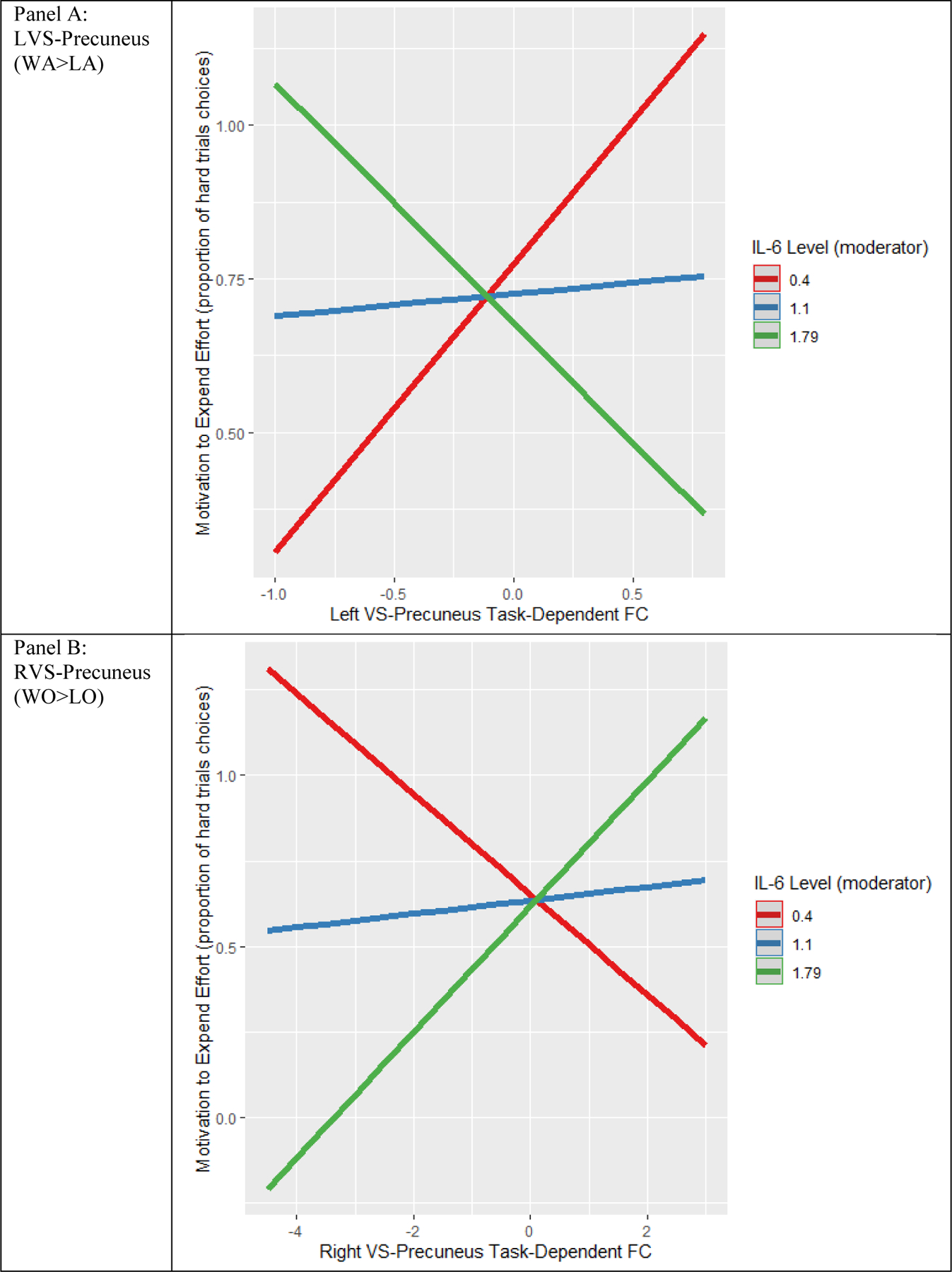
Example of simple slopes moderation effects. This figure illustrates a plot of simple slopes, with moderating effects of IL-6 on LVS-precuneus FC (for WA>LA) and effort relationships in Panel A, and moderating effects of IL-6 on RVS-precuneus FC (for WO>LO) and effort relationships in Panel B. Slope lines for predicted values of the moderator at the mean (m) and mean +/− standard deviation (sd) are plotted.
Table 2.
Moderation of the Association between FC during Reward Processing and Motivation to Expend Effort by Pro-inflammatory Marker IL-6. This table examines the moderation effects of IL-6 on the relationship between the task-dependent FC and effort. Johnson-Neyman intervals are provided, which indicate the values of IL-6 at which the slope of the FC-effort relationship was statistically significant. A table of all seed and ROI FC combinations for moderation effects across all cytokines is available in the Supplement.
| Contrast | FC Seed and ROI | Beta for FC x IL-6 | Unadjusted P value | Adjusted P value | IL-6 values below which FC-Effort association was positive | IL-6 values above which FC-Effort association was negative |
|---|---|---|---|---|---|---|
| WA>LA | ||||||
| LVS-Right PCC | -0.76 | <0.001 | 0.004 | 0.85 | 1.33 | |
| LVS-Precuneus | -0.79 | <0.001 | 0.004 | 0.88 | 1.4 | |
| LVS-Right PCC | -0.79 | 0.002 | 0.01 | 0.86 | 1.44 | |
| RVS-Right PCC | -0.76 | 0.003 | 0.01 | 0.79 | 1.42 | |
| RVS-Precuneus | -0.74 | 0.003 | 0.01 | 0.72 | 1.37 | |
| WO>LO | IL-6 values below which FC-Effort association was negative | IL-6 values above which FC -Effort association was positive | ||||
| RVS-dmPFC | 0.15 | <0.001 | 0.006 | 0.84 | 1.45 | |
| RVS-Precuneus | 0.24 | 0.017 | 0.049 | 0.81 | 2 |
Note: Effort was defined by proportion of hard trials. Unstandardized betas are presented for interaction terms.
Abbreviations: FC, functional connectivity; IL-6, interleukin-6; ROI, region of interest; PCC, posterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; VS, ventral striatum
3.4. Outcome Contrast: Moderation Analysis of PIMs and gPPI in predicting behavioral effort
For the WO>LO contrast, individuals with greater IL-6 and greater task-dependent functional connectivity (from RVS to precuneus and dmPFC) exhibited greater motivation to expend effort (B’s = 0.15 to 0.24, adjusted p’s < 0.05, n = 30). No moderator associations were found in task-dependent functional connectivity for TNFα or CRP (adjusted p’s > 0.1), see Supplement S7.
3.5. Regions of Significance Testing
To examine moderation effects further, we also conducted regions of significance testing, noting that these moderation effects were generally significant at IL-6 values less than 0.72 to 0.88 pg/mL, depending on the ROI, (for anticipation: stronger gPPI-PIM associations; for outcome: weaker gPPI-PIM associations) and at IL-6 values greater than 1.32 – 2 pg/mL, depending on the ROI (for anticipation: weaker gPPI-PIM associations; for outcome: stronger gPPI-PIM associations) (Ambrosia et al., 2018). See Figure 2.
Figure 2.
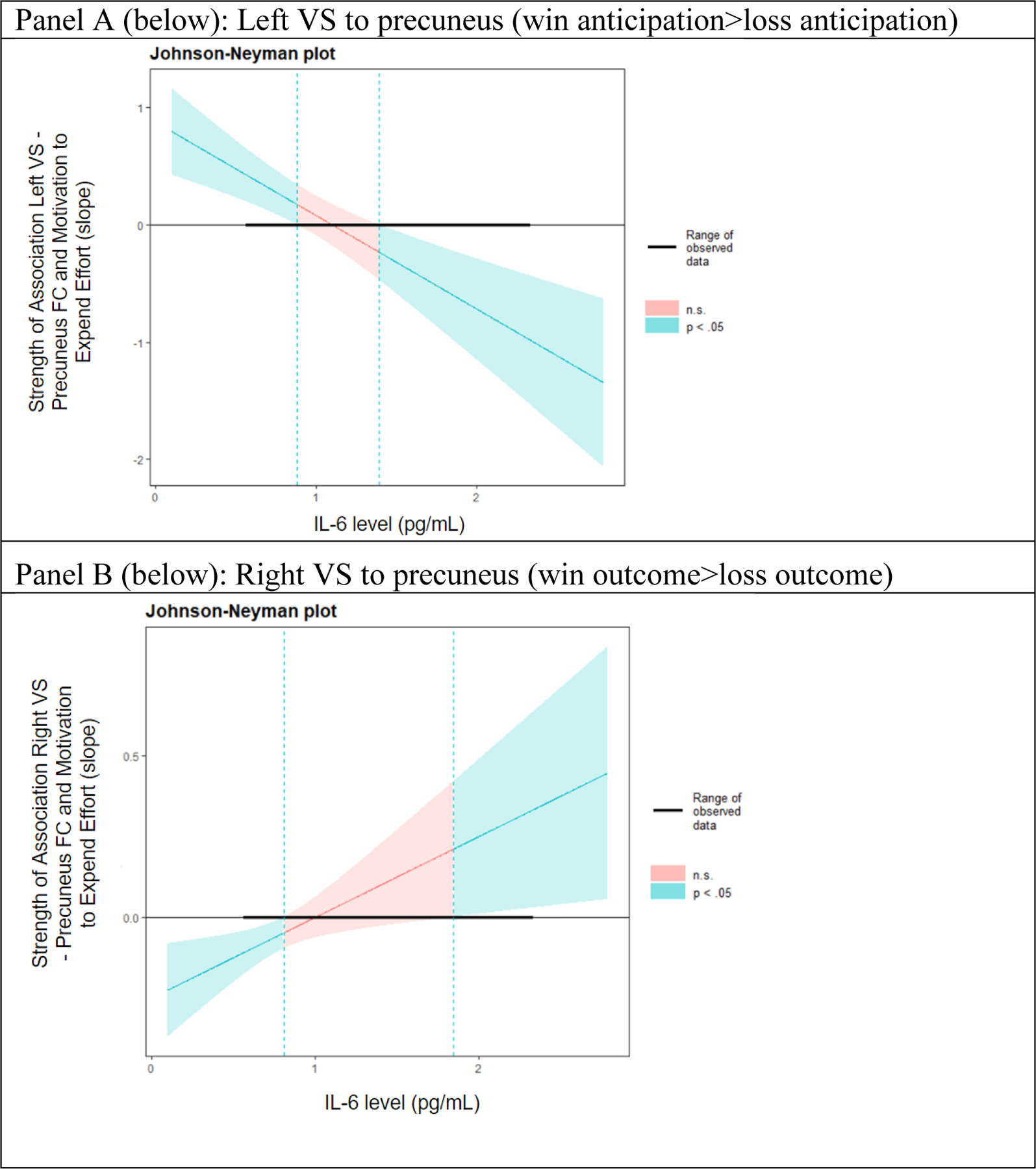
Johnson-Neyman Plot (region of significance). These plots represent analyses examining moderation effects of IL-6 on task-dependent FC in predicting motivation to expend effort. For task-dependent FC during reward anticipation, as IL-6 level increases, the association between the FC and effort becomes weaker (Panel A). For task-dependent FC during reward outcome, as IL-6 level increases, the association between connectivity and effort becomes stronger (Panel B) .
Discussion
In this study of healthy adolescents at high risk for depression, we found that higher levels of TNFα were directly associated with greater task-dependent functional connectivity (win anticipation > loss anticipation) between the ventral striatum and regions of the default mode network (PCC and vmPFC). In a moderation analysis examining neural connectivity during reward outcome, we found that greater motivation to expend effort on a behavioral effort task was predicted by higher IL-6 levels and greater task-dependent FC (for win > loss outcome contrasts) between the LVS and DMN regions (PCC, precuneus). Conversely, when examining reward anticipation, we found that less motivation to expend effort was predicted by greater IL-6 levels and greater task-dependent FC (for win > loss anticipation contrasts) between the RVS and a DMN region (precuneus) along with the dmPFC. To our knowledge, this is the only study examining relationships between PIMs and gPPI related to different subtypes of reward in adolescents. Our results provide valuable insight into the role of PIMs in neural reward processing related to the VS and regions of the DMN, along with dissection of variations of neural connectivity during two distinct temporal periods of reward processing and how these may differentially interact with low-grade inflammatory processes to influence behavior. Ultimately, our findings in this group of adolescents at high-risk of depression (with low rates of any psychiatric diagnosis) could guide research that might modify maladaptive trajectories of neuro- and immuno-development to reduce risk for adolescent psychopathology such as depressive illnesses.
First, we found that higher levels of TNFα were linked to greater connectivity during win anticipation as compared to loss anticipation, specifically between the LVS and three major regions of the DMN (right and left PCC and vmPFC). Given that depressive disorders (associated with elevated PIM levels) are linked to greater dysfunction in reward anticipation and association of PIMs with neurotransmitters specifically relevant to reward anticipation (e.g., dopamine), higher levels of TNFα may result in more profound dysfunction in VS dopaminergic activity during win anticipation (Burrows et al., 2021; Bylsma et al., 2008), leading to compensatory overconnectivity of LVS-DMN circuitry during win anticipation. Alternatively, recent large-scale studies also suggest that elevated PIM levels in healthy individuals (in contrast to depressed individuals) are linked to lower levels of certain depressive symptoms (e.g., depressed mood, suicidal ideation) (Milaneschi et al., 2021) and that depressive symptoms are linked to less VS-DMN connectivity during win anticipation (vs loss anticipation) (Quevedo et al., 2017). Thus, TNFα may help to buffer against such dysfunction in VS-DMN connectivity. Notably, our findings were specific to connectivity during anticipation of reward, which is consistent with conceptualizations of inflammation being associated with “sickness behavior” which affects anticipatory reward processes (e.g., reducing desire for external activities/reward) more than outcome reward processes. Also, our findings were specific to the LVS, which is concordant with the broader literature in depressed patients that tends to identify stronger associations related to inflammatory markers and the LVS (as opposed to the RVS) (Felger et al., 2016; Rengasamy et al., 2022a).
On examination of moderation effects of task-dependent connectivity and PIMs in predicting effort on a behavioral effort task, we found first that greater FC during win > loss anticipation (for LVS and RVS to precuneus/PCC) and higher IL-6 levels were associated with less motivation to expend effort. On the other hand, we also found that greater FC during win > loss outcome (RVS to precuneus and dmPFC) and higher IL-6 levels were associated with greater motivation to expend effort. These opposing set of findings in our sample of high-risk adolescents may be explained by the fact that during anticipatory phases, greater VS-DMN connectivity during win trials may represent greater negative self-rumination about potential reward (e.g., “I never get good things”), while during outcome phases, greater connectivity represents greater positive rumination or learning related to more salient positive outcomes (e.g., “I am getting a good thing”) and self, which is consistent with studies of depressed patients identifying greater deficits in reward anticipation processes as compared to reward outcome processes (Halahakoon et al., 2020; Northoff and Hayes, 2011; Olino et al., 2011). Alternatively, from a neurobiological standpoint, given that IL-6 has neurotrophic and synaptogenetic properties (Islam et al., 2009; Mirabella et al., 2021), it is also possible that IL-6 functions to enhance whichever regions of striatal-DMN circuitry that are more strongly connected, which vary between reward anticipation and outcome. Thus, IL-6 might both facilitate and over-regulate functional connectivity that enhances motivation to expend effort, dependent on the reward process (e.g., outcome or anticipation). These effects could respectively then either increase or decrease motivation to expend effort (Moriarity et al., 2020). Furthermore, prior studies suggest that reward anticipation may be more closely linked to alterations in dopaminergic activity while reward outcome is linked to endogenous opioid activity (Berridge and Robinson, 2016). Given that PIMs are thought to more closely affect dopaminergic activity through direct effects on dopamine reuptake or release (or effects on cofactors) (Felger and Treadway, 2017; Rengasamy et al., 2021b), excess PIM activity and increased FC between VS-DMN regions (reflecting overregulation of such connectivity secondary to VS dysfunction) may synergistically contribute to decreased motivation to expend effort, particularly in adolescence where the dopaminergic system is rapidly changing. Of note, these effects appeared to be more pertinent below IL-6 levels of 0.7 pg/mL and at IL-6 levels greater than 2 pg/mL, which may help guide clinical studies.
Our findings shed light on PIM associations between distinct subtypes of reward processing – reward anticipation and reward outcome—in an important developmental timeperiod that is characterized by significant neuronal remodeling and changing of dopaminergic responsivity (Galvan, 2010; Shaw et al., 2010). Normatively, adolescence is characterized by significant development of the immune system, particularly in regards to microglial programming and astrocyte development . Existing studies suggest that immune cells (e.g., such as microglia, which release central cytokines) play a necessary important role in adaptive neurogenesis, synaptic pruning, synaptic transmission, and axonal guidance (Bilbo and Schwarz, 2012; Willis et al., 2020). Thus, effects of PIMs on circuitry involved with reward anticipation and outcome in adolescence (where reward circuitry is being modeled) may have longer-standing effects across the lifespan. Future studies are needed to better understand neuroimmune interactions in healthy adolescents and long-term effects of dysfunction neuroimmune interactions on reward processing.
It is important to understand how the current findings can contribute to knowledge of risk factors for adolescent depression. Familial history of depression is considered the leading risk factor for depression (Lewinsohn et al., 2000; Thapar et al., 2012), and in adolescents with such risk, perturbations in reward processes are postulated to play a key role in development of depression (Luking et al., 2016). Thus, understanding the biological and behavioral factors that might be contributing to reward processing disturbances in such populations is of import to elucidate heterogeneity of outcome (i.e., who among members of a high-risk population develops depression) and to understand intervention targets to prevent development of depression (e.g., therapy interventions such as behavioral activation targeting anhedonia). Our current findings generally identify that greater levels of inflammatory markers and VS-DMN connectivity differentially (based on reward anticipation or outcome contrast) predict behavioral effort. More immediately, studies could identify (using larger samples and/or longitudinal, prospective study designs) the exact temporal precedence of such moderating findings (e.g., confirming our theoretical hypotheses here that elevations in inflammatory markers and connectivity measures predict future behavioral effort). Ultimately, future studies could utilize anti-inflammatory interventions (e.g., such as has been done with TNFα antagonists(Lee et al., 2020)) in conjunction with neural data (e.g., elevated VS-DMN connectivity) to potentially increase behavioral effort in real-world settings (e.g. engagement with peer groups) in high-risk populations of adolescents to prevent development of depression.
One limitation of our study is the small sample size (and thus risk of Type II error or correlation magnitude inflation), though no other studies to our knowledge have examined such associations in healthy adolescents at high-risk for depression (Marek et al., 2020). Another is the cross-sectional design, as longitudinal designs would elucidate potential temporal pathways relating inflammatory activation, reward processing, and behavioral correlates of motivation. The focus on high-risk adolescents only precludes comparing this population with adolescents with low familial risk for depression, although a robust literature describes dysfunction in reward processing in adolescents at high risk of depression (Luking et al., 2016) and our goals were primarily to understand heterogeneity among high-risk adolescents in characteristics that have relevance to putative mechanisms of depression. In addition, our behavioral effort task (using a monetary reward) reflects a limited class of rewards that elicit real-world motivation to expend effort, though prior studies find this task is related to behavioral measures of anhedonia suggesting its generalizability (Treadway et al., 2009). Thus, future studies could incorporate other classes of reward, such as socially based rewards (e.g., happy or sad face) to capture other meaningful “real-world” reward phenotypes and understanding the neural underpinnings of such processes, with focus on examining internal consistency for neuroimaging indices to enhance reproducibility (Kohls et al., 2009). Furthermore, it could be fruitful to examine moderating effects of biological sex, given that boys and girls may have different behavioral and neural correlates to different types of rewards (Alarcón and Forbes, 2017; Alarcón et al., 2020; Becker and Chartoff, 2019; Greimel et al., 2018). Future studies would also benefit from examining a broader array of cytokines, as our inflammatory markers largely index nonspecific immune system activation.
In summary, in our study of healthy adolescents at high risk for depression, we found direct positive associations of TNFα with task-dependent FC between the VS and DMN regions. In moderation analyses, we found that greater IL-6 levels and greater task-dependent FC were associated with both (1) less motivation to expend effort, when considering FC during reward anticipation, and (2) greater motivation to expend effort, when considering FC during reward receipt. Such associations may be present given the role of inflammatory markers’ involvement in normative immunodevelopment or stronger associations of dopaminergic activity with reward anticipation, and emphasize the importance of understanding differences in neuroimmune interactions between different reward processes. Future longitudinal studies in healthy high-risk adolescents are needed to temporally understand the role of inflammatory markers in both reward processing and motivation to expend effort, to guide efforts aimed at prevention of depression in this high-risk population.
Supplementary Material
Highlights:
Inflammatory markers are linked to neural reward circuitry and behavioral correlates
TNFα may specifically be linked to reward-related functional connectivity
IL-6 interacts with neural connectivity to predict motivational effort
Future research needs to focus on cytokine interactions with neural circuitry
Acknowledgments:
This research was supported by funding from the Ruth L. Kirschstein National Research Service Award Institutional Research Training Grants sponsored by the National Institutes of Health (NIH T32 MH018951: Brent) and NIH Research Grant Program (NIH R01 MH10810). Research was also supported by a NARSAD Independent Investigator Grant sponsored by the Brain and Behavior Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Conflicts: No conflicts of interest are declared for any author.
Conflict of Interest:
There are no conflicts of interest associated with any author.
References
- Alarcón G, Forbes EE, 2017. Prosocial behavior and depression: a case for developmental gender differences. Current behavioral neuroscience reports 4, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón G, Morgan JK, Allen NB, Sheeber L, Silk JS, Forbes EE, 2020. Adolescent gender differences in neural reactivity to a friend’s positive affect and real-world positive experiences in social contexts. Developmental cognitive neuroscience 43, 100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosia M, Eckstrand KL, Morgan JK, Allen NB, Jones NP, Sheeber L, Silk JS, Forbes EE, 2018. Temptations of friends: adolescents’ neural and behavioral responses to best friends predict risky behavior. Social cognitive and affective neuroscience 13, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranger DA, Lindenmuth M, Nance M, Guyer AE, Keenan K, Hipwell AE, Shaw DS, Forbes EE, 2021. The longitudinal stability of fMRI activation during reward processing in adolescents and young adults. NeuroImage 232, 117872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Chartoff E, 2019. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 2016. Liking, wanting, and the incentive-sensitization theory of addiction. American Psychologist 71, 670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM, 2012. The immune system and developmental programming of brain and behavior. Frontiers in neuroendocrinology 33, 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Arbelaez C, Brent D, 2002. Course and outcome of child and adolescent major depressive disorder. Child and adolescent psychiatric clinics of North America. [DOI] [PubMed]
- Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ, 2020. Characterizing anhedonia: a systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cognitive, Affective, & Behavioral Neuroscience 20, 816–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows K, Stewart JL, Kuplicki R, Figueroa-Hall L, Spechler PA, Zheng H, Guinjoan SM, Savitz JB, Teague TK, Paulus MP, 2021. Elevated peripheral inflammation is associated with attenuated striatal reward anticipation in major depressive disorder. Brain, Behavior, and Immunity 93, 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J, 2008. A meta-analysis of emotional reactivity in major depressive disorder. Clinical psychology review 28, 676–691. [DOI] [PubMed] [Google Scholar]
- Chat IK-Y, Nusslock R, Moriarity DP, Bart CP, Mac Giollabhui N, Damme KS, Carroll AL, Miller GE, Alloy LB, 2021. Goal-striving tendencies moderate the relationship between reward-related brain function and peripheral inflammation. Brain, Behavior, and Immunity 94, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Frías NS, Nikolova YS, Michalski LJ, Baranger DA, Hariri AR, Bogdan R, 2015. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychological medicine 45, 2605–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Acunto G, Nageye F, Zhang J, Masi G, Cortese S, 2019. Inflammatory Cytokines in Children and Adolescents with Depressive Disorders: A Systematic Review and Meta-analysis. Journal of child and adolescent psychopharmacology. [DOI] [PubMed]
- Delgado MR, Nystrom LE, Fissell C, Noll D, Fiez JA, 2000. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of neurophysiology 84, 3072–3077. [DOI] [PubMed] [Google Scholar]
- Dobryakova E, Smith DV, 2021. Reward Enhances Connectivity between the Ventral Striatum and the Default Mode Network. bioRxiv [DOI] [PMC free article] [PubMed]
- Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A, 2004. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R)
- Eckstrand KL, Forbes EE, Bertocci MA, Chase HW, Greenberg T, Lockovich J, Stiffler R, Aslam HA, Graur S, Bebko G, Phillips M, 2019a. Anhedonia reduction and the association between left ventral striatal reward response and 6-month improvement in life satisfaction among young adults. Jama Psychiatry 76, 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstrand KL, Hanford LC, Bertocci MA, Chase HW, Greenberg T, Lockovich J, Stiffler R, Aslam HA, Graur S, Bebko G, 2019b. Trauma-associated anterior cingulate connectivity during reward learning predicts affective and anxiety states in young adults. Psychological medicine 49, 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH, 2016. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular psychiatry 21, 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Treadway MT, 2017. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology 42, 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE, 2012. Research review: altered reward function in adolescent depression: what, when and how? Journal of Child Psychology and Psychiatry 53, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, 2010. Adolescent development of the reward system. Frontiers in human neuroscience 4, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greimel E, Bakos S, Landes I, Töllner T, Bartling J, Kohls G, Schulte-Körne G, 2018. Sex differences in the neural underpinnings of social and monetary incentive processing during adolescence. Cognitive, Affective, & Behavioral Neuroscience 18, 296–312. [DOI] [PubMed] [Google Scholar]
- Halahakoon DC, Kieslich K, O’Driscoll C, Nair A, Lewis G, Roiser JP, 2020. Reward-processing behavior in depressed participants relative to healthy volunteers: A systematic review and meta-analysis. JAMA psychiatry 77, 1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey KL, Morgan J, Musselman SC, Olino TM, Forbes EE, 2014. Social anhedonia and medial prefrontal response to mutual liking in late adolescents. Brain and cognition 89, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Xin S, Ou Y, Zhang W, Liang Y, Chen J, Yang X, Chen X, Guo T, Yang X, 2016. Enhanced default mode network connectivity with ventral striatum in subthreshold depression individuals. Journal of Psychiatric Research 76, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam O, Gong X, Rose-John S, Heese K, 2009. Interleukin-6 and neural stem cells: more than gliogenesis. Molecular biology of the cell 20, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieling C, Adewuya A, Fisher HL, Karmacharya R, Kohrt BA, Swartz JR, Mondelli V, 2019. Identifying depression early in adolescence. The Lancet Child & Adolescent Health 3, 211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Herpertz‐ Dahlmann B, Konrad K, 2009. Differential effects of social and non‐ social reward on response inhibition in children and adolescents. Developmental science 12, 614–625. [DOI] [PubMed] [Google Scholar]
- Lee Y, Mansur RB, Brietzke E, Carmona NE, Subramaniapillai M, Pan Z, Shekotikhina M, Rosenblat JD, Suppes T, Cosgrove VE, 2020. Efficacy of Adjunctive Infliximab vs. Placebo in the Treatment of Anhedonia in Bipolar I/II Depression. Brain, Behavior, and Immunity [DOI] [PubMed]
- Lewinsohn PM, Rohde P, Seeley JR, Klein DN, Gotlib IH, 2000. Natural course of adolescent major depressive disorder in a community sample: predictors of recurrence in young adults. American Journal of Psychiatry 157, 1584–1591. [DOI] [PubMed] [Google Scholar]
- Liu S, Seidlitz J, Blumenthal JD, Clasen LS, Raznahan A, 2020. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proceedings of the National Academy of Sciences 117, 18788–18798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Pagliaccio D, Luby JL, Barch DM, 2016. Reward processing and risk for depression across development. Trends in cognitive sciences 20, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, Donohue MR, Foran W, Miller RL, Feczko E, 2020. Towards reproducible brain-wide association studies. BioRxiv
- Marsland AL, Kuan DC, Sheu LK, Krajina K, Kraynak TE, Manuck SB, Gianaros PJ, 2017. Systemic inflammation and resting state connectivity of the default mode network. Brain Behav Immun 62, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, Burgess S, Penninx BW, Khandaker GM, 2021. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Molecular Psychiatry, 1–10. [DOI] [PMC free article] [PubMed]
- Mirabella F, Desiato G, Mancinelli S, Fossati G, Rasile M, Morini R, Markicevic M, Grimm C, Amegandjin C, Termanini A, 2021. Prenatal interleukin 6 elevation increases glutamatergic synapse density and disrupts hippocampal connectivity in offspring. Immunity 54, 2611–2631. e2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Silk JS, Woods BK, Forbes EE, 2019. Differential neural responding to affective stimuli in 6-to 8-year old children at high familial risk for depression: Associations with behavioral reward seeking. Journal of affective disorders 257, 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, Ng T, Titone MK, Chat IK-Y, Nusslock R, Miller GE, Alloy LB, 2020. Reward responsiveness and ruminative styles interact to predict inflammation and mood symptomatology. Behavior therapy 51, 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Hayes DJ, 2011. Is our self nothing but reward? Biological psychiatry 69, 1019–1025. [DOI] [PubMed] [Google Scholar]
- O’Callaghan G, Stringaris A, 2019. Reward processing in adolescent depression across neuroimaging modalities. Zeitschrift fur Kinder-und Jugendpsychiatrie und Psychotherapie 47, 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, Youssef G, Yücel M, Lorenzetti V, 2018. The anticipation and outcome phases of reward and loss processing: A neuroimaging meta‐ analysis of the monetary incentive delay task. Human brain mapping 39, 3398–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Dahl RE, Ryan ND, Silk JS, Birmaher B, Axelson DA, Forbes EE, 2011. “I won, but I’m not getting my hopes up”: Depression moderates the relationship of outcomes and reward anticipation. Psychiatry Research: Neuroimaging 194, 393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Ng R, Scott H, Kodavaganti S, Smyda G, Diwadkar V, Phillips M, 2017. Ventral striatum functional connectivity during rewards and losses and symptomatology in depressed patients. Biological psychology 123, 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy LF, Horan WP, Barch DM, Buchanan RW, Dunayevich E, Gold JM, Lyons N, Marder SR, Treadway MT, Wynn JK, 2015. Effort-based decision-making paradigms for clinical trials in schizophrenia: part 1—psychometric characteristics of 5 paradigms. Schizophrenia bulletin 41, 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy M, Brundin L, Griffo A, Panny B, Capan C, Forton C, Price RB, 2022a. Cytokine and Reward Circuitry Relationships in Treatment-Resistant Depression. Biological Psychiatry Global Open Science 2, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy M, Da Costa E Silva SA, Spada M, Price RB, 2022b. Does the moderator matter? Identification of multiple moderators of the association between peripheral inflammatory markers and depression severity in a large racially diverse community cohort. Neuropsychopharmacology, 1–9. [DOI] [PMC free article] [PubMed]
- Rengasamy M, Marsland A, McClain L, Kovats T, Walko T, Pan L, Price RB, 2021a. Longitudinal relationships of cytokines, depression and anhedonia in depressed adolescents. Brain, Behavior, and Immunity 91, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy M, Marsland A, Spada M, Hsiung K, Kovats T, Price RB, 2021b. A chicken and egg scenario in psychoneuroimmunology: Bidirectional mechanisms linking cytokines and depression. Journal of Affective Disorders Reports 6, 100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepa E, Fisk J, McCabe C, 2017. Blunted neural response to anticipation, effort and consummation of reward and aversion in adolescents with depression symptomatology. Journal of Psychopharmacology 31, 303–311. [DOI] [PubMed] [Google Scholar]
- Schreiter S, Spengler S, Willert A, Mohnke S, Herold D, Erk S, Romanczuk-Seiferth N, Quinlivan E, Hindi-Attar C, Banzhaf C, 2016. Neural alterations of frontostriatal circuitry during reward anticipation in euthymic bipolar disorder. Psychological Medicine 46, 3187–3198. [DOI] [PubMed] [Google Scholar]
- Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM, 2010. Aging of the innate immune system. Curr Opin Immunol 22. [DOI] [PMC free article] [PubMed]
- Sinclair D, Purves-Tyson TD, Allen KM, Weickert CS, 2014. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology 231, 1581–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, Vulser H, Miranda R, Penttilä J, Struve M, 2015. The brain’s response to reward anticipation and depression in adolescence: dimensionality, specificity, and longitudinal predictions in a community-based sample. American Journal of Psychiatry 172, 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Collishaw S, Pine DS, Thapar AK, 2012. Depression in adolescence. The Lancet 379, 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH, 2012. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. Journal of abnormal psychology 121, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH, 2009. Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PloS one 4, e6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Cooper JA, Miller AH, 2019. Can’t or Won’t? Immunometabolic Constraints on Dopaminergic Drive. Trends in Cognitive Sciences [DOI] [PMC free article] [PubMed]
- Waltmann M, Schlagenhauf F, Deserno L, 2022. Sufficient reliability of the behavioral and computational readouts of a probabilistic reversal learning task. Behavior Research Methods, 1–22. [DOI] [PMC free article] [PubMed]
- Willis EF, MacDonald KP, Nguyen QH, Garrido AL, Gillespie ER, Harley SB, Bartlett PF, Schroder WA, Yates AG, Anthony DC, 2020. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell 180, 833–846. e816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


