Abstract
Objective
Children with a supratentorial midline low-grade glioma (LGG) may be at risk for impaired bone health due to hypothalamic-pituitary dysfunction, obesity, exposure to multiple treatment modalities, and/or decreased mobility. The presence of impaired bone health and/or its severity in this population has been understudied. We aimed to identify the prevalence and risk factors for bone problems in children with supratentorial midline LGG.
Materials and methods
A retrospective study was performed in children with supratentorial midline (suprasellar or thalamic) LGG between 1 January 2003 and 1 January 2022, visiting the Princess Máxima Center for Pediatric Oncology. Impaired bone health was defined as the presence of vertebral fractures and/or very low bone mineral density (BMD).
Results
In total, 161 children were included, with a median age at tumor diagnosis of 4.7 years (range: 0.1–17.9) and a median follow-up of 6.1 years (range: 0.1–19.9). Five patients (3.1%) had vertebral fractures. In 99 patients, BMD was assessed either by Dual Energy X-ray Absorptiometry (n = 12) or Bone Health Index (n = 95); 34 patients (34.3%) had a low BMD (≤ −2.0). Impaired visual capacity was associated with bone problems in multivariable analysis (OR: 6.63, 95% CI: 1.83–24.00, P = 0.004).
Conclusion
In this retrospective evaluation, decreased BMD was prevalent in 34.3% of children with supratentorial midline LGG. For the risk of developing bone problems, visual capacity seems highly relevant. Surveillance of bone health must be an aspect of awareness in the care and follow-up of children with a supratentorial midline LGG.
Significance statement
Patients with supratentorial midline LGG may encounter various risk factors for impaired bone health. Bone problems in survivors of childhood supratentorial midline LGG are, however, understudied. This is the first paper to address the prevalence of bone problems in this specific patient population, revealing visual problems as an important risk factor. Diencephalic syndrome historyand/or weight problems associated with hypothalamic dysfunction were related to bone problems in univariate analyses. The results of this study can be used in the development of guidelines to adequately screen and treat these patients to subsequently minimizing bone problems as one of the endocrine complications.
Keywords: bone surveillance, childhood cancer survivors, hypothalamic-pituitary dysfunction, overweight, suprasellar, visual problems
Introduction
Endocrine complications are common in up to 60% of childhood cancer survivors (CCS) (1). Deprived bone health, ranging from low bone mineral density (BMD) to bone pain and fractures, is a prevalent late effect, and brain tumor survivors especially have a higher risk of fractures and low BMD (2, 3). Multiple risk factors for impaired bone health in CCS have been described (4, 5, 6, 7, 8, 9, 10).
Low-grade glioma (LGG) is the most frequent brain tumor in children (11) with a survival rate of more than 90% after multimodal treatment (12). The adverse effects of the tumor and treatment may severely impact quality of life (13). Children with suprasellar LGG may develop hypothalamic-pituitary dysfunction (HD), resulting in pituitary insufficiency, decreased energy expenditure, overweight and obesity, disturbed day–night rhythm, loss of initiative, hyperphagia, and behavioral problems (14). Overweight and obesity are well-known risk factors for impaired bone mineralization (15, 16). Besides the increase in body mass index (BMI), pituitary deficiencies and immobilization (due to visual problems, fatigue, and/or lack of initiative) are common in these patients and may contribute to bone problems (17). Lastly, children with progressive LGG are often exposed to multiple chemotherapy treatments and new targeted therapies such as inhibitors of the RAS/MAPK signaling pathway, which may negatively influence bone health (18, 19, 20).
Negative disruption of the balance between bone formation and bone resorption is a process that may occur gradually and during disease without initially causing pain or other symptoms. Often, deprived bone health is not noticed until a fracture occurs. Bone mineral density (BMD), as a marker of bone health, can be determined by dual-energy x-ray absorptiometry (DXA) (21). BMD is also reflected by the bone health index (BHI), obtained by measuring metacarpal cortical thickness on x-ray using BoneXpert image analyzing software (22, 23). BMD is an indicator of the bone mineral content of bones.
Even though children with supratentorial midline LGG have multiple risk factors for bone problems, its prevalence or severity has to our knowledge not been described. Awareness of impaired bone health and its associated factors, however, would open doors towards surveillance and prevention, such as combined lifestyle programs and early start of vitamin D, calcium, and/or pituitary hormone substitution.
The International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) recommends screening of bone problems in all CCS treated with cranial radiotherapy (2). For children with LGG, rarely treated with radiotherapy, there is no surveillance protocol for bone health. The aim of this study is to give a detailed description of the prevalence and severity of bone problems and impaired bone health in children with supratentorial midline LGG and to describe its associated factors.
Materials and methods
Study design and population
The Dutch supratentorial midline LGG cohort in collaboration with the ‘Dutch Childhood Oncology Group registry’, was used for selection of patients (https://www.skion.nl/). Patients that had all visited the national center for pediatric oncology, the Princess Máxima Center, Utrecht, The Netherlands, between the opening of the center (2018) and 2022 were selected.
Inclusion criteria were: supratentorial midline LGG (suprasellar or thalamic origin), diagnosed between 1 January 2003 until 1 January 2022 (n = 161), aged ≤18 years at time of tumor diagnosis, and minimum follow-up time of 1 year. Thalamic tumors with involvement of the suprasellar area were included. Patients with an optic nerve glioma without involvement of the optic chiasm were excluded.
Data collection
Data were retrospectively collected from medical records.
Operational definitions
Outcomes
‘Any’ bone problems were defined as the single occurrence or combinations of the following situations: vertebral fractures grade I-III according to the Genant semiquantitative method (on MRI scan or x-ray of the spine) (9, 24), ‘very low BMD’ on a DXA scan (21), or ‘very low BMD’ as determined by BHI (22). The presence of a bone problem was assessed as a binary outcome and a patient with multiple bone problems was only scored once.
‘Very low BMD’ was defined as a Z-score of ≤ −2.0 on the DXA scan (21) or a BHI of ≤ −2.0 SDS, corrected for sex and biological age (25). BMD Z-scores from the total body less head from DXA were used and adjusted for height at time of measurement (26). BHI was assessed by digital x-ray radiogrammetry of the hand (22, 23). If both DXA and BHI were assessed at the same time (maximum 3 months apart), without other bone problems, the DXA values were decisive.
‘At risk for low BMD’ was defined as a Z-score on the DXA scan between −1.0 and −2.0 or a BHI between −1.0 and −2.0 SDS, corrected for sex and age (reference population: healthy Dutch children) (27). Prevalence of bone problems could only be described in the children in whom bone assessment was done. As part of the sensitivity analysis, all analyses were repeated in all children with supratentorial midline LGG. For this sensitivity analysis, if information on bone assessment was missing it was assumed that there were no (clinically relevant) bone problems.
Pituitary dysfunction was defined as having any anterior pituitary deficiency, posterior pituitary deficiency, or central precocious puberty (CPP) (Supplementary1 and 2, see section on supplementary materials given at the end of this article).
Hypothalamic dysfunction was defined according to the diagnostic criteria for hypothalamic syndrome (HS) (17).
Associated factors with bone health
Factors were assessed throughout follow-up until the occurrence of bone health complications (Supplementary 1). The last moment of follow-up was defined as the first occurrence of the bone problem for children with a bone problem, or the last hospital visit (outpatient or inpatient) for children without a bone problem.
Statistical analyses
For normally distributed variables, the mean value was calculated with s.d. For non-normally distributed data, the median value was calculated with the full range and interquartile range (IQR). Characteristics were compared between groups with and without bone problems. The Pearson chi-squared test was used for the association between categorical data (and Fisher’s exact for more than 20% of expected values under five). For analyses of continuous variables with a non-normal distribution, the Mann–Whitney U test was performed and for a normal distribution the Student’s t-test. Assessment of normality of the variables was done using a QQ plot and the Shapiro–Wilk test.
The compound outcome ‘any bone problems’ was assessed in binary multivariable logistic regression. Covariates found to be significant in univariate analyses and/or clinically relevant were included in the multivariable model. The maximum number of covariates included was based on the number of patients in which the outcome was present (one covariate for every ten patients). The Wald test was used to calculate the P-values. P-values <0.05 were considered significant. All analyses were performed by using SPSS version 29.0 (IBM, USA) and R version 4.3.0 (R core team, Vienna, Austria) (packages; haven, dplyr, tidyr, tibble, tidyverse, ggplot2).
Ethics
All patients and parents had given written informed consent for the use of medical data. The Dutch IRB METC NedMec, Utrecht, decided that no additional procedures were required.
Results
Study population
In total, 161 patients with a supratentorial midline LGG were included in this study (Supplementary Table 1). Of them, 49.1% (79/161) were female and the median age at diagnosis of the supratentorial midline LGG was 4.7 years (range: 0.1–17.9). Of all patients, 75.0% (111/148) had experienced one or more tumor progressions during follow-up. In total 55.3% (89/161) had been treated with neurosurgery, 70.2% (113/161) with chemotherapy, 27.3% (44/161) with targeted therapy, and 8.1% (13/161) with radiotherapy. At the time of tumor diagnosis 60.9% (70/115) of patients had a normal weight, 16.5% (19/115) were underweight (diencephalic syndrome), 16.5% (19/115) were overweight, and 6.1% of the patients (7/115) were obese.
The median age at follow-up was 12.6 years (range: 2.5–28.1). In total, 56.7% (80/141) children had entered puberty at follow-up, 14 were currently treated with puberty inhibition and four with estrogen or testosterone suppletion. At follow-up, 13.2% (17/129) of children had a height SDS below −2.0. Normal weight was present in 52.9% (81/153), underweight in 2.0% (3/153), overweight in 29.4% (45/153), and obesity in 15.7% (24/153). HS was present in 39.7% (52/131).
Prevalence of bone problems
Of the 161 patients, vertebral fractures were found in five of them. Of these five, one patient had vertebral fractures of grade I-II and four had a vertebral fracture of grade III. Of all, 99 patients had ever been assessed for bone health with DXA (n = 12, 7.5%) or BHI (n = 95, 59.0%). Of these 99, a total of 34 patients had very low BMD. Median BHI SDS of all measurements in patients with very low BMD was −3.0 (IQR −3.7 to −2.4, range −4.6 to −2.0) and median adjusted BMD Z-score on DXA scan was −3.8 (IQR −4.4 to −2.7, range −4.8 to −2.5) (n = 5). Of all measured patients (n = 99), median BHI SDS was −1.4 (IQR −2.4 to −0.4) and median adjusted BMD Z-score was −2.1 (−4.0 to −1.2).
Taken together, 36.3% of the patients (36/99) had developed a bone problem; two patients based on vertebral fractures grade III only, and all others based on very low BMD, of which three combined with a fracture. In total, 22.2% (22/99) patients showed BMD Z-score between −1 and −2 (low BMD) as assessed by BHI in 20 patients and by DXA scan in two patients. Treatment with bisphosphonates had been administered to one patient with a vertebral fracture and two patients with vertebral fracture of grade III.
Factors associated with bone problems
Children with bone problems (n = 36) were compared with children without bone problems, among those with measurements of BHI or BMD available (n = 63) (Table 1). Children with bone problems had a history of diencephalic syndrome (DS) more often (50.0% versus 10.0%, P < 0.001). Children with bone problems were more often blind (21.7% vs 5.6%) than children without bone problems, and more often severely or moderately visually impaired (8.7% vs 1.9% and 26.1% vs 11.1%, respectively) (P = 0.004). Weight problems associated with hypothalamic dysfunction were present more often in children with bone problems (62.5% vs 36.7%, P = 0.038). There was no difference in the number of patients with hypothalamic syndrome in children with bone problems compared to children without bone problems (for those with measurements available) (Supplementary Table 2)(Table 1).
Table 1.
Covariates in children with bone problems compared to without bone problems. Covariates in children with measurements of BMD/BHI specifically assessed for bone problems (n = 99). Definitions of all variables are attached in Supplementary 1. P-value for between-group differences calculated with Pearson chi-squared test and Fisher’s exact test. If more than 20% of expected values were under five, Fisher’s exact test was performed. For continuous variables (with non-normal distribution), the Mann–Whitney U test was used.
| Covariate | Children with bone problems (%, n/N) n = 36 | Children without bone problems (%, n/N) n = 63 | P |
|---|---|---|---|
| Sex at birth, female | 52.8% (19/36) | 42.9% (27/63) | 0.341 |
| Age at brain tumor diagnosis, median in years (IQR) (range) | 3.2 (0.6–7.9) (0.3–16.5) | 4.4 (2.3–7.5) (0.6–15.5) | 0.099 |
| Follow-up time, median in years (IQR) (range) | 3.6 (2.2–5.9) (0.0–13.9) | 8.5 (5.1–13.3) (2.5–19.9) | <0.001* |
| Muscle problems, yes | 25.0% (5/20) | 7.4% (4/54) | 0.050 |
| Inactivity | 0.330 | ||
| Relative | 52.8% (19/36) | 42.9% (27/63) | |
| Total | 11.1% (4/36) | 6.3% (4/63) | |
| Diencephalic syndrome history, yes | 50.0% (13/26) | 10.0% (5/50) | <0.001* |
| Visual problems | 0.004* | ||
| No/mild | 43.5% (10/23) | 81.5% (44/54) | |
| Moderate | 26.1% (6/23) | 11.1% (6/54) | |
| Severe | 8.7% (2/23) | 1.9% (1/54) | |
| Blindness | 21.7% (5/23) | 5.6% (3/54) | |
| Vitamin D deficiency | 0.145 | ||
| Moderate | 23.1% (3/13) | 41.0% (16/39) | |
| Severe | 23.1% (3/13) | 35.9% (14/39) | |
| Pituitary dysfunction, Yes | |||
| GH deficiency | 22.2% (8/36) | 14.3% (9/63) | 0.314 |
| ACTH deficiency | 25.0% (9/36) | 11.1% (7/63) | 0.071 |
| TSH deficiency | 27.8% (10/36) | 15.9% (10/63) | 0.156 |
| LH/FSH deficiency | 2.8% (1/36) | 3.2% (2/63) | 0.912 |
| Arginine vasopressin deficiency with thirst feeling | 11.1% (4/36) | 6.3% (4/63) | 0.457 |
| Arginine vasopressin deficiency without thirst feeling | 8.3% (3/36) | 1.6% (1/63) | 0.135 |
| No pituitary deficiency | 63.9% (23/36) | 77.8% (49/63) | 0.136 |
| CPP | 38.5% (10/26) | 43.8% (21/48) | 0.660 |
| Elevated IGF-1 | 42.4% (14/33) | 25.4% (15/59) | 0.092 |
| Overweight at follow-up/time of bone problem | 25.8% (8/31) | 35.5% (22/62) | 0.347 |
| Obesity at follow-up/time of bone problem | 12.9% (4/31) | 16.1% (10/62) | 0.682 |
| Significant weight gain | 50.0% (12/24) | 36.0% (18/50) | 0.251 |
| ΔBMI SDS from diagnosis to follow-up, median (IQR) (full range) | 2.2 (0.5–4.8) (−1.0–8.7) | 1.3 (0.1–2.4) (−3.3–7.0) | 0.116 |
| Any clinical relevant weight problem associated with hypothalamic dysfunction** | 62.5% (15/24) | 36.7% (18/49) | 0.038* |
| Surgery treatment, yes | 72.2% (26/36) | 55.6% (35/63) | 0.101 |
| Number of surgeries, median (IQR) (range) | 2 (1–2) (1–6) | 2 (1–2) (1–4) | 0.307 |
| Chemotherapy, yes | 72.2% (26/36) | 74.6% (47/63) | 0.796 |
| Number of chemotherapy rounds, median (IQR) (range) | 2 (1–3) (1–4) | 2 (1–3) (1–5) | 0.249 |
| Targeted therapy | 27.8% (10/36) | 36.5% (23/63) | 0.375 |
| Trametinib | 13.9% (5/36) | 14.3% (9/63) | 0.957 |
| Tovorafenib | 0.0% (0/36) | 4.8% (3/63) | 0.096 |
| VCR neuropathy | 25.0% (9/36) | 22.2% (14/63) | 0.753 |
| Tumor recurrence or progression, Yes | 74.3% (26/35) | 82.5% (47/57) | 0.347 |
*indicates a significant P-value (P < 0.05); **Any clinical relevant weight problem associated with hypothalamic dysfunction includes: diencephalic syndrome history or significant weight gain.
ACTH, adrenocorticotropic hormone; GH, growth hormone; IGF-1, insulin-like growth factor 1; LH/FSH, luteinizing hormone/follicle-stimulating hormone; TSH, thyroid-stimulating hormone.
In multivariable analysis, visual impairment was associated with bone problems (OR: 6.63, 95% CI: 1.83–24.00, P = 0.004) (Table 2).
Table 2.
Multivariable analysis of associated factors with bone problems. Multivariable analysis was done in the children specifically assessed for bone problems (n = 99). Definitions of all variables are attached in Supplementary1. Weight problems includes: Diencephalic syndrome history or significant weight gain or overweight at follow-up or obesity at follow-up. Multivariable binary logistic regression. P-value calculated with Wald test.
| Covariate | All children (n = 66) OR (95% CI) | P |
|---|---|---|
| Age at tumor diagnosis | 1.02 (0.81–1.27) | 0.891 |
| Weight problems due to hypothalamic dysfunction or overweight/obesity at follow-up | 1.68 (0.38–7.31) | 0.493 |
| Visual problems (moderate/severe/blindness vs none) | 6.63 (1.83–24.00) | 0.004* |
*indicates a significant P-value (P < 0.05).
Sensitivity analysis: all included children with supratentorial midline LGG
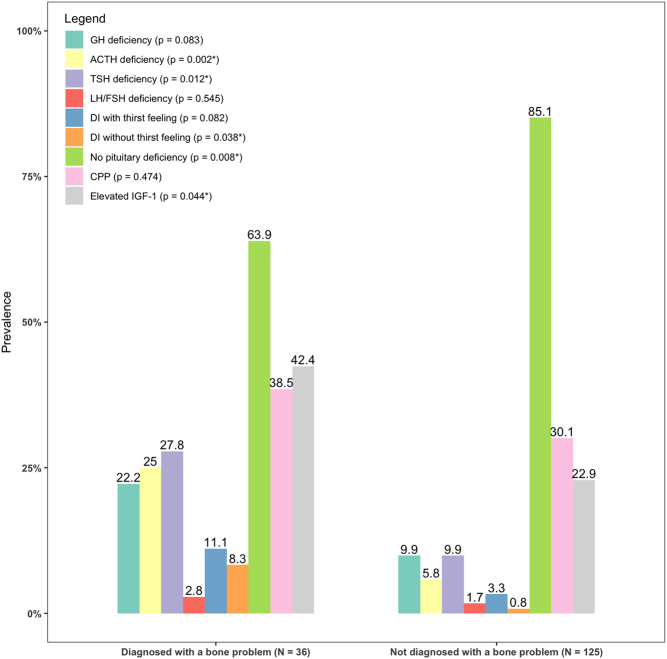
Sensitivity analysis was performed, including all patients (n = 161) (Supplementary Table 3). In this analysis, more bone problems were found in children with any neurosurgical intervention (72.2% vs 50.4%, P = 0.023). Pituitary dysfunction was more prevalent in children with bone problems (Fig. 1). Elevated IGF-1 (without GH replacement therapy) was more often present in children with a bone problem (42.4% vs 22.9%, P = 0.044). In children with bone problems, a total of 60.0% (15/25) had the hypothalamic syndrome compared to 34.9% (37/106) in children without bone problems (P = 0.025) (Table 3). Visual impairment was associated with bone problems in multivariable analysis (SupplementaryTable 4).
Figure 1.
Pituitary deficiencies in children with and without bone problems. Number of pituitary deficiencies in children with clinical relevant bone problems compared to children without clinical relevant bone problems. P-value calculated with Wald test. ACTH, adrenocorticotropic hormone; DI, diabetes insipidus; GH, growth hormone; LH/FSH, luteinizing hormone/follicle-stimulating hormone; TSH, thyroid-stimulating hormone. P-value for between-group differences calculated with Pearson chi-squared test and Fisher’s exact test. If more than 20% of expected values were under five, Fisher’s exact test was performed instead of the chi-squared test.
Table 3.
Covariates of the hypothalamic syndrome in children with bone problems compared to without bone problems, in the whole cohort (n = 161). Definitions of all variables are attached in Supplementary1. P-value for between-group differences calculated with Pearson chi-squared test and Fisher’s exact test. If more than 20% of expected values were under five, Fisher’s exact test was performed. For continuous variables (with non-normal distribution), the Mann–Whitney U test was used.
| Covariate | Children with bone problems (%, n/N) n = 36 | Children without bone problems (%, n/N) n = 125 | P |
|---|---|---|---|
| Hypothalamic syndromeΩ, yes | 60.0% (15/25) | 34.9% (37/106) | 0.025* |
| Hyperphagia | 0.248 | ||
| Mild | 25.0% (5/20) | 19.0% (20/105) | |
| Severe | 0.0% (0/20) | 12.4% (13/105) | |
| Hypophagia | 0.001* | ||
| Mild | 5.0% (1/20) | 1.9% (2/104) | |
| Severe | 20.0% (4/20) | 1.0% (1/104) | |
| BMI | 0.198 | ||
| Normal weight or overweight | 90.3% (28/31) | 78.6% (92/117) | |
| Normal weight after specific intervention for hypothalamic obesity or overweight after specific intervention for hypothalamic obesity or obesity | 9.7% (3/31) | 21.4% (25/117) | |
| Behavioral problems | 0.604 | ||
| Mild | 30.0% (6/20) | 28.0% (26/93) | |
| Severe | 0.0% (0/20) | 7.5% (7/93) | |
| Sleep disorder | 0.148 | ||
| Mild | 25.9% (7/27) | 28.6% (32/112) | |
| Severe | 33.3% (9/27) | 17.0% (19/112) | |
| Temperature dysregulation | 0.059 | ||
| Mild | 37.5% (3/8) | 37.8% (17/45) | |
| Severe | 25.0% (2/8) | 2.2% (1/45) | |
| Pituitary dysfunction | 0.005* | ||
| Partial or complete pituitary dysfunction (with or without DI with adequate thirst feeling) or SIADH or history of central precocious puberty | 45.7% (16/35) | 28.9% (35/121) | |
| (Partial or complete) pituitary dysfunction including DI and adipsia (inadequate thirst feeling) | 8.6% (3/35) | 0.8% (1/121) |
Discussion
Adequate bone health is the prerequisite for normal bone development during childhood (28). Children with oncologic disease are at risk for impaired bone health, frailty, and sarcopenia (29), which in many cases may be prevented with adequate awareness, surveillance, and interventions. Despite multiple risk factors being present in children with supratentorial midline LGG, hardly any studies address this important issue. In our retrospective study, very low BMD was found to be highly prevalent in children with supratentorial midline LGG (34.3%), comparable with the prevalence of bone problems in pediatric chronic diseases (30).
Visual problems were associated with bone problems, most probably due to decreased mobility. We did not find any studies reporting on a higher risk for bone problems in otherwise healthy children with a visual handicap, but one may hypothesize that physical activity will be influenced. Increasing physical activity is considered to benefit BMD (31). The crude retrospective assessment of activity in our study does not reflect everyday life activity and should be studied more in-depth in future prospective studies. Other associations from univariate analysis were hypothalamic dysfunction (diencephalic syndrome at diagnosis and weight problems).
To our knowledge, the association between a history of diencephalic syndrome and a bone problem later in life has not been reported before. Of the children developing a bone problem, 50% has had the diencephalic syndrome. Multiple factors may underlie this association. The poor nutritional state at a young age in children with DS may be accountable for the process of deterioration of bone health at an early age. Similarly, patients with anorexia nervosa also experience deterioration of bone health, with osteoporosis reported in up to 20–30% (32). Next to low fat-mass, low calcium, and vitamin D status, a lower weight also affects physical mobility and therefore lower lean mass (33, 34, 35). The finding that bone health was still impaired after regaining weight in children with DS may be explained by the fact that the weight gain in children after DS is often exceptionally rapid with the development of hypothalamic overweight or obesity (36). Children with hypothalamic obesity often also show loss of initiative and decreased activity, contributing to poor bone health. Also, the fact that patients with elevated IGF-1 concentrations were at increased risk for a bone problem may be a reflection of hypothalamic damage and may be confounding to the presence of DS or age (37, 38). To correct for confounders, multivariable analysis was performed, which showed only vision loss as a significant associated factor for any bone problem. The association between vision loss and a bone problem may be caused by decreased physical activity (8, 39) or disturbance of the circadian rhythm due to visual impairment (7), or more likely, confounded by tumor location and hypothalamic involvement (40). Larger cohorts in future studies are needed to further evaluate the association between hypothalamic dysfunction and bone health.
Although not statistically significant in multivariable analysis, the prevalence of hypothalamic syndrome in the complete cohort of children with supratentorial midline LGG was higher in children with bone problems compared to children without bone problems. Hypothalamic dysfunction may negatively contribute to the homeostasis of the bone tissue (14, 17), which may be caused by pituitary insufficiency (GH or TSH deficiency), obesity, loss of initiative, and decreased mobility.
Lastly, novel targeted therapies, such as RAF or MEK inhibitors, might contribute to bone resorption. Bone renewal no longer occurs sufficiently because cell growth and cell proliferation are inhibited (18, 19). As a result, the quality of the bone tissue decreases. In our study, however, trametinib and tovorafenib were not found to be univariately associated with bone problems. Also vincristine neuropathy was not associated with bone problems.
Limitations of the study
The design of this study may be considered a limitation, due to missing data. Questionnaires could not adequately assess inactivity and muscle problems because of the retrospective nature of this study. As most of the associations were tested univariately, no causal relationship can be drawn. Assessment of bone health during follow-up was not routinely assessed by DXA scanning, the current standard for determination of BMD (only available in 7.5% of the patients). It may be questioned whether the children without BMD or BHI measurements may have normal or lowered BMD as low BMD does not give any clinical signs and symptoms. There were apparently no clinical reasons for the treating physician to measure BMD or BHI and no reasons for the radiologist to suspect low bone quality after assessing the spinal images. Additionally, the fact that no x-ray of the hand had been performed may indicate that in these patients no longitudinal growth problems were present. Therefore, we expect it is reasonable to assume that these children did not have clinically relevant bone problems. However, bone problems can progress silently, and there may have been a misclassification bias in this case for which reason we first analyzed the 99 children with fractures or measured BMD and subsequently repeated the measurement in the total group. Both in the total group and in the 99 children with measurements of BMD/BHI, the same main findings in the multivariable analyses were found. It must be considered however, that also in the 99 cases with measurements of BMD/BHI, selection bias may have been present due to the fact that they had more frequent pituitary and hypothalamic problems. Patients referred to the endocrine department will have more frequent investigations of bone age than those without hypothalamic-pituitary (HP) dysfunction. This also explains that in the subgroup of 99 patients no differences were found in HP dysfunction between children with or without bone problems. It was not possible to draw any conclusions of the timing of bone problem development during follow-up, as no systematic measurements were done and digital x-ray radiogrammetry of the hand before 2010 did not contain the BHI.
Conclusion
Despite the limitations, our study provides new and valuable data on the prevalence and factors associated with bone problems in patients with supratentorial midline LGG. The prevalence of decreased bone health is high and the occurrence of bone problems in children with a supratentorial midline LGG may have a multifactorial origin. The results of our study may be used for future guideline development for surveillance and prevention of (severe) bone problems in patients with supratentorial midline LGG. Physicians should be aware of the risk for bone problems in children with supratentorial midline LGG, especially in case of visual problems and hypothalamic syndrome. Surveillance using DXA scans, supplementation of minerals and vitamins important for bone health (vitamin D, calcium, but also folic acid and vitamin B12) (9), and optimal stimulation of physical activity (increasing mobilization, weight-bearing exercises, combined lifestyle programs, and if indicated referral to a physiotherapist) may help to prevent (further) bone loss in these children in the future (2). Physical therapy should be specifically designed and offered in cases of restricted visual capacity and lack of independent physical freedom and loss of initiative that may be encountered in patients with HS.
Future
Future research on bone health in LGG patients should ideally focus on prevalence and risk factors in prospective cohorts and pave the way to prevent BMD loss. To validate current findings, further studies are needed to evaluate the value of BHI using x-Ray of the hand with DXA, in this specific group of children. As children with a supratentorial midline tumor regularly undergo x-rays of the hand for endocrine evaluation purposes, this would be an ideal screening instrument for this group.
Supplementary Materials
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contribution statement
Conception and design: IMAAR, JEG, BB, LMH, LJ, SMAYN, SHM; Collection and assembly of data: IMAAR, JEG; Data analysis and interpretation: all authors; Manuscript writing: all authors; Final approval: all authors; Accountable for all aspects of the work: all authors
References
- 1.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, et al.Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 20133092371–2381. ( 10.1001/jama.2013.6296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Atteveld JE, Mulder RL, van den Heuvel-Eibrink MM, Hudson MM, Kremer LCM, Skinner R, Wallace WH, Constine LS, Higham CE, Kaste SC, et al.Bone mineral density surveillance for childhood, adolescent, and young adult cancer survivors: evidence-based recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet. Diabetes and Endocrinology 20219622–637. ( 10.1016/S2213-8587(2100173-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Hoed MA, Klap BC, te Winkel ML, Pieters R, van Waas M, Neggers SJCMM, Boot AM, Blijdorp K, van Dorp W, Pluijm SM, et al.Bone mineral density after childhood cancer in 346 long-term adult survivors of childhood cancer. Osteoporosis International 201526521–529. ( 10.1007/s00198-014-2878-z) [DOI] [PubMed] [Google Scholar]
- 4.Kvammen JA Stensvold E Godang K Bollerslev J Myklebust TÅ Brandal P Henriksen C & Bechensteen AG. Bone mineral density and nutrition in long-term survivors of childhood brain tumors. Clinical Nutrition ESPEN 202250162–169. ( 10.1016/j.clnesp.2022.05.025) [DOI] [PubMed] [Google Scholar]
- 5.Mazziotti G Frara S & Giustina A. Pituitary diseases and bone. Endocrine Reviews 201839440–488. ( 10.1210/er.2018-00005) [DOI] [PubMed] [Google Scholar]
- 6.Rossi F Tortora C Paoletta M Marrapodi MM Argenziano M Di Paola A Pota E Di Pinto D Di Martino M & Iolascon G. Osteoporosis in childhood cancer survivors: physiopathology, prevention, therapy and future perspectives. Cancers (Basel) 202214. ( 10.3390/cancers14184349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki N Fujiwara S Yamashita H Ozono R Teramen K & Kihara Y. Impact of sleep on osteoporosis: sleep quality is associated with bone stiffness index. Sleep Medicine 20162573–77. ( 10.1016/j.sleep.2016.06.029) [DOI] [PubMed] [Google Scholar]
- 8.Szmodis M Kälbli K Kaj M Király A Almási G & Csányi T. Bone characteristics and physical fitness in children and adolescents with visual impairment. Journal of Sports Medicine and Physical Fitness 20226281–89. ( 10.23736/S0022-4707.21.12078-X) [DOI] [PubMed] [Google Scholar]
- 9.van Atteveld JE, de Winter DTC, Pluimakers VG, Fiocco M, Nievelstein RAJ, Hobbelink MGG, de Vries ACH, Loonen JJ, van Dulmen-den Broeder E, van der Pal HJ, et al.Risk and determinants of low and very low bone mineral density and fractures in a national cohort of Dutch adult childhood cancer survivors (DCCSS-LATER): a cross-sectional study. Lancet. Diabetes and Endocrinology 20231121–32. ( 10.1016/S2213-8587(2200286-8) [DOI] [PubMed] [Google Scholar]
- 10.van Santen HM Chemaitilly W Meacham LR Tonorezos ES & Mostoufi-Moab S. Endocrine health in childhood cancer survivors. Pediatric Clinics of North America 2020671171–1186. ( 10.1016/j.pcl.2020.08.002) [DOI] [PubMed] [Google Scholar]
- 11.Plant-Fox AS O'Halloran K & Goldman S. Pediatric brain tumors: the era of molecular diagnostics, targeted and immune-based therapeutics, and a focus on long term neurologic sequelae. Current Problems in Cancer 202145100777. ( 10.1016/j.currproblcancer.2021.100777) [DOI] [PubMed] [Google Scholar]
- 12.Collins KL & Pollack IF. Pediatric low-grade gliomas. Cancers (Basel) 202012. ( 10.3390/cancers12051152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gan HW Phipps K Aquilina K Gaze MN Hayward R & Spoudeas HA. Neuroendocrine morbidity after pediatric optic gliomas: A longitudinal analysis of 166 children over 30 years. Journal of Clinical Endocrinology and Metabolism 20151003787–3799. ( 10.1210/jc.2015-2028) [DOI] [PubMed] [Google Scholar]
- 14.Müller HL Tauber M Lawson EA Özyurt J Bison B Martinez-Barbera JP Puget S Merchant TE & van Santen HM. Hypothalamic syndrome. Nature Reviews. Disease Primers 2022824. ( 10.1038/s41572-022-00351-z) [DOI] [PubMed] [Google Scholar]
- 15.Tian Y Ma X Yang C Su P Yin C & Qian AR. The impact of oxidative stress on the bone system in response to the space special environment. International Journal of Molecular Sciences 201718. ( 10.3390/ijms18102132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vranić L Mikolašević I & Milić S. Vitamin D deficiency: consequence or cause of obesity? Medicina (Kaunas) 201955. ( 10.3390/medicina55090541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Santen HM van Schaik J van Roessel IMAA Beckhaus J Boekhoff S & Müller HL. Diagnostic criteria for the hypothalamic syndrome in childhood. European Journal of Endocrinology 2023188. ( 10.1093/ejendo/lvad009) [DOI] [PubMed] [Google Scholar]
- 18.Dumas M, Laly P, Gottlieb J, Vercellino L, Paycha F, Bagot M, Baroudjian B, Madelaine I, Basset-Seguin N, Eftekhari P, et al.Osteopenia and fractures associated with long-term therapy with MEK inhibitors. Melanoma Research 201828641–644. ( 10.1097/CMR.0000000000000490) [DOI] [PubMed] [Google Scholar]
- 19.D'Oronzo S Stucci S Tucci M & Silvestris F. Cancer treatment-induced bone loss (CTIBL): pathogenesis and clinical implications. Cancer Treatment Reviews 201541798–808. ( 10.1016/j.ctrv.2015.09.003) [DOI] [PubMed] [Google Scholar]
- 20.van Dorp W van Beek RD Laven JS Pieters R de Muinck Keizer-Schrama SM & van den Heuvel-Eibrink MM. Long-term endocrine side effects of childhood Hodgkin's lymphoma treatment: a review. Human Reproduction Update 20121812–28. ( 10.1093/humupd/dmr038) [DOI] [PubMed] [Google Scholar]
- 21.Shuhart CR Yeap SS Anderson PA Jankowski LG Lewiecki EM Morse LR Rosen HN Weber DR Zemel BS & Shepherd JA. Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. Journal of Clinical Densitometry 201922453–471. ( 10.1016/j.jocd.2019.07.001) [DOI] [PubMed] [Google Scholar]
- 22.Shalof H Dimitri P Shuweihdi F & Offiah AC. Which skeletal imaging modality is best for assessing bone health in children and young adults compared to DXA? A systematic review and meta-analysis. Bone 2021150116013. ( 10.1016/j.bone.2021.116013) [DOI] [PubMed] [Google Scholar]
- 23.Offiah AC. Current and emerging artificial intelligence applications for pediatric musculoskeletal radiology. Pediatric Radiology 2022522149–2158. ( 10.1007/s00247-021-05130-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genant HK Wu CY van Kuijk C & Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. Journal of Bone and Mineral Research 199381137–1148. ( 10.1002/jbmr.5650080915) [DOI] [PubMed] [Google Scholar]
- 25.Bialo SR & Gordon CM. Underweight, overweight, and pediatric bone fragility: impact and management. Current Osteoporosis Reports 201412319–328. ( 10.1007/s11914-014-0226-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, et al.Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. Journal of Clinical Endocrinology and Metabolism 2011963160–3169. ( 10.1210/jc.2011-1111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schündeln MM, Marschke L, Bauer JJ, Hauffa PK, Schweiger B, Führer-Sakel D, Lahner H, Poeppel TD, Kiewert C, Hauffa BP, et al.A piece of the puzzle: the bone health index of the BoneXpert software reflects cortical bone mineral density in pediatric and adolescent patients. PLoS One 201611e0151936. ( 10.1371/journal.pone.0151936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu J Shin Y Yen MS & Sun SS. Peak bone mass and patterns of change in total bone mineral density and bone mineral contents from childhood into young adulthood. Journal of Clinical Densitometry 201619180–191. ( 10.1016/j.jocd.2014.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Atteveld JE, de Winter DTC, Pluimakers VG, Fiocco M, Nievelstein RAJ, Hobbelink MGG, Kremer LCM, Grootenhuis MA, Maurice-Stam H, Tissing WJE, et al.Frailty and sarcopenia within the earliest national Dutch childhood cancer survivor cohort (DCCSS-LATER): a cross-sectional study. Lancet. Healthy Longevity 20234e155–e165. ( 10.1016/S2666-7568(2300020-X) [DOI] [PubMed] [Google Scholar]
- 30.Högler W & Ward L. Osteoporosis in children with chronic disease. Endocrine Development 201528176–195. ( 10.1159/000381045) [DOI] [PubMed] [Google Scholar]
- 31.Chen CL Chen CY Liaw MY Chung CY Wang CJ & Hong WH. Efficacy of home-based virtual cycling training on bone mineral density in ambulatory children with cerebral palsy. Osteoporosis International 2013241399–1406. ( 10.1007/s00198-012-2137-0) [DOI] [PubMed] [Google Scholar]
- 32.Legroux I & Cortet B. Factors influencing bone loss in anorexia nervosa: assessment and therapeutic options. RMD Open 20195e001009. ( 10.1136/rmdopen-2019-001009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang BY Huang W Zhou GQ Hu N Chen H & Chen C. Body mass index and the risk of low bone mass-related fractures in women compared with men: a PRISMA-compliant meta-analysis of prospective cohort studies. Medicine (Baltimore) 201796e5290. ( 10.1097/MD.0000000000005290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misra M & Klibanski A. Anorexia nervosa and bone. Journal of Endocrinology 2014221R163–R176. ( 10.1530/JOE-14-0039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapses SA & Riedt CS. Bone, body weight, and weight reduction: what are the concerns? Journal of Nutrition 20061361453–1456. ( 10.1093/jn/136.6.1453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Roessel IMAA Schouten-van Meeteren AYN Meijer L Hoving EW Bakker B & van Santen HM. Transition from diencephalic syndrome to hypothalamic obesity in children with suprasellar low grade glioma: a case series. Frontiers in Endocrinology 202213846124. ( 10.3389/fendo.2022.846124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleischman A Brue C Poussaint TY Kieran M Pomeroy SL Goumnerova L Scott RM & Cohen LE. Diencephalic syndrome: a cause of failure to thrive and a model of partial growth hormone resistance. Pediatrics 2005115e742–e748. ( 10.1542/peds.2004-2237) [DOI] [PubMed] [Google Scholar]
- 38.Hawkes CP & Grimberg A. Insulin-like growth factor-I is a marker for the nutritional state. Pediatric Endocrinology Reviews 201513499–511. [PMC free article] [PubMed] [Google Scholar]
- 39.Brian A Pennell A Haibach-Beach P Foley J Taunton S & Lieberman LJ. Correlates of physical activity among children with visual impairments. Disability and Health Journal 201912328–333. ( 10.1016/j.dhjo.2018.10.007) [DOI] [PubMed] [Google Scholar]
- 40.Swanson CM Kohrt WM Buxton OM Everson CA Wright KP Jr Orwoll ES & Shea SA. The importance of the circadian system & sleep for bone health. Metabolism: Clinical and Experimental 20188428–43. ( 10.1016/j.metabol.2017.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a