Abstract
Summary
Familial renal glucosuria (FRG) is a rare renal tubular disorder characterized by increased urinary glucose excretion despite normoglycemia. It is most commonly caused by pathogenic variants in the solute carrier family V member 2 (SLC5A2) gene. This gene encodes the sodium–glucose cotransporter 2, crucial for glucose reabsorption. We report the case of a 44-year-old male referred to the endocrinology outpatient clinic for unexplained glucosuria despite well-controlled diabetes mellitus with metformin and gliclazide therapy. His main complaints were nocturia and an unintentional 5 kg weight loss in 1 year. A 24-h urinary collection revealed overt glucosuria (23.3 g/1.73 m2/24 h), generalized aminoaciduria, and increased uric acid excretion (fractional excretion: 6.4%). Whole-exome sequencing revealed a novel heterozygous c.469-1G>A likely pathogenic variant in the SLC5A2 gene. Specific analysis of the maturity-onset diabetes of the young type (MODY) gene panel showed no pathogenic variants in the hepatocyte nuclear factor-1A (HNF-1A; MODY3) nor in other MODY-associated genes. We assume that the association of glucosuria, aminoaciduria, and increased uric acid excretion can be explained by the combination of diabetes and the likely pathogenic SLC5A2 variant in this patient. In conclusion, we describe a well-controlled diabetic patient with FRG, associated with a novel heterozygous c.469-1G>A likely pathogenic variant in the SLC5A2 gene.
Learning points
The diagnosis of a renal tubular disorder should be considered in patients with unexplained glucosuria and diabetes mellitus, especially if the latter is well controlled.
FRG usually presents with glucosuria but may be associated with generalized aminoaciduria and hyperuricosuria.
Genetic analysis should be considered in patients with young-onset diabetes and glucosuria, particularly with a positive family history.
Background
Familial renal glucosuria (FRG) is a rare hereditary disorder characterized by persistent isolated glucosuria despite normal blood glucose levels, caused by a reduction in the renal tubular reuptake of glucose (1). In most cases, no associated renal tubular dysfunction is observed (e.g. proteinuria, phosphaturia, and uricosuria). The ability of the kidney to reabsorb glucose largely depends on the sodium–glucose cotransporter 2 (SGLT2), located in the proximal convoluted tubule segment S1, which is responsible for 90% of glucose reabsorption (2). The SGLT2 protein consists of 14 transmembrane-spanning domains and is encoded by the solute carrier family V member 2 (SLC5A2) gene. Pathogenic variants in SLC5A2 are responsible for FRG. Both autosomal dominant and recessive inheritance have been described, although it may be a semi-dominant inheritance with incomplete penetrance in heterozygous pathogenic variant carriers and a more severe phenotype in individuals with bi-allelic pathogenic variants (1, 3). FRG is a rare genetic disease with a historically estimated prevalence of 0.29% in the general Caucasian population and of < 0.1% in a screening program for Japanese children. It is considered a benign condition not requiring any specific treatment as its prognosis is usually favorable (4).
Case presentation
We report the case of a 44-year-old male of West African origin, referred to the endocrinology outpatient clinic for unexplained glucosuria despite diabetes mellitus well controlled with metformin and gliclazide therapy. He was diagnosed with diabetes mellitus at the age of 38. His personal history was otherwise unremarkable. In particular, he had no history of urinary tract infections. He reported no familial history of diabetes or kidney disease. His seven children were in good health. He mentioned nocturia and an unintentional 5 kg weight loss in 1 year despite normal appetite. His body mass index was 24.5 kg/m2 (a weight of 62 kg and a height of 1.59 m).
Investigation
Laboratory analysis showed a fasting glycemia of 138 mg/dL, C-peptide of 0.692 nmol/L (normal range (NR): 0.370–1.470 nmol/L), HbA1c of 6.5% (48 mmol/mol), and hypercholesterolemia with total cholesterol of 220 mg/dL, LDL-cholesterol of 156 mg/dL, and HDL-cholesterol of 41 mg/dL. Renal function and uric acid levels were normal. Proteinuria was normal at 0.08 g/24 h (NR: 0.03–0.22 g/24 h). Two 24-h urinary collections revealed overt glucosuria (7.8–23.3 g/1.73 m2/24 h; NR: <0.3 g/1.73 m2/24 h) as well as generalized aminoaciduria (hydroxyproline, serine, glycine, alanine, valine, cysteine, isoleucine, and lysine) and increased uric acid excretion (fractional excretion 6.4%) (Table 1).
Table 1.
Results of the two 24-h urinary collections in the patient.
| Parameter | Patient's collection | Normal values | |
|---|---|---|---|
| Number 1 | Number 2 | ||
| Volume, mL | 1550 | 1150 | |
| Creatinine, g/24 h | 1.27 | 1.32 | 0.8–2.00 |
| Uric acid, mg/24 h | 690 | 557 | 180–550 |
| Glucose, g/24 h | 39.22 | 13.54 | <0.50 |
| Phosphorus, mmol/24 h | 24.0 | 19.3 | 12.9–45.0 |
| Protein, g/24 h | 0.08 | 0.10 | 0.03–0.22 |
| Hydroxyproline, µmol/g creatinine | 34 | 55 | <30 |
| Serine, µmol/g creatinine | 444 | 229 | <400 |
| Glycine, µmol/g creatinine | 2359 | 1358 | <2100 |
| Alanine, µmol/g creatinine | 981 | 602 | <350 |
| Valine, µmol/g creatinine | 112 | 42 | <60 |
| Cysteine, µmol/g creatinine | 62 | 58 | <40 |
| Isoleucine, µmol/g creatinine | 35 | 24 | <20 |
| Lysine, µmol/g creatinine | 270 | 187 | <260 |
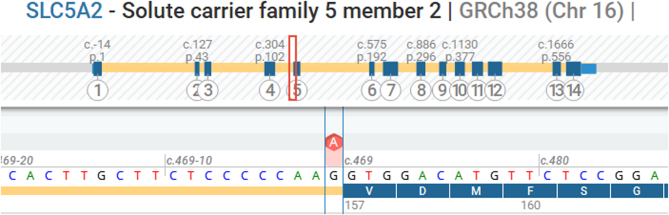
Considering his severe glucosuria and aminoaciduria despite well-controlled diabetes mellitus, genetic testing for an underlying renal tubulopathy was performed. Acquired tubular dysfunction was excluded. Whole-exome sequencing with analysis of a nephropathy gene panel (with specific attention to the SLC5A2 and hepatocyte nuclear factor-1A (HNF-1A) genes) was performed using massive parallel sequencing of the Twist Biosciences Exome CustomV2 kit on NovaSeq6000 (Illumina). Variants were annotated using RefSeq v214, genome build GRCh38, and Alissa Interpret (v5.4; Agilent). Both genes were sequenced with high quality, with exonic and 20 bp intronic regions reliably covered. The analysis revealed a novel heterozygous c.469-1G>A variant in the SLC5A2 gene (NM_003041.4) (Fig. 1). This variant is predicted to alter splicing. More specifically, SpliceAI predicts the loss of the splice acceptor site with a delta score of 1.0. According to the American College of Medical Genetics and Genomics guidelines, the variant can be classified as ‘likely pathogenic’ based on criteria PVS1 and PM2 (pathogenic very strong evidence and pathogenic moderate evidence).
Figure 1.
Mutation analysis of the solute carrier family 5 member 2 (SLC5A2) gene in the patient. Screenshot from ‘Alamut Visual Plus’. The position of the heterozygous c.469-1G>A likely pathogenic variant is indicated in red.
Since the patient was diagnosed with diabetes at a young age, a diagnosis of maturity-onset diabetes of the young type (MODY) was considered. No pathogenic variants were detected in the HNF-1A gene or in other genes included in the MODY gene panel (Table 2). In addition, anti-glutamic acid decarboxylase autoantibodies were not detected (<1.0 IU/mL; NR: <7.5 IU/mL).
Table 2.
The MODY panel performed on the patient.
| Gene | Transcript | Coverage |
|---|---|---|
| ABCC8 | NM_000352.6 | 99.97% |
| GCK | NM_000162.5 | 100.00% |
| GLUD1 | NM_005271.5 | 100.00% |
| HADH | NM_005327.7 | 100.00% |
| HNF1A | NM_000545.8 | 100.00% |
| HNF1B | NM_000458.4 | 99.85% |
| HNF4A | NM_175914.5 | 100.00% |
| INS | NM_000207.3 | 100.00% |
| INSR | NM_000208.4 | 100.00% |
| KCNJ11 | NM_000525.4 | 100.00% |
| SLC16A1 | NM_003051.4 | 100.00% |
Treatment
The patient was advised to take extra care of his diet, avoiding excessive glucose intake. He continued metformin 850 mg three times daily and gliclazide 30 mg once daily. The association of an SGLT2 inhibitor (SGLT2i) was considered but eventually not initiated because of well-controlled diabetes mellitus (HbA1c: < 7.0%), preserved kidney function, and absence of heart failure.
Outcome and follow-up
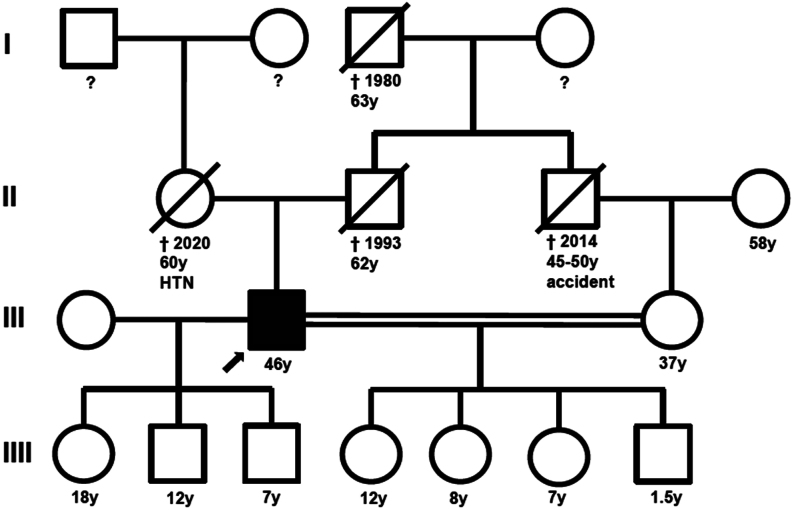
Genetic testing of his children was offered to the patient because renal glucosuria, although most often asymptomatic, can rarely be accompanied by polyuria and polydipsia. The patient’s second wife was his cousin. Given this consanguinity, the risk of a homozygous pathogenic variant in their offspring is increased (Fig. 2).
Figure 2.
Pedigree chart. The patient’s second wife is his cousin (double line). The patient was advised to seek genetic counseling, but he preferred not to inform his children since his condition is benign. HTN, arterial hypertension.
The patient agreed to consult a geneticist but decided to wait to inform his children given the benign nature of the condition. His weight decreased unintentionally to 60 kg and remained stable after a 6-month follow-up. Because of persisting lower urinary tract symptoms, he consulted the urology department, where he was diagnosed with benign prostatic hyperplasia. Treatment with tamsulosin 0.4 mg once daily was initiated.
Discussion
We describe a patient with FRG due to a novel heterozygous likely pathogenic SLC5A2 variant with a presentation of overt glucosuria (associated with aminoaciduria and hyperuricosuria) discordant with his well-controlled type 2 diabetes mellitus.
Glucosuria can be a part of a more global dysfunction of the proximal tubule, such as in renal Fanconi syndrome, with the association of excessive urinary excretion of amino acids, phosphate, bicarbonate, and other solutes normally reabsorbed in the proximal tubule. When isolated normoglycemic glucosuria occurs without tubular dysfunction, it is known as FRG, which is an inherited disorder (2). The pattern of inheritance of FRG was initially thought of as autosomal recessive or autosomal dominant (2, 4). However, familial variant analysis suggested that FRG is best described as semi-dominant with incomplete penetrance in heterozygous pathogenic variant carriers and a more severe phenotype in individuals with biallelic pathogenic variants (1, 3). The variable penetrance might depend on either the compensatory ability of the wild-type allele or otherwise on additional environmental factors (such as diet and climate) influencing glucose reabsorption. This can possibly explain why individuals with the same pathogenic SLC5A2 variant exhibit different degrees of glucosuria (4). Most patients do not develop serious clinical problems, and FRG is therefore considered a benign condition. Patients often complain of polyuria and enuresis, as in our case. Similar to patients taking SGLT2i, episodic dehydration and an increased incidence of urinary tract infections have also been described (2, 4). Weight loss in our patient can be explained by the severity of his glucosuria, with a urinary wasting of 25 g of glucose per day, representing about 4% of his recommended daily calorie intake. To the best of our knowledge, ketoacidosis (a known side effect of SGLT2i) has not been described in patients with SLC5A2 variants.
Generalized or selective aminoaciduria can be a feature of FRG, although it has been suggested that aminoaciduria in this setting is a consequence of the impairment in glucose reabsorption rather than a direct consequence of the pathogenic SLC5A2 variant or a sign of tubular dysfunction (5). Similarly, in patients with type 1 and type 2 diabetes mellitus, it is assumed that the level of aminoaciduria correlates positively with the degree of glucosuria (6). This was also present in our patient's urinary collections, where a trend between the level of glucosuria and aminoaciduria was noticed (Table 1). Bingham et al. suggested that glucosuria causes depolarization and dissipation of the electric gradient of sodium-dependent amino acid transporters in the renal tubule, leading to decreased amino acid resorption (6). Evidence of a direct relation between SGLT2 function and tubular amino acid transport has not been established (5). Renal glucosuria associated with generalized aminoaciduria has also been described in MODY 3, an autosomal dominant form of diabetes occurring at a young age (2). MODY 3 is caused by a pathogenic variant in the HNF-1A gene expressed in the kidney (6). The resulting protein acts as a transcription regulator for SLC5A2, and the consequently reduced SGLT2 expression causes defective renal glucose resorption. Unlike in the case of type 1 and type 2 diabetes, aminoaciduria in MODY 3 is also caused by a direct effect of the pathogenic HNF-1A gene variant on the activity of amino acid transporters (6). The diagnosis of MODY 3 was considered in our patient because he was diagnosed with diabetes mellitus at age 38, was not obese, and had preserved beta-cell function more than 5 years after the initial diagnosis. However, no pathogenic variants in the HNF-1A gene were detected. Few cases of glucosuria and aminoaciduria associated with pathogenic SLC5A2 variants have been reported in patients with biallelic pathogenic variants in consanguineous families (5). To the best of our knowledge, this is the first report of a patient with a heterozygous SLC5A2 variant with aminoaciduria, but the prevalence can be underestimated (if aminoaciduria was not evaluated).
While hyperuricemia is common in type 2 diabetes (because of altered renal uric acid excretion due to insulin resistance and hyperinsulinemia), our patient exhibited normal uric acid levels combined with increased urinary uric acid excretion. The SLC5A2 variant found in our patient may explain this observation, and the mechanism may be similar to the urate-lowering effect of SGLT2i. SGLT2i increases glucosuria, and the elevated urinary glucose activates the excretion of uric acid transported by the apical glucose transporter 9 (GLUT9), leading to hyperuricosuria. In addition, increased glucosuria inhibits uric acid reabsorption by uric acid reabsorption transporter 1 (URAT1) in the proximal tubule and GLUT9 in the collecting duct, promoting hyperuricosuria (Fig. 3) (7). SGLT2i might also suppress uric acid reabsorption by URAT1 by reducing serum insulin concentration.
Figure 3.
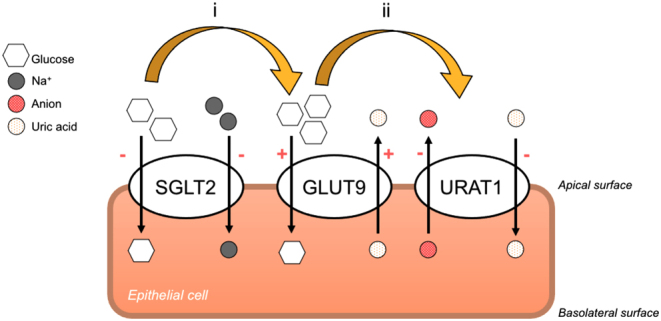
The role of SGLT2 inhibitor (SGLT2i) in hypouricemia induction. Modified from Dong et al. (7). (i) SGLT2i blocks SGLT2 on the apical surface of the epithelial cell, which increases renal glucose excretion. The resulting glucosuria stimulates GLUT9 to reabsorb glucose in exchange for uric acid excretion. (ii) Elevated glucosuria suppresses uric acid reabsorption by uric acid reabsorption transporter 1 (URAT1) in the proximal tubule and GLUT9 in the collecting duct.
Hypothetically, the presence of a loss-of-function pathogenic variant in SLC5A2, leading to lower expression levels of SGLT2 cotransporters, could provide cardiovascular and renal protection, analogous to SGLT2i therapy (8). The genetic defect of the SGLT2 cotransporter results in increased outflow of sodium, chloride, and glucose to the distal parts of the nephron, which activates tubuloglomerular feedback consisting of afferent arteriolar vasoconstriction, reducing blood flow, intraglomerular filtration pressure, and glomerular filtration rate. Neuwirt et al. demonstrated in their case report of a patient with FRG that the sensitivity of the tubuloglomerular feedback was preserved despite long-term activation of this feedback mechanism (9). A cross-sectional study by Fishman et al. found an association between renal glucosuria, lower body weight, and lower rates of arterial hypertension in non-diabetic patients (8). Further research is needed to confirm innate cardiovascular and renal protection in individuals with a likely pathogenic SLC5A2 variant.
Finally, Ren et al. suggested that any variants affecting SLC5A2 function might lower the risk of developing diabetes. These authors have reported on two patients with type 2 diabetes who were diagnosed with FRG. Their literature review of 139 cases revealed two other FRG patients (1.4%) who also had diabetes (10), implicating that the presence of a SLC5A2 variant does not fully protect from diabetes. It was suggested that this variant does not completely inhibit the activity of renal glucose transporters (2, 10), and therefore SGLT2i may still be beneficial for diabetes control in these patients, while the influence of the genetic defect on the binding site of SGLT2i remains to be established (10).
Conclusion
We describe a patient with renal glucosuria despite well-controlled diabetes mellitus, associated with aminoaciduria and hyperuricosuria, due to a novel heterozygous c.469-1G>A likely pathogenic variant in the SLC5A2 gene.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Patient consent
Written informed consent for the publication of his clinical details was obtained from the patient.
Author contribution statement
SI wrote the initial draft of the article, performed a literature review, and included the comments and corrections of the other authors; JV, PJ, and JdF provided clinical follow-up and edited the manuscript; SVD, EVH, and CK performed the genetic analysis, provided valuable guidance for the genetic section, and edited the manuscript. LI edited the manuscript. All authors have read and approved the final manuscript.
Patient’s perspective
I was in good health until about 6 years ago, when I gradually started having more inconvenience when urinating. My health is important to me, and I find it interesting to know what causes this problem. I am pleased to be able to share my story. I am satisfied with the care and follow-up. I prefer not to inform my children, at least not at this time, since my condition is ultimately benign, and I do not want to give them any anxiety. I feel better, but my symptoms are not yet fully resolved despite my current treatment.
References
- 1.Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, Brodehl J, Daschner M, Ehrich JHH, Kemper M, et al.Molecular analysis of the SGLT2 gene in patients with renal glucosuria. Journal of the American Society of Nephrology 2003142873–2882. ( 10.1097/01.asn.0000092790.89332.d2) [DOI] [PubMed] [Google Scholar]
- 2.Santer R & Calado J. Familial renal glucosuria and SGLT2: from a Mendelian trait to a therapeutic target. Clinical Journal of the American Society of Nephrology 20105133–141. ( 10.2215/CJN.04010609) [DOI] [PubMed] [Google Scholar]
- 3.Wang X Yu M Wang T Zhang H Ping F Zhang Q Xu J Feng K & Xiao X. Genetic analysis and literature review of Chinese patients with familial renal glucosuria: identification of a novel SLC5A2 mutation. Clinica Chimica Acta 2017469105–110. ( 10.1016/j.cca.2017.03.027) [DOI] [PubMed] [Google Scholar]
- 4.Li S Yang Y Huang L Kong M & Yang Z. A novel compound heterozygous mutation in SLC5A2 contributes to familial renal glucosuria in a Chinese family, and a review of the relevant literature. Molecular Medicine Reports 2019194364–4376. ( 10.3892/mmr.2019.10110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magen D Sprecher E Zelikovic I & Skorecki K. A novel missense mutation in SLC5A2 encoding SGLT2 underlies autosomal-recessive renal glucosuria and aminoaciduria. Kidney International 20056734–41. ( 10.1111/j.1523-1755.2005.00053.x) [DOI] [PubMed] [Google Scholar]
- 6.Bingham C Ellard S Nicholls AJ Pennock CA Allen J James AJ Satchell SC Salzmann MB & Hattersley AT. The generalized aminoaciduria seen in patients with hepatocyte nuclear factor-1α mutations is a feature of all patients with diabetes and is associated with glucosuria. Diabetes 2001502047–2052. ( 10.2337/diabetes.50.9.2047) [DOI] [PubMed] [Google Scholar]
- 7.Dong M Chen H Wen S Yuan Y Yang L Xu D & Zhou L. The mechanism of sodium-glucose cotransporter-2 inhibitors in reducing uric acid in type 2 diabetes mellitus. Diabetes, Metabolic Syndrome and Obesity 202316437–445. ( 10.2147/DMSO.S399343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishman B Shlomai G Twig G Derazne E Tenenbaum A Fisman EZ Leiba A & Grossman E. Renal glucosuria is associated with lower body weight and lower rates of elevated systolic blood pressure: results of a nationwide cross-sectional study of 2.5 million adolescents. Cardiovascular Diabetology 201918124. ( 10.1186/s12933-019-0929-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuwirt H Burtscher A Cherney D Mayer G & Ebenbichler C. Tubuloglomerular feedback in renal glucosuria: mimicking long-term SGLT-2 inhibitor therapy. Kidney Medicine 2020276–79. ( 10.1016/j.xkme.2019.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren Q Gong S Han X & Ji L. Hereditary renal glycosuria, diabetes and responses to sglt2 inhibitor. Journal of Diabetes 202214216–220. ( 10.1111/1753-0407.13254) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a