Abstract
Antibody-mediated rejection (AMR) is the major cause of graft loss in kidney transplant recipients. The Banff classification defines two classes of AMR, active and chronic active but over time this classification has become increasingly complex. To simplify the approach to AMR, we developed activity and chronicity indices based on kidney transplant biopsy findings and examined their association with graft survival in 147 patients with active or chronic active AMR, all of whom had donor-specific antibodies and were treated for AMR. The activity index was determined as the sum of Banff glomerulitis (g), peritubular capillaritis (ptc), arteritis (v) and C4d scores, with a maximum score of 12. The chronicity index was the sum of interstitial fibrosis (ci), tubular atrophy (ct), chronic vasculopathy (cv), and chronic glomerulopathy (cg) scores, the latter doubled, with a maximum score of 15. While the activity index was generally not associated with graft loss, the chronicity index was significantly associated with graft loss with an optimal threshold value of 4 or greater for predicting graft loss. The association of the chronicity index of 4 or greater with graft loss was independent of other parameters associated with graft loss, including the estimated glomerular filtration rate at the time of biopsy, chronic active (versus active) AMR, AMR with de novo (versus persistent/rebound) donor-specific antibodies, Banff (gDptc) scores, concurrent T cell–mediated rejection and donor-specific antibody reduction post-biopsy. The association of the chronicity index of 4 or greater with graft loss was confirmed in an independent cohort of 61 patients from Necker Hospital, Paris. Thus, our findings suggest that the chronicity index may be valuable as a simplified approach to decision-making in patients with AMR.
Keywords: antibody-mediated rejection, Banff classification, chronicity index, donor-specific antibodies, kidney transplant, transplant glomerulopathy
Antibody-mediated rejection (AMR) represents the major cause of graft loss in kidney transplant recipients.1–4 If unrecognized or not successfully treated in a relatively early stage, AMR typically leads to chronic damage, including transplant glomerulopathy (TG), arterial intimal fibrosis, and interstitial fibrosis/tubular atrophy.5–10 TG in particular is strongly associated with increased rates of graft loss.11–13 Over the past 3 decades, the Banff classification has been the major internationally recognized system for diagnosing and grading rejection in kidney allograft biopsies,14 with diagnostic criteria for AMR first introduced in a 2003 publication15 and having undergone 2 major revisions since.14 The most recent version, Banff 2019,16 recognizes 2 major subtypes of AMR—active and chronic active (CA)—with a third, infrequently encountered subtype of purely chronic AMR. Within these subtypes, there are diagnostic criteria based on active lesions, including glomerulitis (g), peritubular capillaritis (ptc), arteritis (v; which may also be a manifestation of acute T cell–mediated rejection [TCMR]), and C4d deposition in peritubular capillaries, and chronic lesions including TG (cg), peritubular capillary basement membrane multilayering (ptcml), and chronic vasculopathy (cv; which, like arteritis, may be a manifestation of AMR, TCMR, or both). Many pathologists further divide AMR subtypes according to other factors shown to potentially influence graft survival, such as C4d-positive versus C4d-negative, early versus late post-transplantation, associated with de novo donor-specific antibodies (DSAs) versus persistent/rebound DSAs, and pure AMR versus mixed AMR and TCMR.17–22
These modifications, although aimed at improving the diagnostic and prognostic value of the classification and helping to guide therapy, have also come with a price: increased complexity. A 2019 survey, completed by 95 clinicians and 72 pathologists, found the classification to be complex and vulnerable to misinterpretation, leading to heterogeneity in whether patients, particularly those with CA AMR, received treatment directed at AMR.23
To address this, a major objective of the Banff 2019 meeting report16 was clarification of definitions for individual histologic lesion scores and all diagnostic categories. Still, a more straightforward approach to guiding clinicians with respect to prognosis of and therapeutic approach to patients diagnosed with AMR would likely be helpful. In this study, we investigate the potential value of one such approach, scoring biopsies from DSA-positive patients meeting Banff 2019 criteria for AMR according to activity and chronicity indices (AI and CI) similar to those proposed for lupus nephritis24 but incorporating the Banff histologic lesion scores. In 2 separate patient cohorts from Cedars-Sinai Medical Center and Necker Hospital with an initial diagnosis of active or CA AMR, we found that a CI composed of the sum of ci, ct, cv, and cg scores, the latter doubled because of the well-documented impact of TG on graft outcomes, was strongly associated with graft survival with an optimal threshold value of <4 versus ≥4.
METHODS
Cedars-Sinai patients and biopsies
Computerized records of the Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, were searched to identify all renal transplant biopsies performed from January 2015 to December 2018 with a diagnosis of active or CA AMR (including mixed AMR/TCMR), according to Banff 2013 or 2017 criteria,25,26 including documentation of the presence of anti–human leukocyte antigen (HLA) DSA within 10 days of the biopsy. DSA were assessed using Luminex (One Lambda, Canoga Park, CA) single antigen testing, and this was routinely performed at 1, 3, 6, and 12 months post-transplantation and then annually on all sensitized patients (calculated panel reactive antibodies ≥30% and/or prior transplant), annually on remaining patients, plus whenever a biopsy showed histologic changes of AMR. For each DSA present, a relative intensity score was assigned on the basis of the mean fluorescence intensity, and the relative intensity sum score was determined as the sum of the individual scores as previously described.20 Biopsies of ABO-incompatible grafts and those showing recurrent or de novo glomerulonephritis were excluded, as were biopsies from patients with a diagnosis of AMR or suspicious for AMR before the study period. In addition, for patients with >1 biopsy showing AMR during the study period, only the first such biopsy was included, so that for any given patient only a single biopsy was included. A total of 68 such biopsies were identified, all performed for graft dysfunction or increased protein excretion rate. These were added to 79 biopsies meeting the same criteria that were performed from January 2010 through December 2014 and included in a previous study,20 giving a total of 147 biopsies from 147 different patients.
Processing of biopsies and diagnosis of AMR
Each biopsy was studied by routine light microscopy (hematoxylineosin, periodic acid–Schiff, Masson’s trichrome, and Jones silver methenamine stains); direct immunofluorescence for IgG, IgA, IgM, C3, C1q, albumin, fibrin, and kappa and lambda light chains; and indirect immunfluorescence for C4d, performed using a monoclonal anti-C4d antibody as previously described.27 Electron microscopy was performed in all but 12 cases. The latter included recording the maximum number of basement membrane layers in the most severely involved peritubular capillaries.
For each biopsy, histologic slides were reviewed by a renal pathologist (MH) and the following lesion scores (0–3) were graded according to Banff 2019 criteria16: interstitial inflammation in nonscarred cortex (i), tubulitis (t), arteritis (v), glomerulitis (g), peritubular capillaritis (ptc), chronic glomerulopathy (cg), interstitial fibrosis (ci), tubular atrophy (ct), arterial intimal fibrosis (cv), total inflammation (ti), and interstitial inflammation in scarred cortex (i-interstitial fibrosis/tubular atrophy) with the modification that cg scores were based solely on light microscopy (i.e., cg1a was considered as cg = 0). Four biopsies were classified as showing CA AMR with a cg score of 0 by light microscopy; 2 had severe ptcml and 2 had new-onset arterial intimal fibrosis with no prior TCMR or documented hypertension; 3 of these biopsies and 5 others showed cg1a lesions by electron microscopy. C4d staining results were taken from the biopsy reports and scored as 0 to 3 according to Banff criteria.
Finally, computerized patient records were searched, blinded to biopsy findings, and the following information recorded: days post-transplantation of the biopsy, estimated glomerular filtration rate (eGFR) at the time of biopsy (determined using the 2009 Chronic Kidney Disease Epidemiology Collaboration creatinine equation), whether graft loss occurred and the interval between biopsy and time of graft loss (return to dialysis), interval between the biopsy and last follow-up for patients not developing graft loss and serum creatinine level at last follow-up, and treatment given after the biopsy. For patients included in the previous study,20 an updated records search was performed. Follow-up was available for all 147 patients, including 4 who died with a functioning graft, although eGFR at the time of biopsy was available only for 143. A total of 61 patients developed graft loss at a median postbiopsy interval of 28 months (interquartile range 8–46 months; total range 1–109 months); for patients not developing graft loss, the median time from biopsy to last follow-up was 37 months (interquartile range 18–64 months; total range 4–147 months). All patients were treated for AMR directly after the biopsy results were reported, with all but 2 receiving i.v. Ig, 117 rituximab, and varying numbers plasmapheresis, tocilizumab, eculizumab, bortezomib, alemtuzumab, and obinutuzumab. Patients with acute or CA TCMR (Banff 1A grade or higher) also received corticosteroids, with or without thymoglobulin. All study procedures were approved by the Institutional Review Board of Cedars-Sinai Medical Center.
Necker Hospital cohort
A total of 61 biopsies (from 61 patients) from Necker Hospital, Paris, with a diagnosis of active or CA AMR, with or without concurrent TCMR, performed from 2010 through 2016, were identified in which the following criteria were met: (i) there were no previous biopsies showing AMR; (ii) anti-HLA DSA were present at the approximate time of biopsy; and (iii) the patient was treated specifically for AMR. Banff 2019 indices from these biopsies were scored by a renal pathologist from that center (MR), and clinical and serologic data were recorded as described above.
Statistical analysis of data
Morphologic and clinical parameters are expressed as mean ± SD for normally distributed variables and as median and interquartile range for non-normally distributed variables. Differences in the development of graft loss between patient groups were analyzed using the Kaplan-Meier method with the log-rank test to determine significance as well as Cox proportional hazards models to determine hazard ratios (HRs) and their 95% confidence intervals. All tests were 2-tailed, and significance was defined as P < 0.05. SAS version 9.4 (SAS Institute) was used for calculations.
Thresholds for each discrete numerical variable (AI, CI, and AI + CI) were based on the model fit statistics Akaike information criterion (AIC) and Bayesian information criterion (BIC) obtained from the Cox regression models, with smaller AIC and BIC indicating a better fitting model. AIC and BIC are estimators of the prediction error. Given a collection of models for the data, AIC and BIC estimate the quality of each model, relative to each of the other models, and thus provide a means for model selection. For example, AI had discrete values from 2 to 11 (2, 3, 4, …, 11), thus creating potential thresholds of 3 (AI ≥ 3), 4, 5, …, 10 (AI ≥ 10). Individual Cox regression models were estimated for each AI threshold, and the AIC and BIC were obtained for each model. The AI threshold with the smallest AIC and BIC was selected as the “best fitting” model. Initial multivariable Cox models were based on stepwise selection. Addition of a predictor to the resulting stepwise Cox model was based on the −2LogLikelihood fit statistic using the change (Δ) in −2LogLikelihood as the test statistic. Δ(−2LogLikelihood) is approximately chi-square distributed with degrees of freedom equal to the number of additional predictors in the larger model.
RESULTS
Cedars-Sinai cohort
Features of the 147 patients and biopsies are listed in Table 1. The majority had DSAs directed against HLA class II, with or without antibodies against class I. Slightly more than half of the patients had >1 DSA, and 91 of 147 had de novo DSAs (type 2 AMR). Fifty-three percent had active AMR compared with 47% with CA AMR; 98 of 147 biopsies (67%) were C4d-positive, and 45 of 147 (31%) showed TCMR (acute or CA, Banff grade 1A or higher), mainly acute. Only 9 of 147 (6%) biopsies showed changes of thrombotic microangiopathy in glomeruli and/or arterioles. The median AI (g + ptc + v + C4d; maximum score 12) was 5, and the median CI (ci + ct + cv + cg[x2]; maximum score 15) was 3 (Table 1). Not surprisingly, the CI increased with time post-transplantation of the biopsy (slope of the linear regression plot of CI vs. months post-transplantation 0.0406 ± 0.005; intercept 2.35 ± 0.33; r2 = 0.31; P < 0.0001).
Table 1 |.
Demographic, serologic, and pathologic features
| Variable | Value |
|---|---|
|
| |
| Age at the time of biopsy, yr | 42.4 ± 16.9 |
| Patient sex (M/F) | 85/62 |
| Donor type | |
| Deceased | 93 (63) |
| Living related | 24 (16) |
| Living unrelated | 30 (20) |
| Months, transplant to biopsy | 30.0 (6.0–72.0) |
| eGFR at the time of biopsy, ml/mina | 47.5 ± 25.0 |
| Graft loss (yes/no) | 61/86 |
| Nonadherence (reported, yes/no) | 12/135 |
| HLA DSA class | |
| I only | 27 |
| II only | 84 |
| I and II | 36 |
| Number of DSAs | |
| 1 | 72 |
| 2 | 47 |
| 3 | 13 |
| >3 | 15 |
| Immunodominant DSA strength | |
| Weak (MFI <5000) | 39 |
| Moderate (MFI 5000–10,000) | 60 |
| Strong (MFI >10,000) | 48 |
| DSA sum strength | |
| Weak (MFI <5000) | 38 |
| Moderate (MFI 5000–10,000) | 50 |
| Strong (MFI >10,000) | 59 |
| DSA type | |
| De novo | 91 |
| Persistent/rebound | 56 |
| AMR type | |
| Active | 78 |
| Chronic active | 69 |
| TCMR grade | |
| None/borderline | 102 |
| 1A acute | 13 |
| 1B acute | 10 |
| 1A chronic active | 3 |
| 1B chronic active | 3 |
| 2A acute | 13 |
| 2B acute | 3 |
| C4d in peritubular capillaries | |
| Negative (C4d 0–1) | 49 |
| Positive (C4d 2–3) | 98 |
| Thrombotic microangiopathy (yes/no) | 9/138 |
| g score | |
| 0 | 26 |
| 1 | 67 |
| 2 | 33 |
| 3 | 21 |
| ptc score | |
| 0 | 6 |
| 1 | 31 |
| 2 | 79 |
| 3 | 31 |
| (ci + ct) score | |
| 0–1 | 68 |
| 2 | 44 |
| 3–4 | 26 |
| 5–6 | 9 |
| cg score | |
| 0b | 86 |
| 1 | 27 |
| 2 | 13 |
| 3 | 21 |
| cv score | |
| 0 | 72 |
| 1 | 44 |
| 2 | 26 |
| 3 | 5 |
| AI | 5 (4–7) |
| CI | 3 (1–7) |
| (AI + CI) | 9 (6–13) |
AI, activity index; AMR, antibody-mediated rejection; cg, Banff chronic glomerulopathy score, ci, Banff interstitial fibrosis score; CI, chronicity index; ct, Banff tubular atrophy score; cv, Banff chronic arteriopathy score; DSA, donor-specific antibody; eGFR, estimated glomerular filtration rate; F, female; g, Banff glomerulitis score; HLA, human leukocyte antigen; M, male; MFI, mean fluorescence intensity; ptc, Banff peritubular capillaritis score; TCMR, T cell-mediated rejection.
Based on 143 cases; other parameters based on 147.
Includes 8 biopsies with cg1a.
All Banff scores are graded as 0–3 according to criteria detailed in Banff 2019,16 and C4d was determined by immunofluorescence on frozen sections with Banff C4d scores assigned accordingly. Data are expressed as mean ± SD, median (interquartile range), or n (%).
Our previous studies of AMR20 showed that several parameters were significant predictors of graft loss postbiopsy by univariate analysis, including de novo DSA versus persistent/rebound DSA (type 2 AMR vs. type 1 AMR), interval between transplantation and biopsy (per month increase), interval from transplantation to biopsy of ≥84 (vs. <84) months, concurrent TCMR grade 1A or higher, and failure of DSA relative intensity sum score to decrease by >2 (or to 0 if this score at the time of biopsy was 2). Table 2 presents the results of the univariate analysis of the impact of these factors as well as eGFR at the time of biopsy, active versus CA AMR, AI (per 1-unit increase), CI (per 1-unit increase), the sum of AI and CI, and Banff chronic lesion scores (cg, [ci + ct], and cv) on death-censored graft survival. Of these factors, all but AI were significantly associated with graft loss. Both CI and (AI + CI) were significantly associated with graft loss, with optimal predictive cutoff values being CI ≥4 versus CI <4 (Table 3 and Figure 1) and (AI + CI) ≥13 versus (AI + CI) <13 (Table 3). Similar analysis for AI alone showed that AI ≥9 (but no other AI threshold) was associated with graft loss by univariate analysis (HR 2.78; 95% confidence interval 1.10–7.02; P = 0.030); however, only 7 of the 147 biopsies had AI ≥9 and this variable is therefore not appropriate for a multivariable model. When only biopsies with active AMR, CA AMR, pure AMR (no acute or CATCMR with Banff grade ≥1A), and mixed AMR + TCMR were considered separately, in each case CI per 1-unit increase and CI ≥4 remained significantly associated with graft loss whereas AI was not. Table 2 also presents that Banff chronic lesion scores were significantly associated with graft loss; however as shown in Table 3, the association of CI ≥4 versus CI <4 with graft loss was stronger than cg ≥1 (vs. 0) or cg ≥2 (vs. <2). Thus, adding additional chronic elements to TG to formulate the CI, even with the double weighting of the cg score in the CI, appears to improve the value of TG alone as a predictor of graft outcome.
Table 2 |.
Variables associated with death-censored graft loss by univariate analysis
| Variable | Hazard ratio | 95% confidence interval | P |
|---|---|---|---|
|
| |||
| CI per 1-unit increase | 1.28 | 1.19–1.37 | <0.0001 |
| CI ≥4 vs. CI <4 | 6.93 | 3.79–12.65 | <0.0001 |
| AI per 1-unit increase | 1.09 | 0.96–1.24 | 0.19 |
| AI ≥9 vs. AI <9 | 2.78 | 1.10–7.02 | 0.030 |
| (AI + CI) per 1-unit increase | 1.26 | 1.18–1.35 | <0.0001 |
| (AI + CI) ≥13 vs. (AI + CI) <13 | 4.99 | 2.97–8.38 | <0.0001 |
| cg score per 1-unit increase | 1.54 | 1.26–1.88 | <0.0001 |
| (ci + ct) per 1-unit increase | 1.48 | 1.29–1.71 | <0.0001 |
| cv score per 1-unit increase | 2.34 | 1.74–3.15 | <0.0001 |
| TCMR grade 1A or higher | 2.32 | 1.41–3.85 | <0.0001 |
| Active AMR vs. chronic active AMR | 0.30 | 0.17–0.52 | <0.0001 |
| AMR type 2 vs. AMR type 1 | 2.21 | 1.23–3.96 | 0.008 |
| Biopsy ≥84 mo vs. biopsy <84 mo | 2.51 | 1.47–4.30 | 0.0008 |
| Biopsy post-transplant time (per month) | 1.009 | 1.005–1.014 | 0.0002 |
| eGFR (per ml/min)a | 0.958 | 0.943–0.972 | <0.0001 |
| eGFR (per 10 ml/min)a | 0.649 | 0.557–0.756 | <0.0001 |
| ΔRIS >−2 | 4.00 | 2.33–7.14 | <0.0001 |
AI, activity index; AMR, antibody-mediated rejection; cg, Banff chronic glomerulopathy score; ci, Banff interstitial fibrosis score; CI, chronicity index; ct, Banff tubular atrophy score; cv, Banff chronic vasculopathy score; eGFR, estimated glomerular filtration rate; ΔRIS, change in donor-specific antibody relative intensity sum score; TCMR, T cell–mediated rejection.
Type 1 AMR indicates persistent/rebound donor-specific antibody, whereas type 2 AMR indicates de novo donor-specific antibody.
Development of graft loss in patient groups was analyzed using the Kaplan-Meier method with the log-rank test to determine significance, and Cox proportional hazards models were used to determine hazard ratios and their 95% confidence intervals.
Based on 143 cases; other determinations based on 147.
Table 3 |.
Determination of optimal CI and (AI + CI) thresholds for predicting death-censored graft loss
| Threshold value | Hazard ratio | 95% confidence interval | P | AIC | BIC |
|---|---|---|---|---|---|
|
| |||||
| CI ≥3 | 6.92 | 3.37–14.19 | <0.0001 | 493.34 | 495.45 |
| CI ≥4 | 6.93 | 3.79–12.65 | <0.0001 | 484.91 | 487.02 |
| CI ≥5 | 5.70 | 3.27–9.95 | <0.0001 | 491.75 | 493.86 |
| (AI + CI) ≥11 | 4.18 | 2.49–7.03 | <0.0001 | 503.63 | 505.74 |
| (AI + CI) ≥12 | 4.61 | 2.74–7.74 | <0.0001 | 500.84 | 502.95 |
| (AI + CI) ≥13 | 4.99 | 2.97–8.38 | <0.0001 | 499.03 | 501.14 |
| (AI + CI) ≥14 | 4.27 | 2.53–7.23 | <0.0001 | 508.24 | 510.35 |
| cg ≥1 | 1.17 | 1.91–5.48 | <0.0001 | 512.78 | 514.89 |
| cg ≥2 | 2.75 | 1.64–4.63 | <0.0001 | 519.85 | 521.96 |
AI, activity index; AIC, Akaike information criterion; BIC, Bayesian information criterion; cg, Banff chronic glomerulopathy score; CI, chronicity index.
Differences in the development of graft loss between different patient groups was analyzed using the Kaplan-Meier method with the log-rank test to determine significance; Cox proportional hazards models were used to determine hazard ratios and their 95% confidence intervals. Thresholds for CI and (AI + CI) were based on the model fit statistics AIC and BIC obtained from the Cox regression models, with smaller AIC and BIC indicating a better fitting model.
Figure 1 |. Kaplan-Meier analysis of death-censored graft survival in patients with chronicity index (CI) <4 (n = 82) versus CI ≥4 (n = 65) in the Cedars-Sinai cohort.
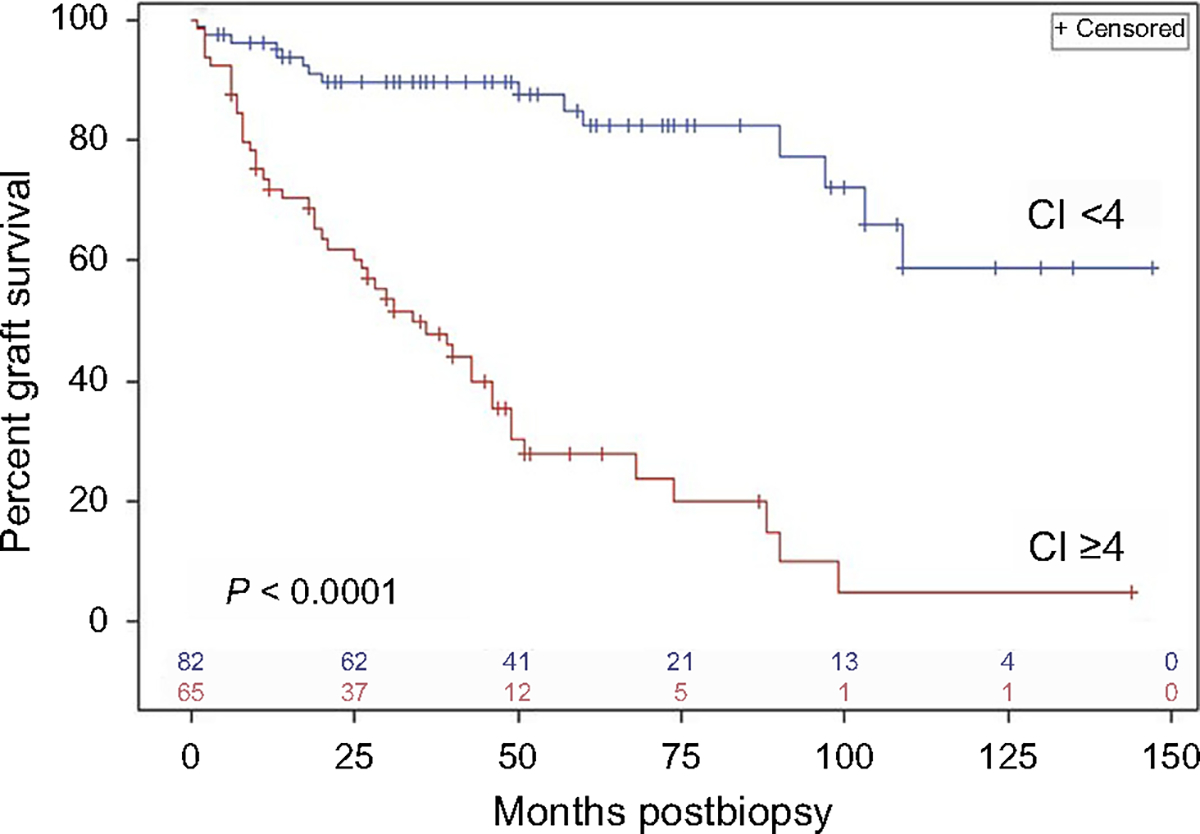
Vertical ticks on each curve indicate censored values (time post-transplantation of last follow-up without graft loss), and the numbers of remaining uncensored cases are indicated just above the x-axis. The 2 curves are significantly different (P = 0.0001) by log-rank analysis.
In a multivariable model including each of the morphologic and serologic variables in Table 2 that were significantly associated with graft loss, only 3 remained significant: CI ≥4, TCMR (acute or CA) of grade 1A or higher, and failure of the HLA antibody relative intensity sum score to decline by >2 (Table 4). Adding the sum of Banff (g + ptc) scores (which per 1-unit increase were significantly associated with graft loss by univariate analysis: HR 1.23; 95% confidence interval 1.01–1.51; P = 0.041) to the multivariable analysis did not change these findings. In a separate multivariable analysis, CI ≥4 and eGFR at the time of biopsy were each significantly associated with graft loss after adjusting for the other (Table 5).
Table 4 |.
Predictors of death-censored graft loss by multivariable analysis
| Variable | Hazard ratio | 95% confidence interval | P |
|---|---|---|---|
|
| |||
| CI ≥4 vs. CI <4 | 6.30 | 3.36–11.84 | <0.0001 |
| TCMR grade 1A or higher | 2.68 | 1.58–4.55 | 0.0002 |
| ΔRIS ≥−2 | 3.72 | 2.08–6.67 | <0.0001 |
AI, activity index; CI, chronicity index; g, Banff glomerulitis score; ptc, Banff peritubular capillaritis score; ΔRIS, change in donor-specific antibody relative intensity sum score; TCMR, T cell–mediated rejection.
The multivariable Cox model included the following variables in addition to those listed above that were the only variables found to be significant (P < 0.05): active AMR vs. chronic active AMR, AMR type 1 vs. AMR type 2, biopsy ≥84 mo vs. biopsy <84 mo post-transplantation, and AI per 1-unit increase. Similar results were obtained with the sum of Banff (g + ptc) scores substituting for AI. When time post-transplantation of biopsy per month was substituted for biopsy ≥84 mo vs. biopsy <84 mo post-transplantation, the former was also not significantly associated with graft loss in this model, while CI ≥4 remained significant. Analysis is based on 147 cases.
Table 5 |.
Predictors of death-censored graft loss by multivariable analysis
| Variable | Hazard ratio | 95% confidence interval | P |
|---|---|---|---|
|
| |||
| CI ≥4 vs. CI <4 | 5.23 | 2.65–10.32 | <0.0001 |
| eGFR (per 1 ml/min increase) | 0.958 | 0.941–0.976 | <0.0001 |
| CI ≥4 vs. CI <4 | 5.23 | 2.65–10.32 | <0.0001 |
| eGFR (per 10 ml/min increase) | 0.652 | 0.542–0.784 | <0.0001 |
CI, chronicity index; eGFR, estimated glomerular filtration rate.
eGFR is at the time of biopsy. Analysis is based on 143 cases.
Necker Hospital cohort
To determine whether the above findings were applicable to an independent group of patients with DSA-positive AMR, biopsies from 61 patients from Necker Hospital, Paris, performed from 2010 through 2016, were scored by a pathologist from that center (MR), and the findings correlated with graft outcomes. As shown in Table 6, this cohort differed from the Cedars-Sinai cohort in that biopsies in the former were performed earlier post-transplantation with a higher fraction of patients having persistent/rebound DSA and active AMR. Biopsies from the Necker cohort also included 18 protocol biopsies (30%) performed at 3 or 12 months post-transplantation, whereas all the Cedars-Sinai biopsies were indication biopsies. A higher fraction of Necker patients also had a single DSA and a lower sum of DSA mean fluorescence intensity values, and there were fewer graft losses in this cohort. There were also notable differences in treatments given for AMR at the 2 centers. As shown in Tables 7 and 8, the association between CI and graft survival (per 1-unit increase) did not reach statistical significance, perhaps related to the relatively early timing of the biopsies. However, as with the Cedars-Sinai cohort, there was a strong and significant association between CI ≥4 and graft loss (Figure 2); this was the optimal cutoff value for CI in predicting graft loss (Table 8). AI was not significantly associated with graft loss, but (AI + CI) was; the optimal cutoff value for (AI + CI) was 10 in the Necker cohort compared with 13 in the Cedars-Sinai cohort, perhaps reflecting the higher median value for (AI + CI) in the latter (Table 6). The only other parameter that was close to being significantly associated with graft loss was AMR type 2 versus AMR type 1 (de novo DSA vs. persistent/rebound DSA) (Tables 7 and 8). In multivariable analysis including the latter, CI ≥4 remained significantly associated with graft loss (HR 3.50; 95% confidence interval 1.34–9.18; P = 0.011) but (AI + CI) ≥10 did not (HR 2.20; 95% confidence interval 0.84–5.80; P = 0.11).
Table 6 |.
Comparison of Cedars-Sinai and Necker cohorts
| Variable | Cedars-Sinai | Necker |
|---|---|---|
|
| ||
| Number of cases | 147 | 61 |
| Age at the time of biopsy, yr | 42.4 ± 16.9 | 51.4 ± 14.8 |
| Months, transplant to biopsy | 30.0 (6.0–72.0) | 3.0 (1.0–6.0) |
| Graft loss (yes/no) | 61/86 (41/59) | 16/45 (26/74) |
| HLA DSA class | ||
| I only | 27 (18) | 14 (23) |
| II only | 84 (57) | 34 (56) |
| I and II | 36 (24) | 13 (21) |
| Number of DSAs | ||
| 1 | 72 (49) | 37 (61) |
| >1 | 75 (51) | 24 (39) |
| DSA sum strength (sum of MFIs) | ||
| <5000 | 38 (26) | 40 (66) |
| 5000–10,000 | 50 (34) | 10 (16) |
| >10,000 | 59 (40) | 11 (18) |
| DSA type | ||
| De novo | 91 (62) | 15 (25) |
| Persistent/rebound | 56 (38) | 46 (75) |
| AMR type | ||
| Active | 78 (53) | 58 (95) |
| Chronic active | 69 (47) | 3 (5) |
| TCMR (acute or chronic active) | ||
| None/borderline | 102 (69) | 48 (79) |
| ≥1A | 45 (31)a | 13 (21)a |
| C4d in peritubular capillaries | ||
| Positive/negative | 98/49 | 35/23b |
| % positive | 67 | 60 |
| Thrombotic microangiopathy | ||
| Present/absent | 9/138 | 3/58 |
| % present | 6 | 5 |
| Biopsy indication | ||
| For cause | 147 (100) | 43 (70) |
| Protocol | 0 (0) | 18 (30) |
| Postbiopsy treatments | ||
| IVIG | 145 (99) | 48 (79) |
| Rituximab | 117 (80) | 35 (57) |
| Corticosteroids | 69 (47) | 57 (93) |
| PP/PE | 36 (24) | 50 (82) |
| Eculizumab | 6 (4) | 0 (0) |
| Tocilizumab | 11 (7) | 0 (0) |
| Othersc | 24 (16) | 2 (3) |
| AI | 5 (4–7) | 5 (4–6) |
| CI | 3 (1–7) | 2 (0–4) |
| (AI + CI) | 9 (6–13) | 7 (5–10) |
AI, activity index; AMR, antibody-mediated rejection; CI, chronicity index; DSA, donor-specific antibody; HLA, human leukocyte antigen; IVIG, i.v. immunoglobulin; MFI, mean fluorescence intensity; PE, plasma exchange; PP, plasmapheresis; TCMR,T cell-mediated rejection.
Five biopsies from each cohort listed as showing TCMR ≥ Banff grade 1A had isolated intimal arteritis, defined as Banff v (arteritis) score of 1 or 2 with i (interstitial inflammation score) = 0, t (tubulitis score) = 0, or both. C4d was determined by immunofluorescence on frozen sections with Banff C4d scores assigned accordingly as negative (C4d 0–1) or positive (C4d 2–3).
C4d staining was not performed for 3 of the Necker cases.
Other treatments for AMR included bortezomib, alemtuzumab, and obinutuzumab.
Data are expressed as mean ± SD, median (interquartile range), or n (%).
Table 7 |.
Predictors of death-censored graft loss in the Necker cohort
| Variable | Hazard ratio | 95% confidence interval | P |
|---|---|---|---|
|
| |||
| CI per 1-unit increase | 1.18 | 0.98–1.41 | 0.073 |
| CI ≥4 vs. CI <4 | 4.06 | 1.65–10.06 | 0.002 |
| AI per 1-unit increase | 1.09 | 0.86–1.37 | 0.48 |
| AI ≥7 vs. AI <7 | 1.65 | 0.59–4.62 | 0.34 |
| (AI + CI) per 1-unit increase | 1.26 | 1.03–1.39 | 0.022 |
| (AI + CI) ≥10 vs. (AI + CI) <10 | 3.21 | 1.28–8.06 | 0.013 |
| AMR type 2 vs. AMR type 1 | 2.52 | 0.99–6.44 | 0.053 |
| TCMR grade 1A or higher | 0.64 | 0.20–1.82 | 0.37 |
AI, activity index; AMR, antibody-mediated rejection; CI, chronicity index; TCMR, T cell–mediated rejection.
Development of graft loss in patient groups was analyzed by univariate analysis using the Kaplan-Meier method with the log-rank test to determine significance, and Cox proportional hazards models were used to determine hazard ratios and their 95% confidence intervals.
Table 8 |.
Predictors of death-censored graft loss in the Necker cohort
| Threshold value | Hazard ratio | 95% confidence interval | P | AIC | BIC |
|---|---|---|---|---|---|
|
| |||||
| CI ≥3 | 2.39 | 0.96–5.97 | 0.061 | 139.48 | 140.42 |
| CI ≥4 | 4.07 | 1.65–10.06 | 0.002 | 134.28 | 135.22 |
| CI ≥5 | 1.37 | 0.40–4.69 | 0.62 | 142.81 | 143.76 |
| (AI + CI) ≥9 | 2.69 | 1.08–6.65 | 0.033 | 138.71 | 139.66 |
| (AI + CI) ≥10 | 3.21 | 1.28–8.06 | 0.013 | 137.46 | 138.40 |
| (AI + CI) ≥11 | 2.44 | 0.88–6.79 | 0.087 | 140.54 | 141.48 |
AI, activity index; AIC, Akaike information criterion; BIC, Bayesian information criterion; CI, chronicity index.
Thresholds for CI and (AI + CI) were based on the model fit statistics AIC and BIC obtained from the Cox regression models, with smaller AIC and BIC indicating a better fitting model.
Figure 2|. Kaplan-Meier analysis of death-censored graft survival in patients with chronicity index (CI) <4 (n = 45) versus CI ≥4 (n = 16) in the Necker Hospital cohort.
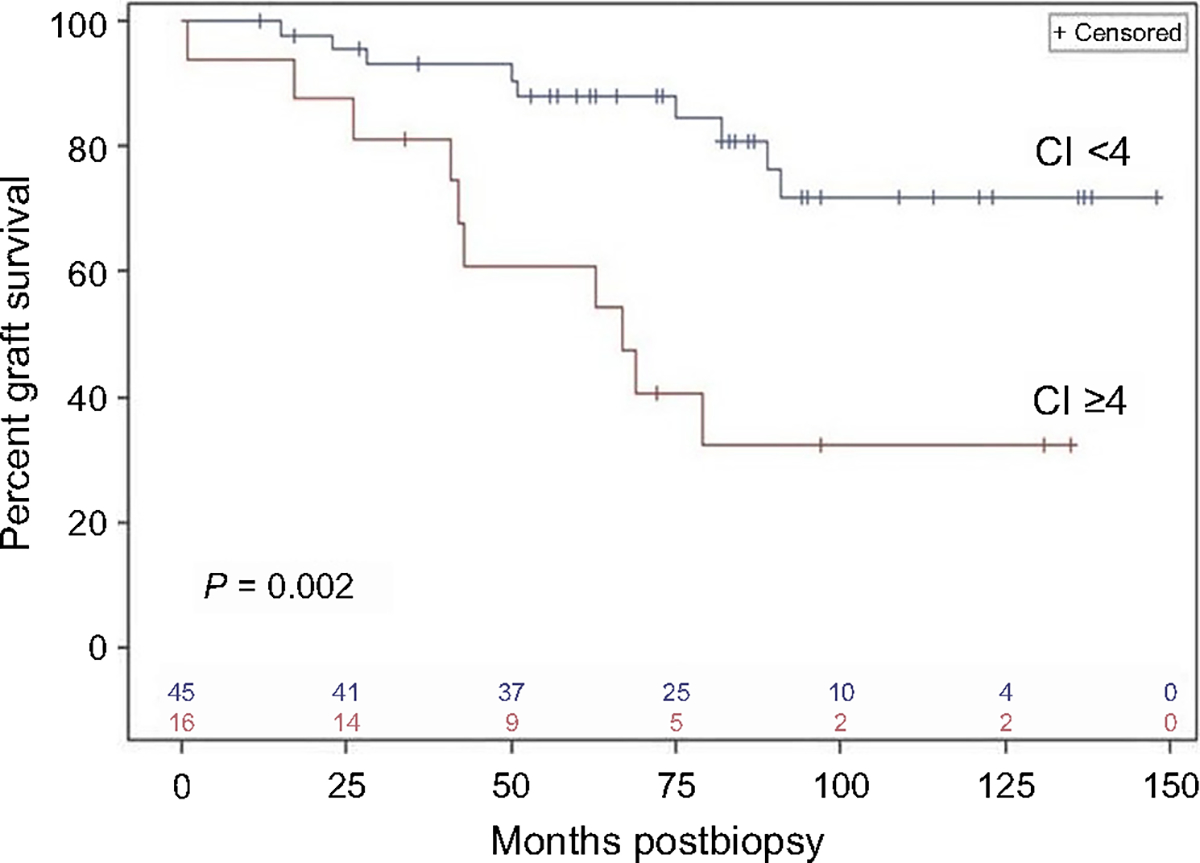
Vertical ticks on each curve indicate censored values (time post-transplantation of last follow-up without graft loss), and the numbers of remaining uncensored cases are indicated just above the x-axis. The 2 curves are significantly different (P = 0.0024) by log-rank analysis.
DISCUSSION
In the current era of immunosuppression, AMR has been identified as the leading cause of long-term kidney allograft failure.1–4 TG, the most identifiable morphologic lesion of AMR progression,7–9,11 is well established as a predictor of poor graft outcomes,12,13,28 so much so that clinicians are often reluctant to aggressively treat patients with documented TG for AMR, although there are newer treatments that show promise for these patients.29
Pathologic features that help distinguish different archetypes of AMR and TG are contained in the most recent (2019) version of the Banff classification.16 This classification, and particularly criteria dealing with AMR, has evolved considerably over the past 2 decades as knowledge has expanded through clinical and molecular studies, with subcategories that in some cases are specifically designated by Banff (e.g., active and CA) and others that, although not officially designated (e.g., C4d-positive vs. C4d-negative, de novo DSA vs. persistent/rebound DSA), may nonetheless be associated with differences in graft outcomes.17–22 Although these subcategories may be valuable to some clinicians in making treatment decisions, the added complexity of the classification has proven difficult to others. In this regard, a 2019 survey completed by 95 clinicians and 72 pathologists found the Banff criteria for AMR to be vulnerable to misinterpretation, leading to heterogeneity in whether patients, particularly those with CA AMR, received treatment specifically directed at AMR.23
To try and help simplify this classification as a prognostic indicator (but not with respect to diagnostic criteria), we examined the potential value of scoring biopsies from patients meeting Banff 2019 criteria for AMR according to AI and CI similar to those proposed for lupus nephritis24 but incorporating the Banff histologic lesion scores (g, ptc, v, C4d, cg, ci, ct, and cv). Like Banff, the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification for lupus nephritis is complex and includes lesions with a wide range of activity and chronicity within the same subcategory (e.g., class IV),24,30 leading to heterogeneity in how the same biopsy findings are approached by different clinicians. For this reason, a recently proposed revision of the ISN/RPS classification recommended the use of AI and CI on the basis of specific pathologic findings rather than broader subcategories of active, active and chronic, and chronic.24
We initially determined AI and CI in 147 DSA-positive patients with an initial diagnosis of active or CA AMR on kidney allograft biopsy. All were treated for AMR postbiopsy, although treatment was not uniform. Although AI was not associated with graft loss except at an extremely high (≥9) and rarely seen level, CI was strongly and significantly associated with death-censored graft loss, with an optimal threshold value of ≥4, independent of factors previously shown20 and confirmed here to also be associated with graft loss by univariate and in some cases multivariable analysis, including eGFR at the time of biopsy. Although the Banff cg score is heavily weighed within the CI because of the well-documented association of TG with poor graft outcomes,12,13,19 our data analysis showed that the association of CI ≥4 with graft loss was stronger than that for cg scores of ≥1 or ≥2. The latter is noteworthy as it suggests that mild TG does not necessarily imply a poor graft outcome in patients treated for AMR, especially if there are little or no other chronic changes on the biopsy. Furthermore, in the Necker Hospital cohort where only 3 of 61 patients had a cg score of ≥1, CI ≥4 was still predictive of graft loss. Thus, the presence of more than mild chronic changes in grafts showing active inflammation, whether it be microvascular13,22 or tubulointerstitial,31–33 is associated with worse graft outcomes. Consistent with this, unit increases in (AI + CI) were significantly associated with graft loss in both the Cedars-Sinai and Necker cohorts.
The confirmation of the association of CI ≥4 with a significantly higher risk of graft loss in the Necker cohort is also noteworthy in that the latter differed from the Cedars-Sinai cohort with respect to a number of parameters including time from transplantation to biopsy, fraction of patients having persistent/rebound (as opposed to de novo) DSA and active (as opposed to CA) AMR, inclusion of protocol biopsies, DSA sum scores, and frequency of graft losses. Thus, the predictive value of CI <4 versus CI ≥4 appears to remain valid for a wide range of patients diagnosed with AMR.
The finding that CI is associated with graft loss is not surprising. In the initial studies of AI and CI in lupus nephritis, CI was strongly associated with kidney failure rates.34 Chronicity sum scores based on interstitial fibrosis/tubular atrophy, glomerulosclerosis, and chronic vascular changes have also been shown to be predictive of loss of kidney function across a large spectrum of native kidney diseases.35,36 In addition to TG, interstitial fibrosis/tubular atrophy has been shown to negatively affect renal graft survival33 and arterial intimal fibrosis may be a manifestation of persistent exposure to DSA.10 By contrast, the finding that AI was generally not predictive of graft survival is somewhat surprising, noting that previous studies in de novo DSA-positive patients found (g + ptc) ≥3 (but not 1–2) to be predictive of graft loss compared with (g + ptc) = 0.37 In the Cedars-Sinai cohort of 147 DSA-positive patients, 1-unit increases in (g + ptc) were significantly associated with graft loss by univariate but not multivariable analysis, although there was not a statistically significant cutoff value for (g + ptc) for predicting graft loss. Perhaps the impact of (g + ptc) is weakened by its inclusion in the AI with other parameters (C4d and v scores), although arteritis (v > 0) in patients with other diagnostic changes of AMR was shown to be predictive of graft loss,38 and some, but not all, studies have shown an impact of diffuse C4d staining on graft survival, albeit usually by univariate analysis only and often not statistically significant.17,37,39,40 Still, molecular studies in cases of AMR without concurrent TCMR showed that transcripts associated with fibrosis and tissue injury, but not those associated with active inflammation, were most strongly associated with graft survival.41
This study does have significant limitations. It is retrospective, albeit with similar findings in 2 independent patient cohorts from different centers. A detailed statistical analysis of the weighting of the different parameters comprising AI and CI was not conducted, and it should be emphasized that these indices are not meant to represent a statistically based prediction tool similar to the iBox42 or the IgA nephropathy prediction tool.43 All patients were treated for AMR, although this was not uniform. Still, inclusion of only treated patients may have contributed to the lack of association of AI with graft loss. Furthermore, the time of initial biopsy showing AMR spanned a rather long interval (2010–2018 for Cedars-Sinai, 2010–2016 for Necker), during which new treatments for AMR, such as tocilizumab,29 were introduced into practice at Cedars-Sinai. The size of the Necker cohort was also relatively small with rather few cases showing CA AMR and mixed AMR/TCMR, limiting multivariable analyses that could be performed. Finally, we limited our study to DSA-positive patients with biopsies fully diagnostic of AMR according to Banff criteria,16,26 excluding DSA-negative patients with microvascular inflammation termed “suspicious for AMR” in Banff 2013,25 as reports of the clinical significance of the latter lesions are quite variable.44–46 Notably, 1 study of such lesions diagnosed early post-transplantation found that these patients had excellent graft outcomes, far better than those in patients with DSA-positive AMR, despite lack of treatment for AMR.45 Indeed, microvascular inflammation in the absence of DSA may represent a response to missing self or another factor related to HLA mismatches rather than undetected HLA DSA or non-HLA antibodies.47,48
In summary, we found that a CI (ci + ct + cv + cg[x2]) of ≥4 on a renal allograft biopsy with an initial AMR diagnosis was strongly associated with graft loss in 2 separate cohorts of 147 and 61 DSA-positive patients, respectively. The 2 cohorts differed with respect to a number of features, although each consisted of patients and biopsies with features that have been shown in different studies to be predictors of graft outcomes in AMR. For clinicians making treatment decisions for patients diagnosed with AMR, particularly CA AMR, a relatively simple pathologic score that is highly associated with graft survival in patients treated for AMR after the biopsy would appear to be useful in this process.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health National Center for Advancing Translational Science UCLA Clinical and Translational Science Institute grant number UL1TR001881 (JM) and by a mini-grant from the Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center (MH).
No part of this manuscript was prepared by a commercial organization, and the work described in the manuscript was not funded by a commercial organization.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Djamali A, Kaufman DB, Ellis TM, et al. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520, 2331. [DOI] [PubMed] [Google Scholar]
- 3.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. [DOI] [PubMed] [Google Scholar]
- 4.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68–74. [DOI] [PubMed] [Google Scholar]
- 5.Sis B, Jhangri GS, Riopel J, et al. A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant. 2012;12:1168–1179. [DOI] [PubMed] [Google Scholar]
- 6.Loupy A, Suberbielle-Boissel C, Hill GS, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009;9:2561–2570. [DOI] [PubMed] [Google Scholar]
- 7.Smith RN, Kawai T, Boskovic S, et al. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant. 2008;8:1662–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas M, Mirocha J. Early ultrastructural changes in renal allografts: correlation with antibody-mediated rejection and transplant glomerulopathy. Am J Transplant. 2011;11:2123–2131. [DOI] [PubMed] [Google Scholar]
- 9.Bagnasco SM, Zachary AA, Racusen LC, et al. Time course of pathologic changes in kidney allografts of positive crossmatch HLA-incompatible transplant recipients. Transplantation. 2014;97:440–445. [DOI] [PubMed] [Google Scholar]
- 10.Hill GS, Nochy D, Bruneval P, et al. Donor-specific antibodies accelerate arteriosclerosis after renal transplantation. J Am Soc Nephrol. 2011;22:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gloor JM, Sethi S, Stegall MD, et al. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant. 2007;7:2124–2132. [DOI] [PubMed] [Google Scholar]
- 12.Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant. 2008;8:492–496. [DOI] [PubMed] [Google Scholar]
- 13.Kieran N, Wang X, Perkins J, et al. Combination of peritubular capillary C4d and transplant glomerulopathy predicts late renal allograft failure. J Am Soc Nephrol. 2009;20:2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—an addition to the Banff ‘97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. [DOI] [PubMed] [Google Scholar]
- 16.Loupy A, Haas M, Roufosse C, et al. Banff 2019 Kidney Meeting Report I: updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020;20:2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orandi BJ, Alachkar N, Kraus ES, et al. Presentation and outcomes of C4d-negative antibody-mediated rejection after kidney transplantation. Am J Transplant. 2016;16:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halloran PF, Merino Lopez M, Barreto Pereira A. Identifying subphenotypes of antibody-mediated rejection in renal allografts. Am J Transplant. 2016;16:908–920. [DOI] [PubMed] [Google Scholar]
- 19.Aubert O, Loupy A, Hidalgo L, et al. Antibody-mediated rejection due to preexisting versus de novo donor-specific antibodies in kidney allograft recipients. J Am Soc Nephrol. 2017;28:1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas M, Mirocha J, Reinsmoen NL, et al. Differences in pathologic features and graft outcomes in antibody-mediated rejection of renal allografts due to persistent/recurrent versus de novo donor-specific antibodies. Kidney Int. 2017;91:729–737. [DOI] [PubMed] [Google Scholar]
- 21.Matignon M, Muthukumar T, Seshan SV, et al. Concurrent acute cellular rejection is an independent risk factor for renal allograft failure in patients with C4d-positive antibody-mediated rejection. Transplantation. 2012;94:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aubert O, Higgins S, Bouatou Y, et al. Archetype analysis identifies distinct profiles in renal transplant recipients with transplant glomerulopathy associated with allograft survival. J Am Soc Nephrol. 2019;30:625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schinstock CA, Sapir-Pichhadze R, Naesens M, et al. Banff survey on antibody-mediated rejection clinical practices in kidney transplantation: diagnostic misinterpretation has potential therapeutic implications. Am J Transplant. 2019;19:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. [DOI] [PubMed] [Google Scholar]
- 25.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. [DOI] [PubMed] [Google Scholar]
- 26.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 kidney meeting report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas M, Rahman MH, Racusen LC, et al. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: correlation with histologic findings. Am J Transplant. 2006;6:1829–1840. [DOI] [PubMed] [Google Scholar]
- 28.Wu K, Schmidt D, Lopez del Moral C, et al. Poor outcomes in patients with transplant glomerulopathy independent of Banff categorization or therapeutic interventions. Front Med. 2022;9:889648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. 2017;17:2381–2389. [DOI] [PubMed] [Google Scholar]
- 30.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of lupus nephritis revisited. Kidney Int. 2004;65:521–530. [DOI] [PubMed] [Google Scholar]
- 31.Park WD, Griffin MD, Cornell LD, et al. Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc Nephrol. 2010;21:1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chekuri A, Mehra R, Sood P, Hariharan S. Early allograft inflammation and scarring associate with graft dysfunction and poor outcomes in renal transplant recipients with delayed graft function: a prospective single center cohort study. Transplant Int. 2018;31:1369–1379. [DOI] [PubMed] [Google Scholar]
- 33.Halloran PF, Matas A, Kasiske BL, et al. Molecular phenotype of kidney transplant indication biopsies with inflammation in scarred areas. Am J Transplant. 2019;19:1356–1370. [DOI] [PubMed] [Google Scholar]
- 34.Austin HA III, Muenz LR, Joyce KM, et al. Prognostic factors in lupus nephritis: contribution of renal histologic data. Am J Med. 1983;75:382–391. [DOI] [PubMed] [Google Scholar]
- 35.Sethi S, D’Agati VD, Nast CC, et al. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int. 2017;91:787–789. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava A, Palsson R, Kaze AD, et al. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston kidney biopsy cohort study. J Am Soc Nephrol. 2018;29:2213–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Kort H, Willicombe M, Brookes P, et al. Microcirculation inflammation associates with outcome in renal transplant patients with de novo donor-specific antibodies. Am J Transplant. 2013;13:485–492. [DOI] [PubMed] [Google Scholar]
- 38.Lefaucheur C, Loupy A, Vernerey D, et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381:313–319. [DOI] [PubMed] [Google Scholar]
- 39.Regele H, Exner M, Watschinger B, et al. Endothelial C4d deposition is associated with inferior kidney allograft outcome independently of cellular rejection. Nephrol Dial Transplant. 2001;16:2058–2066. [DOI] [PubMed] [Google Scholar]
- 40.Nickeleit V, Zeiler M, Gudat F, et al. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol. 2002;13:242–251. [DOI] [PubMed] [Google Scholar]
- 41.Einecke G, Reeve J, Gupta G, et al. Factors associated with kidney graft survival in pure antibody-mediated rejection at the time of indication biopsy: importance of parenchymal injury but not disease activity. Am J Transplant. 2021;21:1391–1401. [DOI] [PubMed] [Google Scholar]
- 42.Loupy A, Aubert O, Orandi BJ, et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: international derivation and validation study. BMJ. 2019;366:l4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbour SJ, Coppo R, Zhang H, et al. International IgA nephropathy network: evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sablik KA, Clahsen-van Groningen MC, Looman CWN, et al. Chronic-active antibody-mediated rejection with or without donor-specific antibodies has similar histomorphology and clinical outcome—a retrospective study. Transplant Int. 2018;31:900–908. [DOI] [PubMed] [Google Scholar]
- 45.Senev A, Coemans M, Lerut E, et al. Histological picture of antibody-mediated rejection without donor-specific anti-HLA antibodies: clinical presentation and implications for outcome. Am J Transplant. 2019;19:763–780. [DOI] [PubMed] [Google Scholar]
- 46.Callemeyn J, Ameye H, Lerut E, et al. Revisiting the changes in the Banff classification for antibody-mediated rejection after kidney transplantation. Am J Transplant. 2021;21:2413–2423. [DOI] [PubMed] [Google Scholar]
- 47.Callemeyn J, Senev A, Coemans M, et al. Missing self-induced microvascular rejection of kidney allografts: a population-based study. J Am Soc Nephrol. 2021;32:2070–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senev A, Lerut E, Coemans M, et al. Association of HLA mismatches and histology suggestive of antibody-mediated injury in the absence of donor-specific anti-HLA antibodies. Clin J Am Soc Nephrol. 2022;17:1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]


