Abstract
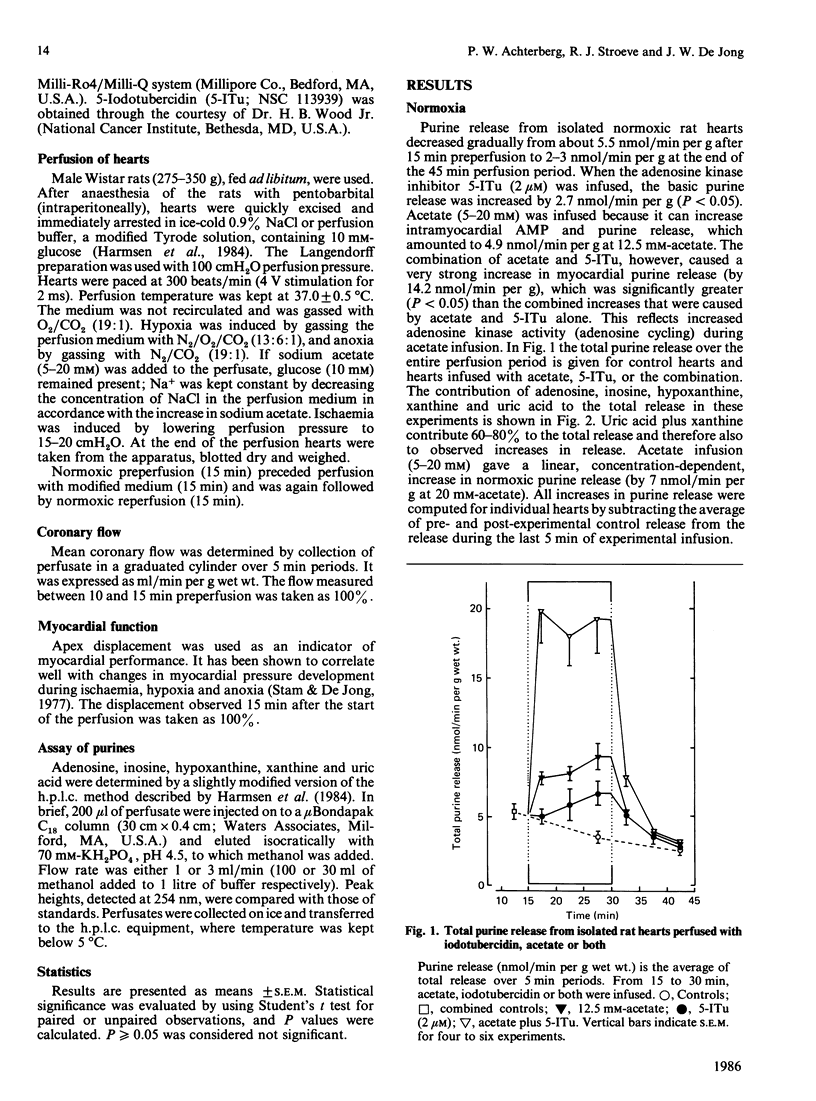
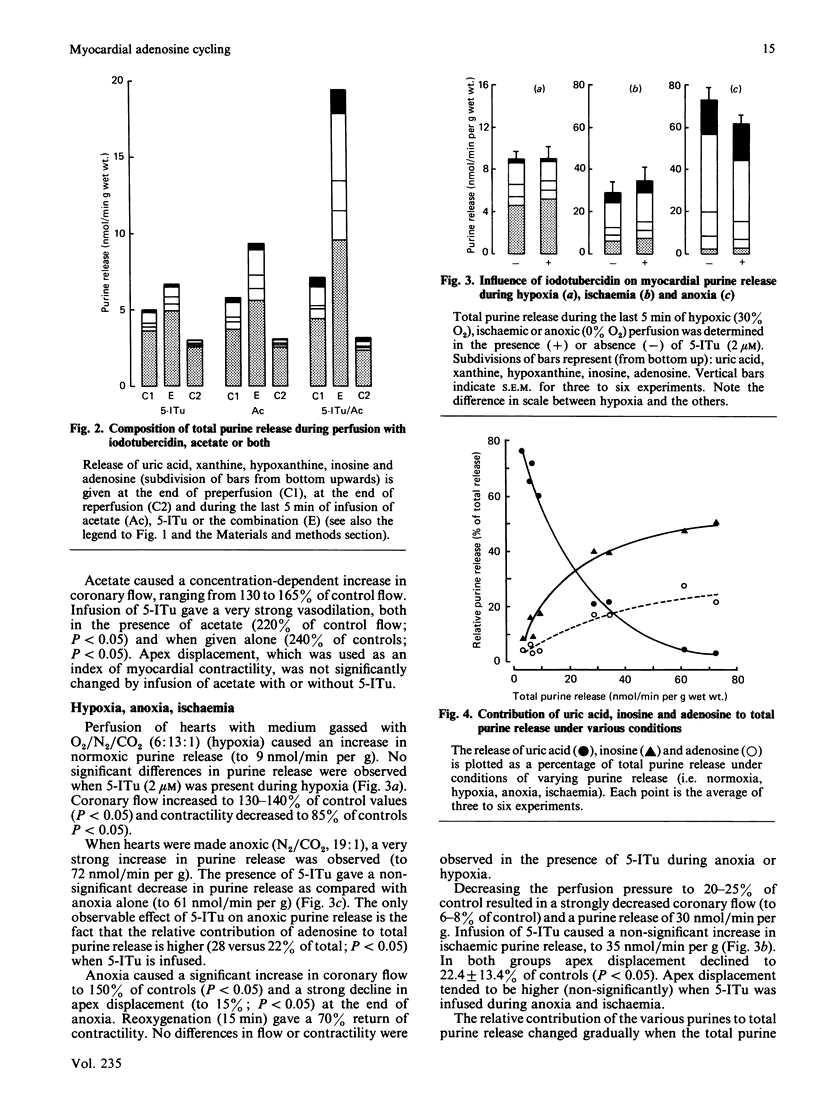
Formation and rephosphorylation of adenosine (adenosine cycling) was studied in isolated rat hearts during normoxia and under conditions of stimulated purine formation. Hearts were infused with an inhibitor of adenosine kinase (5-iodotubercidin, 2 microM). In addition, perfusions were carried out with or without acetate, which is converted into acetyl-CoA, with simultaneous breakdown of ATP to AMP and purines. We found a linear, concentration-dependent, increase in normoxic purine release by acetate (5-20 mM). Differences in total purine release with or without iodotubercidin were taken as a measure of adenosine cycling. In normoxic hearts, iodotubercidin caused a minor increase in purine release (2.7 nmol/min per g wet wt.). Acetate (12.5 mM) increased purine release by 4.9 nmol/min per g, and its combination with inhibitor gave a large increase, by 14.2 nmol/min per g. This indicates a strongly increased adenosine cycling rate during acetate infusion. However, no significant differences in purine release were observed when iodotubercidin was infused during hypoxia, anoxia or ischaemia. The hypothesis that adenosine cycling is near-maximal during normoxia was not confirmed. Increased myocardial adenosine formation appears to be regulated by the availability of AMP and not by inhibition of adenosine kinase. This enzyme mainly functions to salvage adenosine in order to prevent excessive loss of adenine nucleotides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achterberg P. W., Harmsen E., de Jong J. W. Adenosine deaminase inhibition and myocardial purine release during normoxia and ischaemia. Cardiovasc Res. 1985 Oct;19(10):593–598. doi: 10.1093/cvr/19.10.593. [DOI] [PubMed] [Google Scholar]
- Achterberg P. W., de Tombe P. P., Harmsen E., de Jong J. W. Myocardial S-adenosylhomocysteine hydrolase is important for adenosine production during normoxia. Biochim Biophys Acta. 1985 Jul 5;840(3):393–400. doi: 10.1016/0304-4165(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978 Sep 15;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch J. R., Newsholme E. A. The control of the metabolism and the hormonal role of adenosine. Essays Biochem. 1978;14:82–123. [PubMed] [Google Scholar]
- Aussedat J., Verdys M., Rossi A. Synthèse des nucléotides adényliques à partir de l'adénosine exogène dans le coeur de rat perfusé dans des conditions de normoxie et après ischémie. Arch Int Physiol Biochim. 1984 Oct;92(3):203–217. doi: 10.3109/13813458409104501. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Belloni F. L., Rubio R., Berne R. M. Atrioventricular conduction disturbances during hypoxia. Possible role of adenosine in rabbit and guinea pig heart. Circ Res. 1980 Nov;47(5):684–691. doi: 10.1161/01.res.47.5.684. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowditch J., Brown A. K., Dow J. W. Accumulation and salvage of adenosine and inosine by isolated mature cardiac myocytes. Biochim Biophys Acta. 1985 Feb 21;844(2):119–128. doi: 10.1016/0167-4889(85)90082-5. [DOI] [PubMed] [Google Scholar]
- De Jong J. W. Partial purification and properties of rat-heart adenosine kinase. Arch Int Physiol Biochim. 1977 Aug;85(3):557–569. doi: 10.3109/13813457709069872. [DOI] [PubMed] [Google Scholar]
- Fisher M. N., Newsholme E. A. Properties of rat heart adenosine kinase. Biochem J. 1984 Jul 15;221(2):521–528. doi: 10.1042/bj2210521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen E., de Tombe P. P., de Jong J. W., Achterberg P. W. Enhanced ATP and GTP synthesis from hypoxanthine or inosine after myocardial ischemia. Am J Physiol. 1984 Jan;246(1 Pt 2):H37–H43. doi: 10.1152/ajpheart.1984.246.1.H37. [DOI] [PubMed] [Google Scholar]
- Hülsmann W. C. Coronary vasodilation by fatty acids. Basic Res Cardiol. 1976 Mar-Apr;71(2):179–191. doi: 10.1007/BF01927870. [DOI] [PubMed] [Google Scholar]
- Liang C. S., Lowenstein J. M. Metabolic control of the circulation. Effects of acetate and pyruvate. J Clin Invest. 1978 Nov;62(5):1029–1038. doi: 10.1172/JCI109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namm D. H. Myocardial nucleotide synthesis from purine bases and nucleosides. Comparison of the rates of formation of purine nucleotides from various precursors and identification of the enzymatic routes for nucleotide formation in the isolated rat heart. Circ Res. 1973 Dec;33(6):686–695. doi: 10.1161/01.res.33.6.686. [DOI] [PubMed] [Google Scholar]
- Newby A. C., Holmquist C. A., Illingworth J., Pearson J. D. The control of adenosine concentration in polymorphonuclear leucocytes, cultured heart cells and isolated perfused heart from the rat. Biochem J. 1983 Aug 15;214(2):317–323. doi: 10.1042/bj2140317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C. The role of adenosine kinase in regulating adenosine concentration. Biochem J. 1985 Feb 15;226(1):343–344. doi: 10.1042/bj2260343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibel D. K., Rovetto M. J. Myocardial adenosine salvage rates and restoration of ATP content following ischemia. Am J Physiol. 1979 Aug;237(2):H247–H252. doi: 10.1152/ajpheart.1979.237.2.H247. [DOI] [PubMed] [Google Scholar]
- Ronca-Testoni S., Borghini F. Degradation of perfused adenine compounds up to uric acid in isolated rat heart. J Mol Cell Cardiol. 1982 Mar;14(3):177–180. doi: 10.1016/0022-2828(82)90116-x. [DOI] [PubMed] [Google Scholar]
- Schoutsen B., De Jong J. W., Harmsen E., De Tombe P. P., Achterberg P. W. Myocardial xanthine oxidase/dehydrogenase. Biochim Biophys Acta. 1983 Jul 14;762(4):519–524. doi: 10.1016/0167-4889(83)90055-1. [DOI] [PubMed] [Google Scholar]
- Schrader J., Baumann G., Gerlach E. Adenosine as inhibitor of myocardial effects of catecholamines. Pflugers Arch. 1977 Nov 25;372(1):29–35. doi: 10.1007/BF00582203. [DOI] [PubMed] [Google Scholar]
- Schrader J., Schütz W., Bardenheuer H. Role of S-adenosylhomocysteine hydrolase in adenosine metabolism in mammalian heart. Biochem J. 1981 Apr 15;196(1):65–70. doi: 10.1042/bj1960065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz W., Schrader J., Gerlach E. Different sites of adenosine formation in the heart. Am J Physiol. 1981 Jun;240(6):H963–H970. doi: 10.1152/ajpheart.1981.240.6.H963. [DOI] [PubMed] [Google Scholar]
- Stam H., De Jong J. W. Sephadex-induced reduction of coronary flow in the isolated rat heart: a model for ischemic heart disease. J Mol Cell Cardiol. 1977 Aug;9(8):633–650. doi: 10.1016/s0022-2828(77)80359-3. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H., Hems R., Krebs H. A. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980 Mar 15;186(3):701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland P. M., Saebø J. Sequestration of adenosine in crude extract from mouse liver and other tissues. Biochim Biophys Acta. 1979 Oct 18;587(3):341–352. doi: 10.1016/0304-4165(79)90438-0. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- de Jong J. W., Keijzer E., Uitendaal M. P., Harmsen E. Further purification of adenosine kinase from rat heart using affinity and ion-exchange chromatography. Anal Biochem. 1980 Jan 15;101(2):407–412. doi: 10.1016/0003-2697(80)90206-7. [DOI] [PubMed] [Google Scholar]



