Abstract
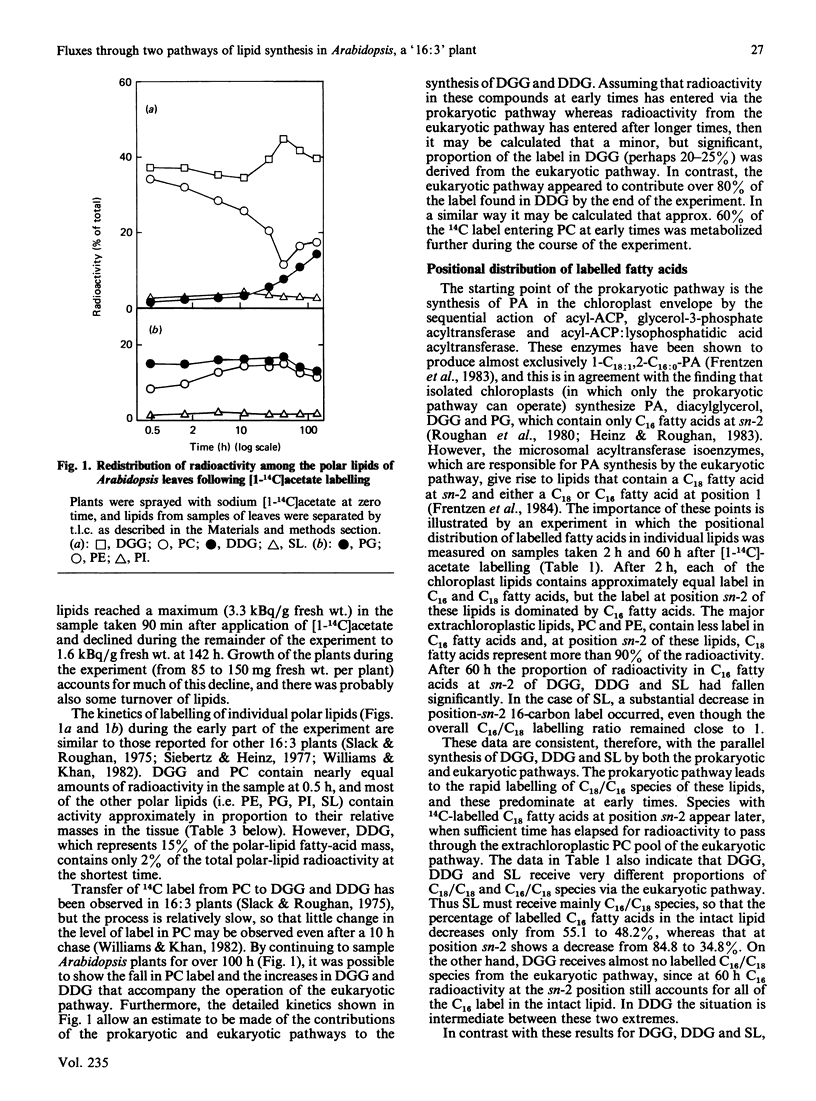
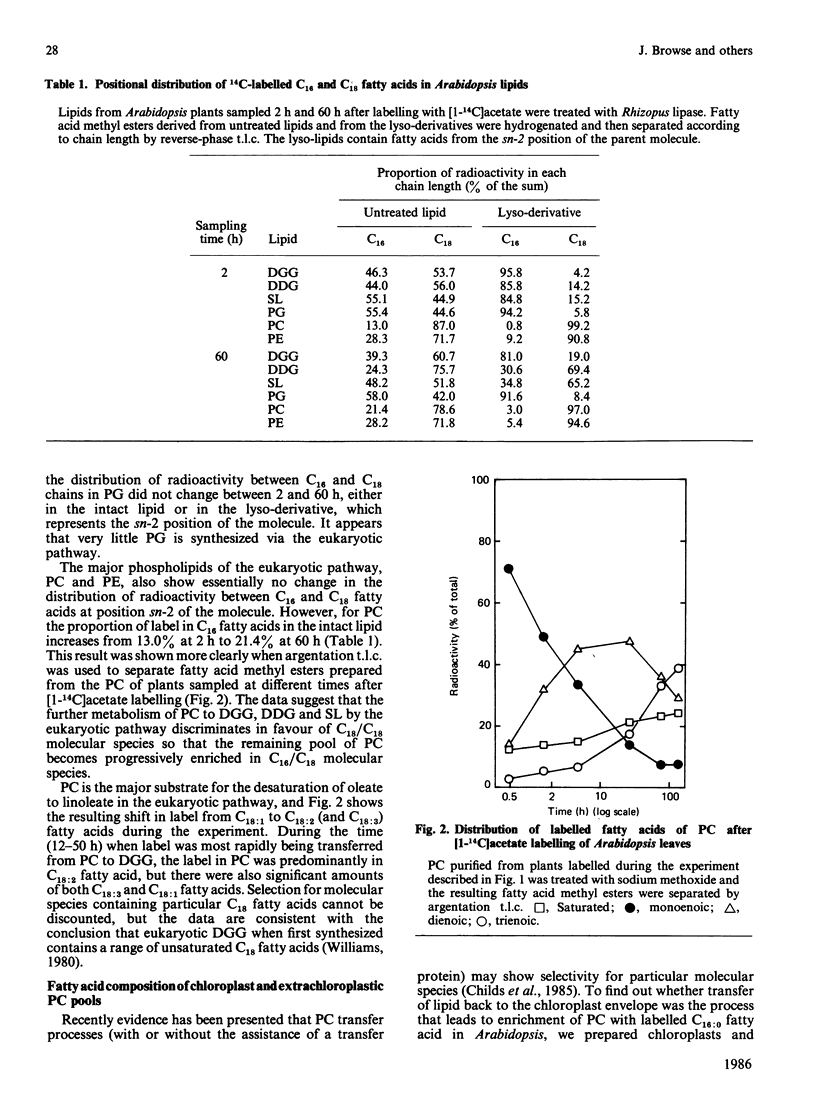
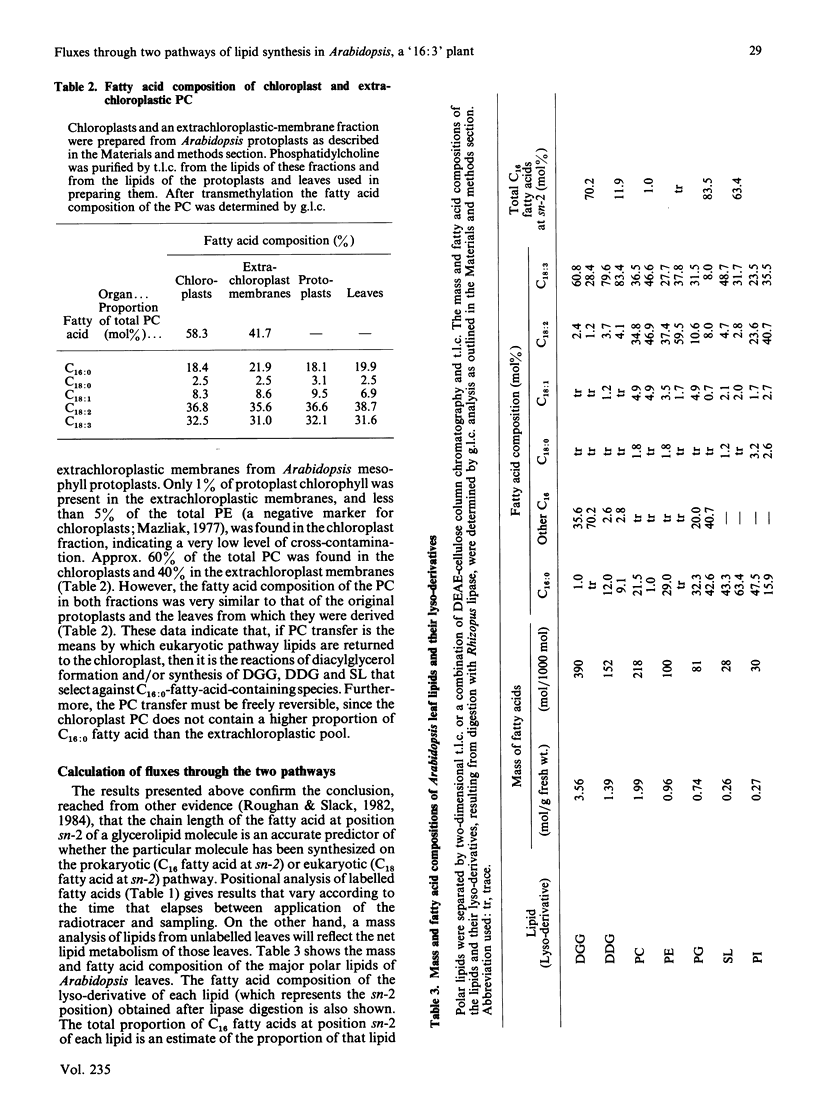
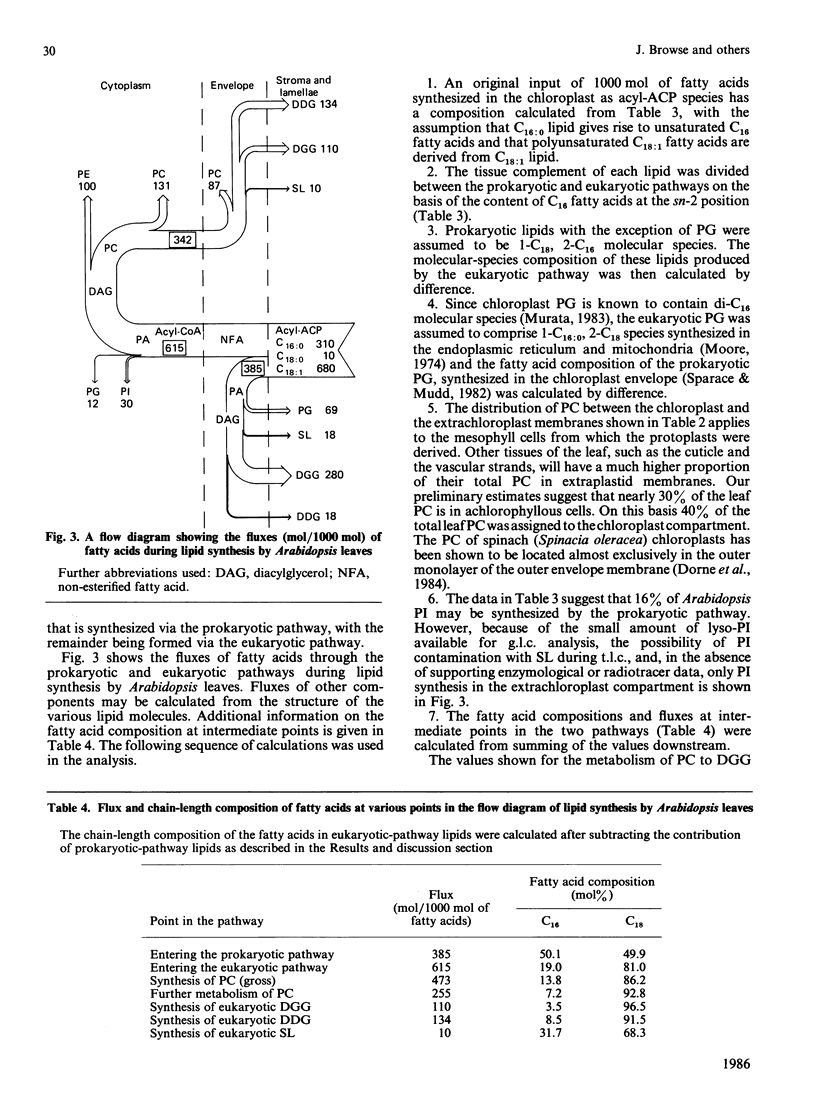
The kinetics of [1-14C]acetate incorporation in Arabidopsis thaliana L. (Heyn) showed almost equal labelling of phosphatidylcholine (PC) and diacylgalactosylglycerol (DGG) at early times and the transfer of radioactivity from PC to DGG and diacyldigalactosylglycerol (DDG) at longer times. These kinetics demonstrated the parallel operation of the prokaryotic and eukaryotic pathways of lipid synthesis [Roughan & Slack (1982) Annu. Rev. Plant Physiol. 33, 97-132] in this tissue. At 2 h after the application of [1-14C]acetate, more than 85% of the radioactivity at the sn-2 position of each chloroplast lipid was in 16-carbon fatty acids. However, after 60 h, molecular species containing labelled C18 fatty acids at position sn-2 and presumably derived from microsomal PC made a large contribution (20-70%) to each chloroplast lipid except phosphatidylglycerol. These findings are consistent with the contention that the chain length of the fatty acid at the sn-2 position of glycerol is an accurate predictor of whether a particular lipid molecule has been synthesized by the prokaryotic or eukaryotic pathway. At 30 min after the start of [1-14C]acetate labelling, only 12.3% of the radioactivity in PC was in saturated fatty acids, but the proportion increased steadily to 24.3% after 142 h. It is suggested that steps involved in the conversion of PC to chloroplast lipids on the eukaryotic pathway discriminate against palmitate-containing species. The step involved does not appear to be transfer of PC to the chloroplast because extrachloroplastic and chloroplast membranes purified from Arabidopsis mesophyll protoplasts each contained PC with a fatty acid composition similar to that of the same lipid from leaves. Positional analysis of unlabelled lipids, together with the information summarized above, is used to construct a quantitative scheme of the fluxes through the prokaryotic and eukaryotic pathways during lipid synthesis in Arabidopsis. This scheme shows that 38% of the fatty acids synthesized de novo in the chloroplast enter the prokaryotic pathway in the chloroplast envelope. Of the 62% which are exported as acyl-CoA species to enter the eukaryotic pathway, 56% (34% of the total) are returned to complete synthesis of the chloroplast's complement of glycerolipids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelqvist L. A. A simple and convenient procedure for the hydrogenation of lipids on the micro- and nanomole scale. J Lipid Res. 1972 Jan;13(1):146–148. [PubMed] [Google Scholar]
- Appleby R. S., Safford R., Nichols B. W. The involvement of lecithin and monogalactosyl diglyceride in linoleate synthesis by green and blue-green algae. Biochim Biophys Acta. 1971 Nov 5;248(2):205–211. doi: 10.1016/0005-2760(71)90008-7. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Child P., Myher J. J., Kuypers F. A., Op den Kamp J. A., Kuksis A., Van Deenen L. L. Acyl selectivity in the transfer of molecular species of phosphatidylcholines from human erythrocytes. Biochim Biophys Acta. 1985 Jan 25;812(2):321–332. doi: 10.1016/0005-2736(85)90306-2. [DOI] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Heinz E., Roughan P. G. Rates and products of long-chain Fatty Acid synthesis from [1-C]acetate in chloroplasts isolated from leaves of 16:3 and 18:3 plants. Plant Physiol. 1984 Apr;74(4):890–896. doi: 10.1104/pp.74.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. E., Roughan P. G., Slack C. R. Manipulating the incorporation of [1-C]acetate into different leaf glycerolipids in several plant species. Plant Physiol. 1982 Nov;70(5):1316–1320. doi: 10.1104/pp.70.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K. On extraction of acyl and alkyl dihydroxyacetone phosphate from incubation mixtures. Lipids. 1974 Aug;9(8):502–505. doi: 10.1007/BF02532495. [DOI] [PubMed] [Google Scholar]
- Heinz E., Roughan P. G. Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 1983 Jun;72(2):273–279. doi: 10.1104/pp.72.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Khan M. U., Williams J. P. Improved thin-layer chromatographic method for the separation of major phospholipids and glycolipids from plant lipid extracts and phosphatidyl glycerol and bis(monoacylglyceryl) phosphate from animal lipid extracts. J Chromatogr. 1977 Oct 11;140(2):179–185. doi: 10.1016/s0021-9673(00)88412-5. [DOI] [PubMed] [Google Scholar]
- Moore T. S. Phosphatidylglycerol synthesis in castor bean endosperm: kinetics, requirements, and intracellular localization. Plant Physiol. 1974 Aug;54(2):164–168. doi: 10.1104/pp.54.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. W. Fatty acid metabolism in the chloroplast lipids of green and blue-green algae. Lipids. 1968 Jul;3(4):354–360. doi: 10.1007/BF02530939. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. The role of chloroplasts and microsomal fractions in polar-lipid synthesis from [1-14C]acetate by cell-free preparations from spinach (Spinacia oleracea) leaves. Biochem J. 1980 Apr 15;188(1):17–24. doi: 10.1042/bj1880017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G., Mudd J. B., McManus T. T., Slack C. R. Linoleate and alpha-linolenate synthesis by isolated spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Dec 15;184(3):571–574. doi: 10.1042/bj1840571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Phosphatidylglycerol and chilling sensitivity in plants. Plant Physiol. 1985 Mar;77(3):740–746. doi: 10.1104/pp.77.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. E., Williams J. P. Galactolipid Synthesis in Vicia faba Leaves: IV. Site(s) of Fatty Acid Incorporation into the Major Glycerolipids. Plant Physiol. 1979 Apr;63(4):674–676. doi: 10.1104/pp.63.4.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling studies in vivo on the metabolism of the acyl and glycerol moieties of the glycerolipids in the developing maize leaf. Biochem J. 1977 Feb 15;162(2):289–296. doi: 10.1042/bj1620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Terpstra J. Some properties of a microsomal oleate desaturase from leaves. Biochem J. 1976 Apr 1;155(1):71–80. doi: 10.1042/bj1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. The kinetics of incorporation in vivo of (14C)acetate and (14C)carbon dioxide into the fatty acids of glycerolipids in developing leaves. Biochem J. 1975 Nov;152(2):217–228. doi: 10.1042/bj1520217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparace S. A., Mudd J. B. Phosphatidylglycerol synthesis in spinach chloroplasts: characterization of the newly synthesized molecule. Plant Physiol. 1982 Nov;70(5):1260–1264. doi: 10.1104/pp.70.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. P. Galactolipid synthesis in Vicia faba leaves. V. Redistribution of 14C-labelling in the polar moieties and the 14C-labelling kinetics of the fatty acids of the molecular species of mono- and digalactosyl diacylglycerols. Biochim Biophys Acta. 1980 Jun 23;618(3):461–472. doi: 10.1016/0005-2760(80)90264-7. [DOI] [PubMed] [Google Scholar]
- Williams J. P., Watson G. R., Leung S. P. Galactolipid Synthesis in Vicia faba Leaves: II. Formation and Desaturation of Long Chain Fatty Acids in Phosphatidylcholine, Phosphatidylglycerol, and the Galactolipids. Plant Physiol. 1976 Feb;57(2):179–184. doi: 10.1104/pp.57.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]



