Abstract
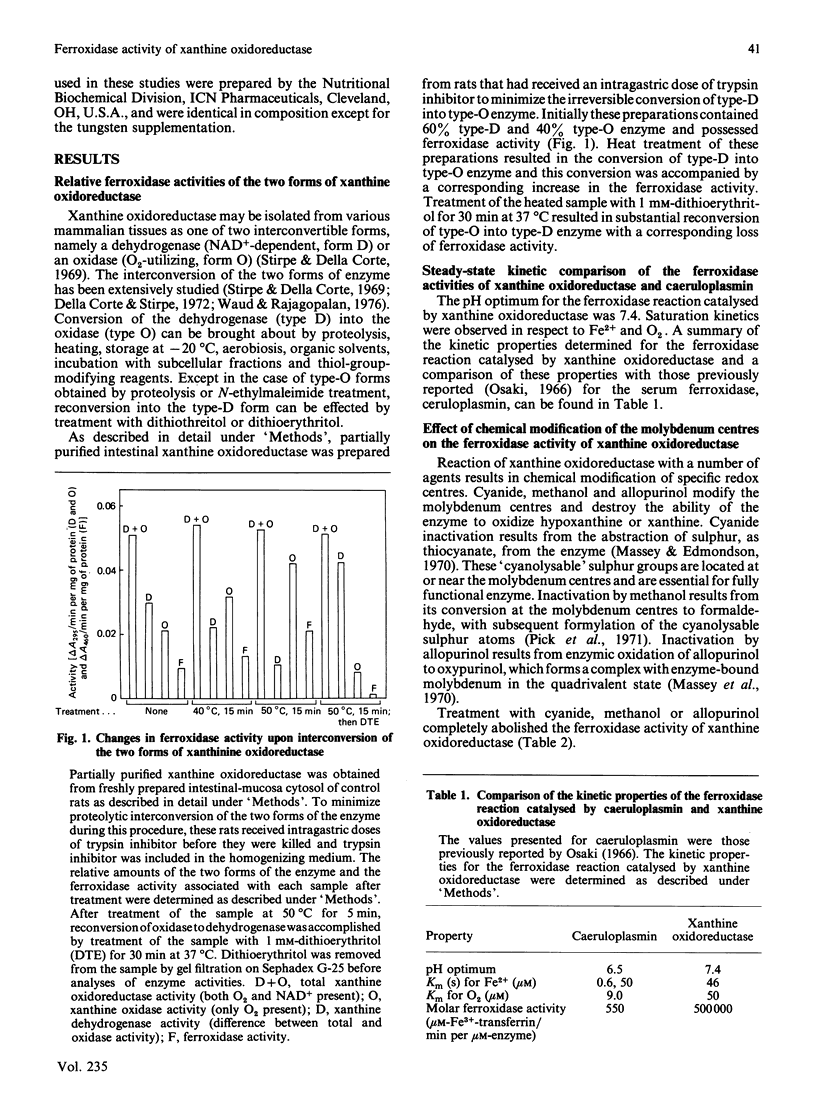
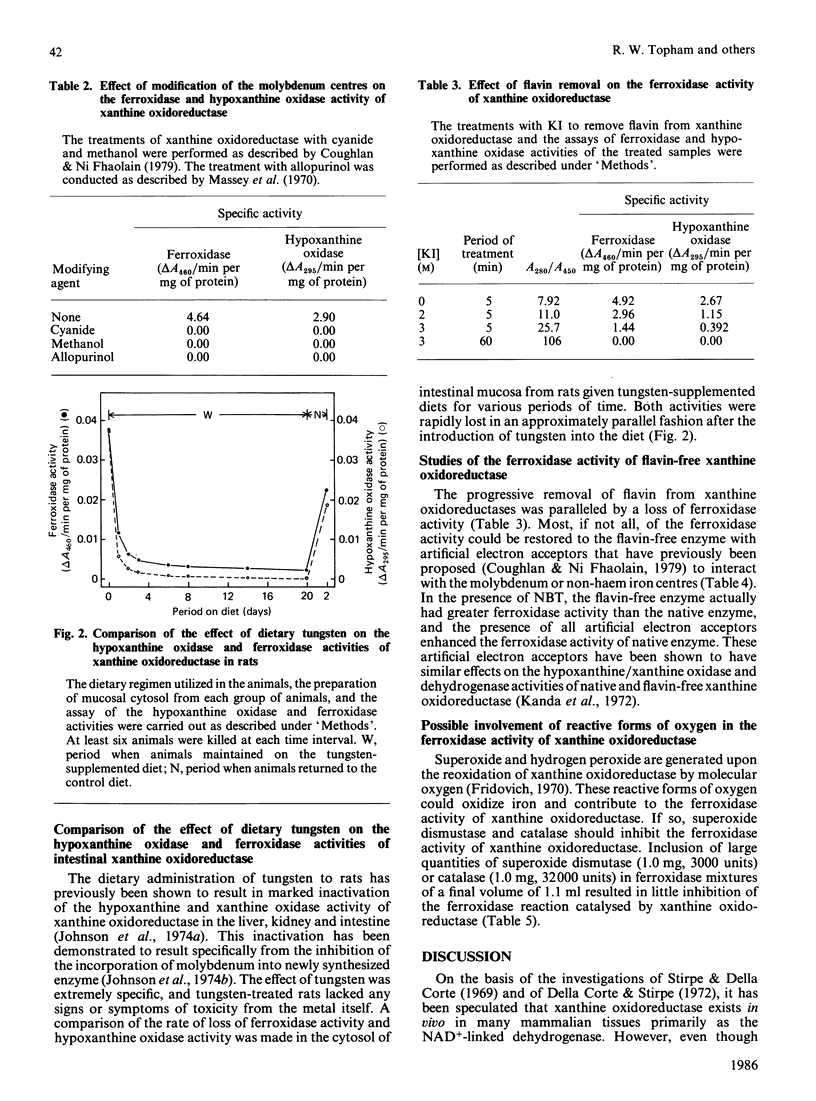
The O2-utilizing (type O, oxidase) form of xanthine oxidoreductase is primarily responsible for its ferroxidase activity. This form of xanthine oxidoreductase has 1000 times the ferroxidase activity of the serum ferroxidase caeruloplasmin. It has the ability to catalyse the oxidative incorporation of iron into transferrin at very low Fe2+ and O2 concentrations. Furthermore, the pH optimum of the ferroxidase activity of the enzyme is compatible with the conditions of pH that normally exist in the intestinal mucosa, where it has been proposed that xanthine oxidoreductase may facilitate the absorption of ionic iron. Modification of the molybdenum (Mb) centres of the enzyme in vitro by treatment with cyanide, methanol or allopurinol completely abolishes its ferroxidase activity. The feeding of dietary tungsten to rats, which prevents the incorporation of molybdenum into newly synthesized intestinal xanthine oxidoreductase, results in the progressive loss of the ferroxidase activity of intestinal-mucosa homogenates. Removal of the flavin centres from the enzyme also results in the complete loss of ferroxidase activity; however, the ferroxidase activity of the flavin-free form of the enzyme can be restored with artificial electron acceptors that interact with the molybdenum or non-haem iron centres. The presence of superoxide dismutase or catalase in the assay system results in little inhibition of the ferroxidase activity of xanthine oxidoreductase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battelli M. G., Corte E. D., Stirpe F. Xanthine oxidase type D (dehydrogenase) in the intestine and other organs of the rat. Biochem J. 1972 Feb;126(3):747–749. doi: 10.1042/bj1260747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittin G. M., Chee Q. T. Relation of ferroxidase (ceruloplasmin) to iron absorption. J Lab Clin Med. 1969 Jul;74(1):53–59. [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D., Ballou D., Van Heuvelen A., Palmer G., Massey V. Kinetic studies on the substrate reduction of xanthine oxidase. J Biol Chem. 1973 Sep 10;248(17):6135–6144. [PubMed] [Google Scholar]
- El-Shobaki F. A., Rummel W. Mucosal transferrin and ferritin factors in the regulation of iron absorption. Res Exp Med (Berl) 1977 Dec 15;171(3):243–253. doi: 10.1007/BF01851508. [DOI] [PubMed] [Google Scholar]
- Evans J. L., Abraham P. A. Anemia, iron storage and ceruloplasmin in copper nutrition in the growing rat. J Nutr. 1973 Feb;103(2):196–201. doi: 10.1093/jn/103.2.196. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Fried R., Fried L. W., Babin D. R. Biological role of xanthine oxidase and tetrazolium-reductase inhibitor. Eur J Biochem. 1973 Mar 15;33(3):439–445. doi: 10.1111/j.1432-1033.1973.tb02701.x. [DOI] [PubMed] [Google Scholar]
- Halliday J. W., Powell L. W., Mack U. Iron absorption in the rat: the search for possible intestinal mucosal carriers. Br J Haematol. 1976 Oct;34(2):237–250. doi: 10.1111/j.1365-2141.1976.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Huebers H., Huebers E., Forth W., Rummel W. Binding of iron to a non-ferritin protein in the mucosal cells of normal and iron-deficient rats during absorption. Life Sci I. 1971 Oct 15;10(20):1141–1148. doi: 10.1016/0024-3205(71)90274-8. [DOI] [PubMed] [Google Scholar]
- Huebers H., Huebers E., Rummel W., Crichton R. R. Isolation and characterization of iron-binding proteins from rat intestinal mucosa. Eur J Biochem. 1976 Jul 15;66(3):447–455. doi: 10.1111/j.1432-1033.1976.tb10569.x. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Osaki S., Frieden E. A micromethod for the determination of ferroxidase (ceruloplasmin) in human serums. Clin Chem. 1967 Feb;13(2):142–150. [PubMed] [Google Scholar]
- Johnson J. L., Rajagopalan K. V., Cohen H. J. Molecular basis of the biological function of molybdenum. Effect of tungsten on xanthine oxidase and sulfite oxidase in the rat. J Biol Chem. 1974 Feb 10;249(3):859–866. [PubMed] [Google Scholar]
- Johnson J. L., Waud W. R., Cohen H. J., Rajagopalan K. V. Molecular basis of the biological function of molybdenum. Molybdenum-free xanthine oxidase from livers of tungsten-treated rats. J Biol Chem. 1974 Aug 25;249(16):5056–5061. [PubMed] [Google Scholar]
- Kamiński Z. W., Jezewska M. M. Intermediate dehydrogenase-oxidase form of xanthine oxidoreductase in rat liver. Biochem J. 1979 Jul 1;181(1):177–182. doi: 10.1042/bj1810177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M., Brady F. O., Rajagopalan K. V., Handler P. Studies on the dissociation of flavin adenine dinucleotide from metalloflavoproteins. J Biol Chem. 1972 Feb 10;247(3):765–770. [PubMed] [Google Scholar]
- Komai H., Massey V., Palmer G. The preparation and properties of deflavo xanthine oxidase. J Biol Chem. 1969 Apr 10;244(7):1692–1700. [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V. Xanthine oxidase activities: evidence for two catalytically different types. Arch Biochem Biophys. 1978 Jan 30;185(2):370–375. doi: 10.1016/0003-9861(78)90179-0. [DOI] [PubMed] [Google Scholar]
- Lee G. R., Nacht S., Lukens J. N., Cartwright G. E. Iron metabolism in copper-deficient swine. J Clin Invest. 1968 Sep;47(9):2058–2069. doi: 10.1172/JCI105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970 Dec 25;245(24):6595–6598. [PubMed] [Google Scholar]
- Massey V., Komai H., Palmer G., Elion G. B. On the mechanism of inactivation of xanthine oxidase by allopurinol and other pyrazolo[3,4-d]pyrimidines. J Biol Chem. 1970 Jun 10;245(11):2837–2844. [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J Biol Chem. 1971 May 10;246(9):3018–3023. [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966 Jun 25;241(12):2746–2751. [PubMed] [Google Scholar]
- Osaki S., Johnson D. A. Mobilization of liver iron by ferroxidase (ceruloplasmin). J Biol Chem. 1969 Oct 25;244(20):5757–5758. [PubMed] [Google Scholar]
- Osaki S. Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase (ceruloplasmin). J Biol Chem. 1966 Nov 10;241(21):5053–5059. [PubMed] [Google Scholar]
- Pick F. M., McGartoll M. A., Bray R. C. Reaction of formaldehyde and of methanol with xanthine oxidase. Eur J Biochem. 1971 Jan 1;18(1):65–72. doi: 10.1111/j.1432-1033.1971.tb01215.x. [DOI] [PubMed] [Google Scholar]
- Ragan H. A., Nacht S., Lee G. R., Bishop C. R., Cartwright G. E. Effect of ceruloplasmin on plasma iron in copper-deficient swine. Am J Physiol. 1969 Nov;217(5):1320–1323. doi: 10.1152/ajplegacy.1969.217.5.1320. [DOI] [PubMed] [Google Scholar]
- Roeser H. P., Lee G. R., Nacht S., Cartwright G. E. The role of ceruloplasmin in iron metabolism. J Clin Invest. 1970 Dec;49(12):2408–2417. doi: 10.1172/JCI106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savin M. A., Cook J. D. Mucosal iron transport by rat intestine. Blood. 1980 Dec;56(6):1029–1035. [PubMed] [Google Scholar]
- Stirpe F., Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O). J Biol Chem. 1969 Jul 25;244(14):3855–3863. [PubMed] [Google Scholar]
- Topham R. W. Isolation of an intestinal promoter of Fe3+ - transferrin formation. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1339–1345. doi: 10.1016/0006-291x(78)91150-6. [DOI] [PubMed] [Google Scholar]
- Topham R. W., Johnson D. A. Kinetic studies of the Fe(II) oxidation with human serum ferroxidase-II. Arch Biochem Biophys. 1974 Feb;160(2):647–654. doi: 10.1016/0003-9861(74)90442-1. [DOI] [PubMed] [Google Scholar]
- Topham R. W., Walker M. C., Calisch M. P., Williams R. W. Evidence for the participation of intestinal xanthine oxidase in the mucosal processing of iron. Biochemistry. 1982 Sep 14;21(19):4529–4535. doi: 10.1021/bi00262a002. [DOI] [PubMed] [Google Scholar]
- Topham R. W., Woodruff J. H., Neatrour G. P., Calisch M. P., Russo R. B., Jackson M. R. The role of ferroxidase-II and a ferroxidase inhibitor in iron mobilization from tissue stores. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1532–1539. doi: 10.1016/0006-291x(80)91348-0. [DOI] [PubMed] [Google Scholar]
- Topham R. W., Woodruff J. H., Walker M. C. Purification and characterization of the intestinal promoter of iron(3+)-transferrin formation. Biochemistry. 1981 Jan 20;20(2):319–324. doi: 10.1021/bi00505a014. [DOI] [PubMed] [Google Scholar]
- Valberg L. S., Sorbie J., Ludwig J. Mucosal iron-binding proteins in mice with X-linked anaemia. Br J Haematol. 1977 Feb;35(2):321–330. doi: 10.1111/j.1365-2141.1977.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. The mechanism of conversion of rat liver xanthine dehydrogenase from an NAD+-dependent form (type D) to an O2-dependent form (type O). Arch Biochem Biophys. 1976 Feb;172(2):365–379. doi: 10.1016/0003-9861(76)90088-6. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Lee G. R., Cartwright G. E. Ferroxidase activity of rat ceruloplasmin. Am J Physiol. 1974 Nov;227(5):1094–1097. doi: 10.1152/ajplegacy.1974.227.5.1094. [DOI] [PubMed] [Google Scholar]
- Worwood M., Jacobs A. Absorption of 59 Fe in the rat: iron binding substances in the soluble fraction of intestinal mucosa. Life Sci I. 1971 Dec 1;10(23):1363–1373. doi: 10.1016/0024-3205(71)90337-7. [DOI] [PubMed] [Google Scholar]


