Abstract
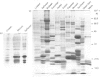
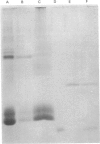
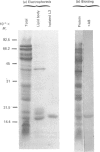
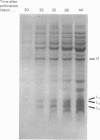
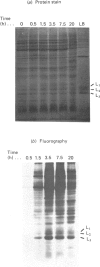
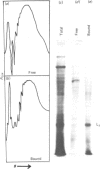
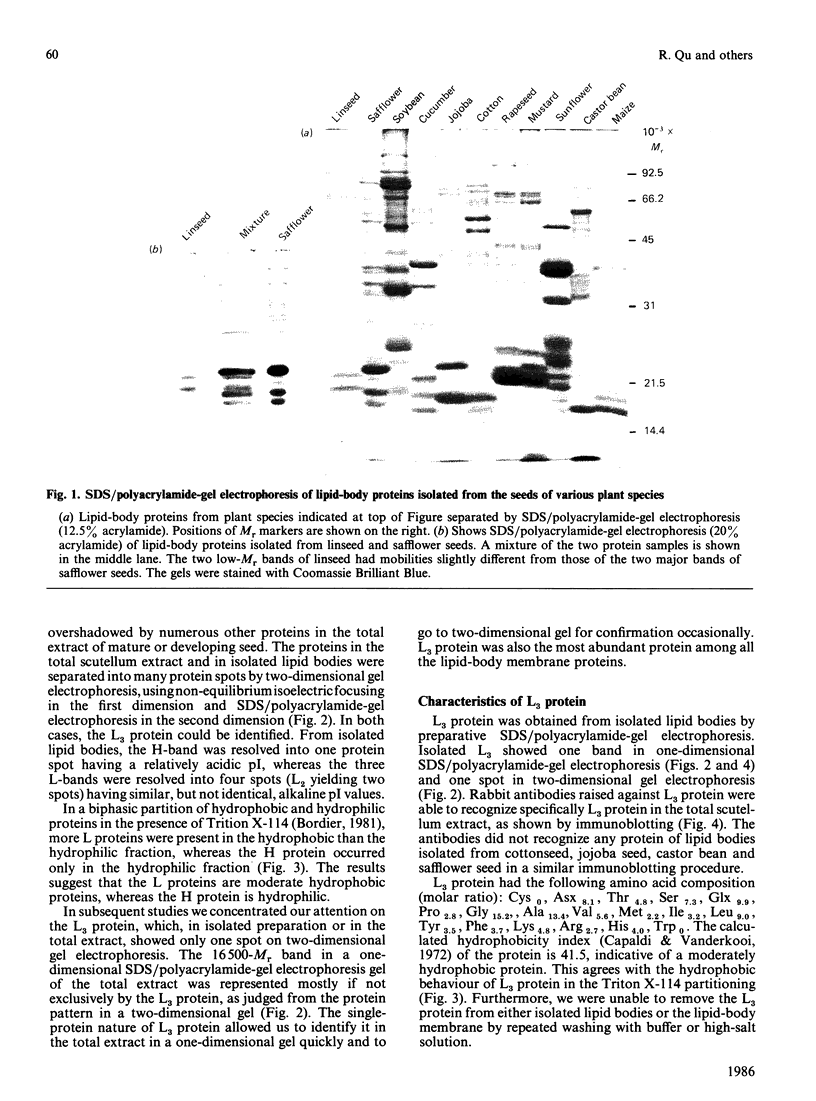
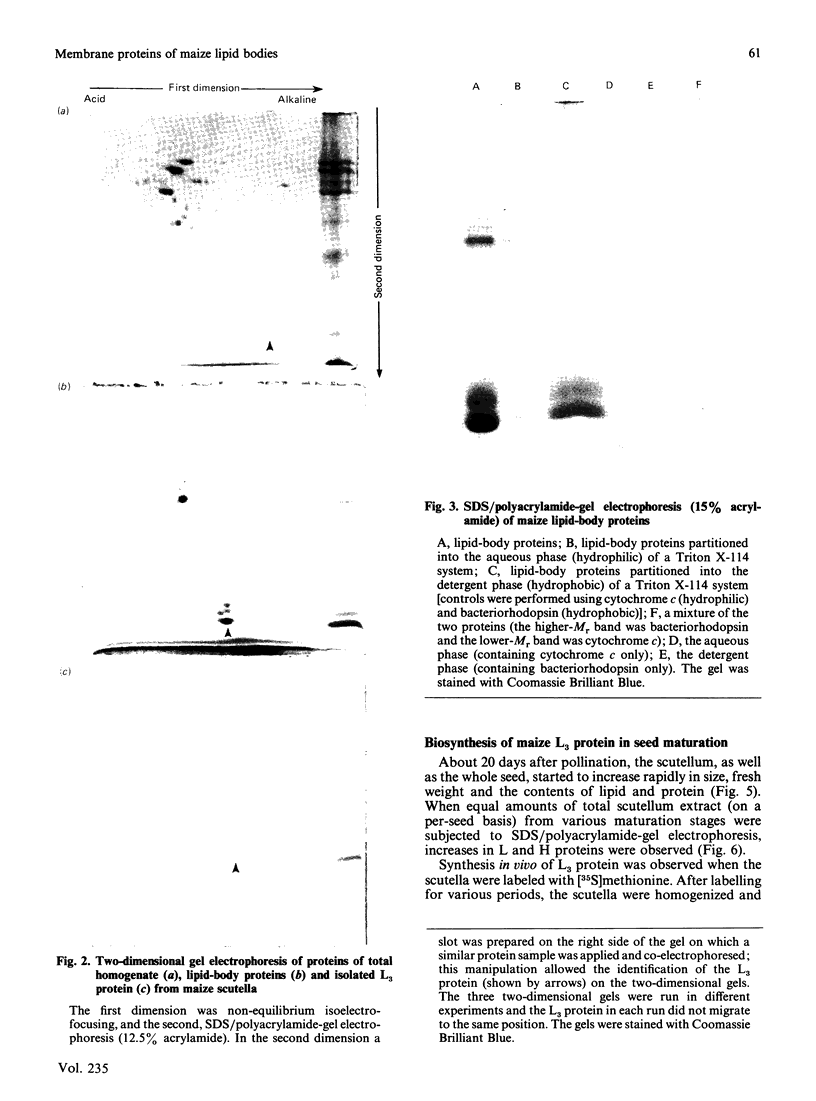
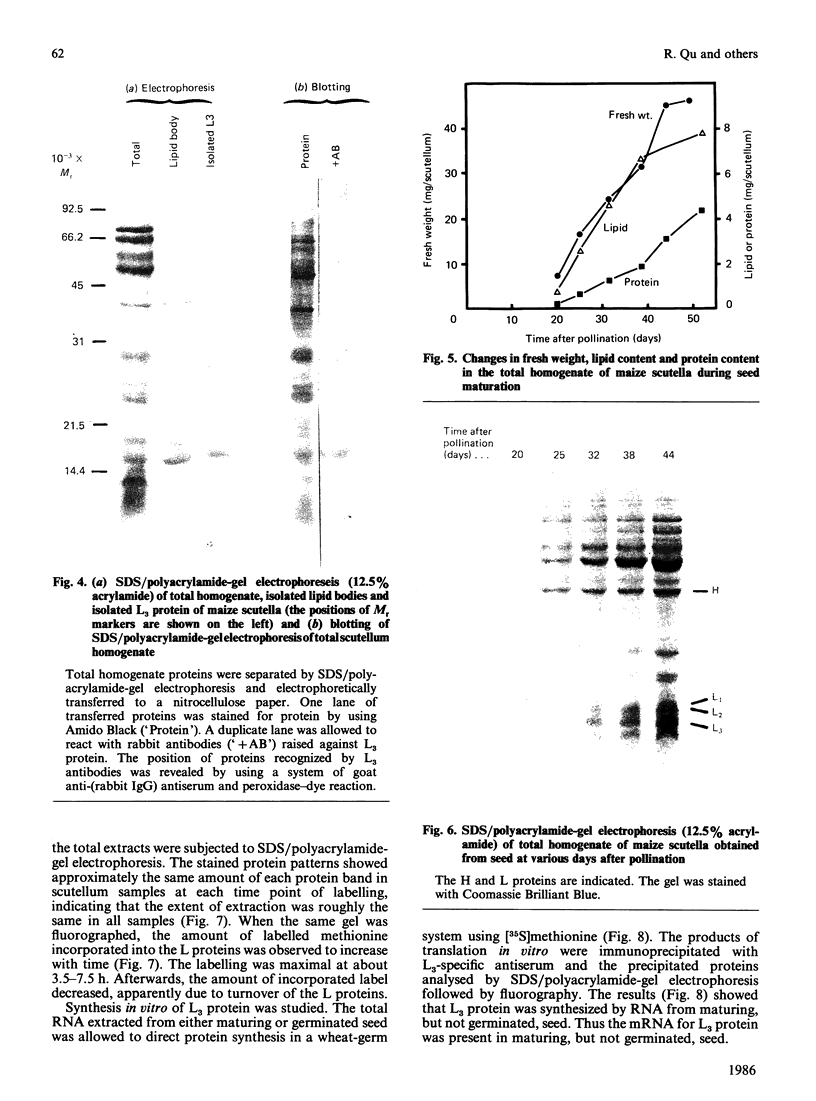
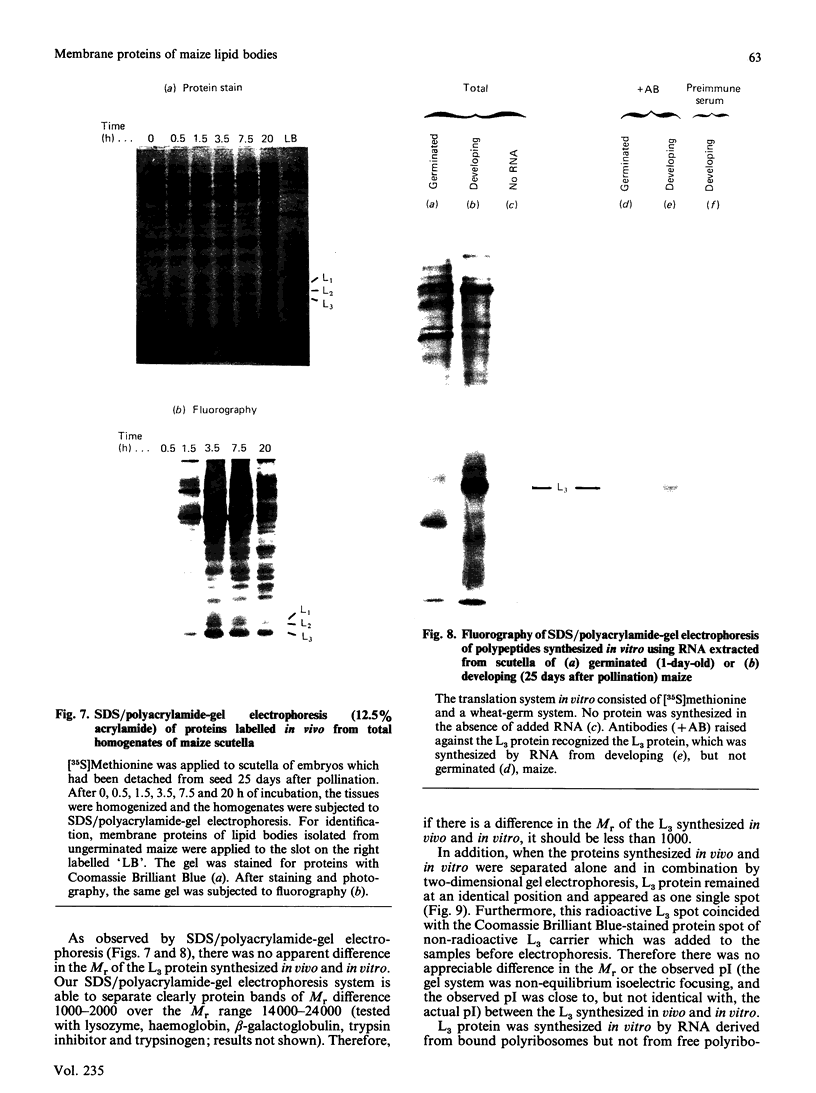
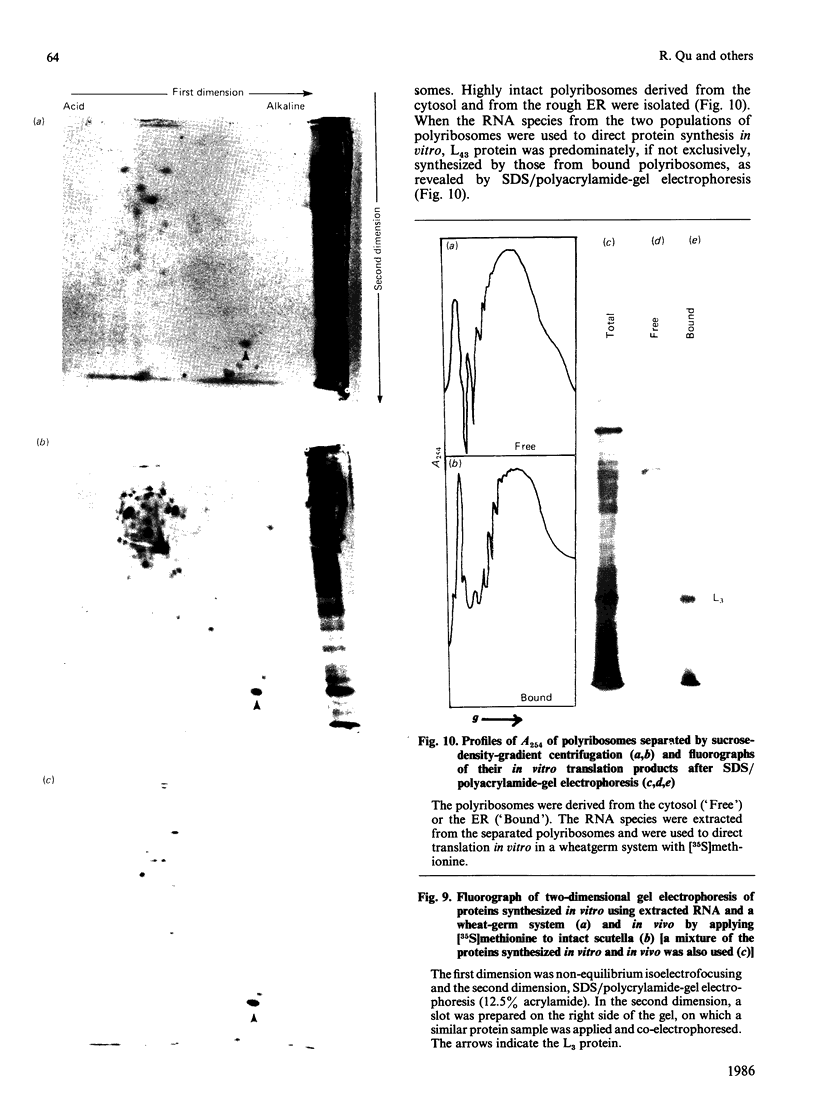
Storage lipid bodies, which are prominent organelles present in the storage tissues of most seeds, have not been subjected to intensive biochemical investigation. In the present studies the major proteins in lipid bodies isolated from eleven taxonomically diverse species were shown to be distinctly different, as revealed by SDS/polyacrylamide-gel electrophoresis. The lipid-body membrane of maize (Zea mays L.) contained three major proteins of low Mr (19,500, 18,000 and 16,500), and they were chosen for further study. They all had alkaline pI values and behaved as hydrophobic integral proteins, as shown by their resistance to solubilization after repeated washing, amino acid composition and partitioning in a Triton X-114 system. Labelling in vivo with [35S]methionine and translation in vitro using extracted RNA in a wheat-germ system showed that the proteins were synthesized during seed maturation and not germination. The proteins synthesized in vivo and in vitro exhibited no appreciable difference in their mobilities in two-dimensional gel electrophoresis (isoelectric focusing and molecular sieving). The most abundant protein, that of Mr 16,500, was shown to be synthesized predominantly, if not exclusively, by RNA derived from bound polyribosomes and not from free polyribosomes. The implication of the results on the biosynthesis of the lipid bodies is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Donaldson R. P. Membrane lipid metabolism in germinating castor bean endosperm. Plant Physiol. 1976 Apr;57(4):510–515. doi: 10.1104/pp.57.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: V. An Attempt to Characterize the Total Free and Membrane-bound Polysomal Population. Plant Physiol. 1975 Apr;55(4):749–756. doi: 10.1104/pp.55.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. H., Wimer L. T., Huang A. H. Lipase in the Lipid Bodies of Corn Scutella during Seedling Growth. Plant Physiol. 1983 Oct;73(2):460–463. doi: 10.1104/pp.73.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau R. A., Huang A. H. Gluconeogenesis from storage wax in the cotyledons of jojoba seedlings. Plant Physiol. 1977 Aug;60(2):329–333. doi: 10.1104/pp.60.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau R. A., Liu K. D., Huang A. H. Spherosomes of Castor Bean Endosperm: MEMBRANE COMPONENTS, FORMATION, AND DEGRADATION. Plant Physiol. 1980 Jun;65(6):1176–1180. doi: 10.1104/pp.65.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Slack C. R., Bertaud W. S., Shaw B. D., Holland R., Browse J., Wright H. Some studies on the composition and surface properties of oil bodies from the seed cotyledons of safflower (Carthamus tinctorius) and linseed (Linum ustatissimum). Biochem J. 1980 Sep 15;190(3):551–561. doi: 10.1042/bj1900551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Spherosome membranes: half unit-membranes. Plant Physiol. 1972 Jun;49(6):937–943. doi: 10.1104/pp.49.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]