Abstract
Human immunodeficiency virus (HIV)-induced immunodeficiency is characterized by progressive loss of CD4+ T cells associated with functional abnormalities of the surviving lymphocytes. Increased susceptibility to apoptosis and loss of proper cell cycle control can be observed in lymphocytes from HIV-infected individuals and may contribute to the lymphocyte dysfunction of AIDS patients. To better understand the relation between T-cell activation, apoptosis, and cell cycle perturbation, we studied the effect of exogenous interleukin-2 (IL-2) administration on the intracellular turnover of phase-dependent proteins. Circulating T cells from HIV-infected patients display a marked discrepancy between a metabolic profile typical of G0 and a pattern of expression of phase-dependent proteins that indicates a more-advanced position within the cell cycle. This discrepancy is enhanced by in vitro activation with ConA and ultimately results in a marked increase of apoptotic events. Conversely, treatment of lymphocytes with IL-2 alone restores the phase-specific pattern of expression of cell cycle-dependent proteins and is associated with low levels of apoptosis. Interestingly, exogenous IL-2 administration normalizes the overall intracellular protein turnover, as measured by protein synthesis, half-life of newly synthesised proteins, and total protein ubiquitination, thus providing a possible explanation for the effect of IL-2 on the intracellular kinetics of cell cycle-dependent proteins. The beneficial effect of IL-2 administration is consistent with the possibility of defective IL-2 function in vivo, which is confirmed by the observation that lymphocytes from HIV-infected patients show abnormal endogenous IL-2 paracrine/autocrine function upon in vitro mitogen stimulation. Overall these results confirm that perturbation of cell cycle control contributes to HIV-related lymphocyte dysfunction and, by showing that IL-2 administration can revert this perturbation, suggest a new mechanism of action of IL-2 therapy in HIV-infected patients.
The profound immunodeficiency observed in AIDS patients is related to progressive T-lymphocyte depletion, which is caused by human immunodeficiency virus (HIV)-mediated killing of infected CD4+ T cells as well as by massive apoptotic death of uninfected bystander lymphocytes (20–23, 39, 40, 42). The high level of apoptosis occurring in vivo is consistent with the finding that lymphocytes isolated from HIV-infected patients are abnormally susceptible to apoptotic stimuli in vitro (1, 23, 44). Interestingly, apoptotic phenomena of uninfected lymphocytes during HIV infection involve both CD4 and CD8 T cells, are correlated with the level of immune activation and lymphocyte turnover, and appear to be reverted by effective antiretroviral therapy (4, 7, 22, 23, 29, 37, 42). The presence of this exaggerated propensity to apoptosis indicates that HIV disease is not only a syndrome of virus-mediated CD4+ T-cell destruction but also a state of qualitative impairment of lymphocyte function. Consistent with this hypothesis, abnormalities in lymphocyte function in HIV-infected patients have been described by several authors using various experimental systems (21, 41, 49, 50).
Under normal circumstances T-cell activation and progression through the cell cycle are dependent on (i) environmental stimuli, (ii) sequential synthesis and degradation of regulatory proteins, and (iii) metabolic status of the cell. Among the external stimuli, a simple distinction can be made between those that induce a state of “competence,” such as antigens, superantigens, anti-CD3, phytohemagglutinin, concanavalin A (ConA), etc., and those that determine the final progression through repeated cell cycling (e.g., interleukin 2 [IL-2]) (33). When physiological T-cell activation takes place, both stimuli are provided in the lymph node environment by a combination of factors related to antigens, antigen-presenting cells, costimulatory signals, and cytokines. The final result of this process is the proliferation and differentiation of antigen-specific lymphocyte T-cell clones, with generation of memory and effector lymphocytes. Conversely, apoptosis or anergy occurs in cells which have low affinity for the antigen or which receive defective and/or inappropriate costimulation and cytokine signaling. This finely tuned process usually leads to antigen elimination via either humoral or cellular immune responses and eliminates potentially autoreactive cells (33).
In the setting of HIV infection many factors impact on the physiological regulation of lymphocyte activation, including (i) high levels of background antigenic stimuli, (ii) disruption of the lymph node architecture, (iii) excessive presence of proinflammatory or proapoptotic cytokines, and (iv) abnormal pressure on the regenerative capacity of T cells in order to maintain the numeric homeostasis of the lymphoid system in compensation for HIV-induced cell losses (8, 11, 24–28, 30, 38, 45, 55). In this situation it is conceivable that a persistent state of lymphocyte activation may affect the capability of T lymphocytes to properly coordinate the sequential expression of phase-dependent proteins. As a result, the perturbation of cell cycle control becomes itself a cause of lymphocyte dysfunction, probably by lowering the threshold for activation-induced apoptosis.
In an attempt to find a link between the high susceptibility to apoptosis and the high level of immune activation and T-cell turnover during HIV infection, we focused our attention on the intracellular kinetics of cell cycle-dependent proteins. In previous studies we have shown that lymphocytes from HIV-infected individuals express high levels of cyclin B and AgNOR proteins, which are characteristic of the advanced phases of the cell cycle (i.e., G1/S to G2/M), despite a DNA content and a metabolic profile that are typical of a G0 phase (6, 47). This apparent conflict between cell cycle and metabolic profiles was reverted when patients were treated with antiretroviral therapy. Importantly, the abnormal functional status of the cell cycle machinery in peripheral blood lymphocytes (PBLs) from HIV-infected patients becomes more evident after in vitro treatment with mitogens. Following activation of lymphocytes from healthy individuals, the intracellular turnover of various cell cycle-dependent parameters (cyclin B1 and cdc25 expression and ubiquitination, p34 cdc2 activity, NOR morphology, and C23/nucleolin localization) shows a cyclic pattern, which leads to a biological state similar to that observed in the same lymphocytes prior to activation (6). In contrast, complex but consistent changes of the same indices are seen in T lymphocytes from HIV-infected patients, resulting in a fivefold increase in activation-induced apoptosis (6, 47). We thus concluded that this perturbation of cell cycle-dependent proteins is part of the functional lymphocyte impairment caused by HIV infection and may represent a novel biological link between immune activation, accelerated lymphocyte turnover, and increased apoptosis during HIV infection.
We now hypothesize that the cell cycle abnormalities observed in HIV-infected patients may be related to an in vivo imbalance between the different stimuli that are required for proper T-cell activation and proliferation. IL-2 is a very well-characterized T-cell growth factor that plays a crucial role in determining the fate of T-cell proliferation in vitro and in vivo. It is therefore conceivable that a defect in IL-2 production and/or signaling may play a significant role in the loss of cell cycle control that is associated with chronic HIV infection. To test this hypothesis, we studied the effect of exogenous IL-2 administration on the intracellular kinetics of cell cycle-dependent proteins.
MATERIALS AND METHODS
Patient population.
Twenty HIV-infected patients were included in this study. All patients were asymptomatic and were not receiving antiretroviral treatment at the time of blood collection; they had an average viremia of 41,000 copies/ml and an average CD4 count of 334/mm3. Ten uninfected individuals were used as controls. After informed consent was obtained, blood samples were collected, and HIV viremia was measured by the branched DNA technique (Quantiplex; Chiron).
Lymphocytes.
Immunological phenotyping was performed on FACScaliber (Becton Dickinson, San Jose, Calif.). after direct staining with human CD4-fluorescein isothiocyanate monoclonal antibodies (Becton Dickinson). For the in vitro activation studies, PBLs were cultured in 10% fetal calf serum–RPMI medium at an initial density of 106 cells/ml. Following ConA (5 ng/ml) and/or recombinant IL-2 (50 IU/ml) administration, cells were monitored for ornithine decarboxylase (ODC) activity, proline uptake, IL-2 production, and activity of cell machinery for protein and DNA synthesis (data not shown). All cell cycle-related metabolic parameters were measured as previously detailed (6, 47).
Western blotting.
ODC, 19S regulator subunit 7 of the proteasome (Mss-1), nucleolin, and cyclin B1 expression was measured by Western blotting (monoclonal antibodies from Santa Cruz Biotechnology Inc., Santa Cruz, Calif.), and the bands were analyzed using SigmaGel (Handel Scientific Co., San Rafael, Calif.). The numerical values, on a scale from 0 to 250, indicate the absolute area of the band, i.e., the total calibrated pixel intensity values of each band. Two to five replicates were performed for each sample. In all measurements, internal controls were always performed, including lysis of equal cell numbers, by loading into each lane equal volumes of equal protein concentrations (15 μg/lane) and, after electrophoresis, by performing Coomassie staining. If different protein concentrations were observed at this time, the whole procedure was repeated and the initial-loading protein concentration was adjusted based on the actin band.
AgNOR staining.
AgNOR staining was performed as previously described (6). Briefly, lymphocytes were washed with phosphate-buffered saline, suspended in 95% alcohol, and transferred to a coverglass. After alcohol evaporation, coverglasses were stained (2 parts 50% AgNO3 aqueous solution and 1 part 2% gelatin in 1% formic acid) for 12 min in the dark. AgNORs appeared as black intranuclear dots, and their number per cell was evaluated in at least 500 cells. AgNOR area per cell was measured using Image-Pro Plus software (Media Cybernetics, Silver Spring, Md.). After definition of the grey threshold corresponding to AgNOR alone, the AgNOR area was measured automatically.
Confocal microscopy.
Peripheral blood mononuclear cells were fixed on slides (Labtech) using 4% paraformaldehyde for 15 min. Cells were then permeabilized with 0.5% Triton X-100 and washed with phosphate-buffered saline. Unconjugated mouse anti-C23 antibody (Santa Cruz Biotechnology; sc-8031) was added (1/100; 45 min at 37°C). After washes, fluorecein isothiocyanate-conjugated goat anti-mouse immunoglobulin (GAM-Ig; Sigma) was added (1/200 dilution) and propidium iodine (5 g/ml)–RNase (200 g/ml) was added for 30 min. Following further washing, polyvinyl alcohol (Moviol) was added and slides were covered by a coverslip. Confocal microscopy was with a ×63 zoom 1.6 objective (Leica).
IL-2 production studies.
IL-2 production by cultured lymphocytes was measured by enzyme-linked immunosorbent assay (ELISA) (Immunotech International, Marseille, France); values are in nanograms of protein produced by 106 cells. IL-2 accumulation in the conditioned media was measured as follows. After 4, 8, 12, 16, 20, and 24 h of in vitro mitogen activation, cells and media were collected and, after centrifugation, the supernatant was used to measure IL-2 levels by ELISA and biological activity. At the end of the procedure cells were reincubated with fresh medium at the original concentration on a 24-well plate. Initial velocity of IL-2 production was measured after 4, 16, and 24 h of ConA activation. Cultured cells were washed in complete medium, and the concentration of the newly produced IL-2 was determined by ELISA at 1, 5, and 10 min and expressed as picograms per minute per 106 cells. IL-2 biological activity in conditioned media was measured as proliferation index (arbitrary units per picogram) of the IL-2-dependent CTLL cell line. Duration of IL-2 biological activity in conditioned medium is expressed as percentage of the post-ConA peak biological activity after 4, 16, and 24 h of incubation of the cell-free medium at 37°C.
Protein kinetics.
General protein synthesis was measured as initial velocity by determining [3H]leucine (2 μCi of RPMI medium–10% fetal calf serum) incorporation in trichloracetic acid (TCA)-precipitable fractions of cultured peripheral blood mononuclear cells. The half-life of newly synthesized proteins were determined as follows. After ConA activation, cells were labeled with [3H]leucine as described above, washed, and incubated at 106 cells/ml in fresh medium with ConA but in the absence of labeled leucine. Aliquots of 106 cells were collected every 10 h, and the radioactivity still linked to the TCA-precipitable fraction was determined.
Protein ubiquitination.
Electrophoresis and Western blotting of ubiquitinated proteins were performed as follows. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was carried out using a minigel apparatus (Bio-Rad, Hercules, Calif.). Samples were boiled at 100°C for 5 min in 2% mercaptoethanol buffer. Coomassie blue R-250 (Sigma) was the stain used. Molecular mass standards used were 200, 116, 97, 66, 45, 31, and 21 kDa (Bio-Rad). The gels were electroblotted, and the blots were incubated first with rabbit anti-ubiquitin antibody (Sigma) and then with a peroxidase-conjugated goat anti-rabbit antibody (Bio-Rad). Enhanced chemiluminiscence was used as a detection system (Amersham, Little Chalfont, United Kingdom). Each lane received the protein content of 1.7 × 105 cells.
Statistical analysis.
A two-tailed, two-sample Student t test was used to calculate the P value for differences in means and standard deviations of various parameters between HIV-infected patients and uninfected controls.
RESULTS
Lymphocytes from HIV-infected patients show abnormally high expression of phase-dependent proteins, including ODC and the 19S subunit of the proteasome.
PBLs isolated from HIV-infected individuals with active viral replication consistently show overexpression of cyclin B and AgNORs, which suggests that cells have advanced through the cell cycle (i.e., G1/S to G2/M), despite a metabolic profile and a DNA content which indicate a G0 phase (6, 47). To better characterize this discrepancy, we now extended our analysis of the expression of cell cycle-dependent proteins to ODC and the 19S regulator subunit 7 of the proteasome (Mss-1). The expression of ODC, the key regulatory enzyme of the polyamine cycle, is typically increased soon after the cell enters the cycle, and this increase is usually followed by a corresponding increase in ODC activity (43). The 19S regulator subunit 7 is part of the proteasome, the intracellular proteolytic organelle whose expression and activity increase with progression through the cell cycle and determine the phase-dependent, ubiquitin-mediated degradation of cyclins and other proteins (54).
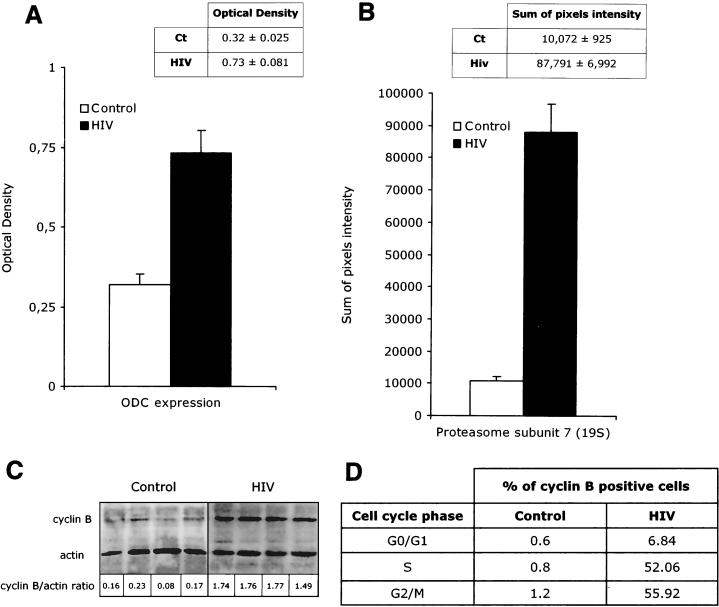
As shown in Fig. 1, freshly isolated PBLs from HIV-infected individuals show expression of ODC, the ATPase proteasome subunit 7 (46 kDa), cyclin B, and AgNORs that is consistently and significantly elevated compared to that of uninfected controls. The intracellular concentrations of ODC were 0.73 ± 0.081 optical units per densitometric area in HIV-infected patients and 0.32 ± 0.025 optical units per densitometric area in healthy individuals (Fig. 1A; P < 0.001), while the intracellular concentrations of the 19S regulator subunit 7 of the proteasome were 87,781 ± 6,992 pixels per densitometric area in HIV-infected patients and 10,872 +/− 925 pixels per densitometric area in healthy individuals (Fig. 1B; P < 0.001). Figure 1C to E show how cyclin B expression and AgNOR number and area of distribution are increased in PBLs from HIV-infected patients, thus confirming our previous observations (6). Interestingly the abnormal microscopic NOR pattern of lymphocytes from HIV-infected patients was associated with intracellular levels of C23/nucleolin, as detected by Western blotting, that were not consistently increased compared to those of normal controls (Fig. 1E). This result indicates that the abnormal pattern of AgNOR staining observed during HIV infection is not a mere consequence of an increased expression of nucleolin but rather is due to a different localization of this molecule. This possibility is confirmed by the observation that in freshly isolated PBLs from HIV-infected patients nucleolin staining in confocal microscopy shows a consistently enlarged area of distribution (see also Fig. 4 and 5). Overall these results further confirm the abnormal pattern of expression of phase-dependent proteins in PBLs from HIV-infected patients. It is of note, however, that this peculiar functional situation does not appear to represent simply the biological equivalent of a more “activated” lymphocyte phenotype in vivo. Indeed, while high expression of ODC, 19S regulator subunit 7 of the proteasome, cyclin B1, and AgNORs suggests that cells are committed to the G1/S transition (10, 43, 51–54, 56), the analysis of the in vitro metabolic profile (proline uptake, protein synthesis, DNA synthesis, and IL-2 production) suggests that most PBLs from HIV-infected patients are resting in G0 (data not shown) (6, 47).
FIG. 1.
Abnormal expression of cell cycle-dependent proteins in PBLs from HIV-infected patients. Experiments were performed on samples collected from 20 HIV-infected individuals and 10 uninfected controls; results are expressed as means and standard deviations. (A) ODC expression as measured by optical density in spectrophotometry. (B) Intracellular concentration of the 19S regulator subunit 7 of the proteasome as measured by Western blotting. (C) Cyclin B intracellular concentration by Western blotting (D) Percentage of cyclin B-positive cells by flow cytometry as measured in the subpopulations of cells with DNA contents of 2n (G0/G1 phase), between 2n and 4n (S phase), and and 4n (G2/M phase). PBLs from HIV-infected patients and controls were costained with anti-cyclin B antibody and propidium iodide for DNA content. (E) Total intracellular nucleolin concentration (left) and AgNOR areas (right) in PBLs from HIV-infected patients and controls.
FIG. 4.
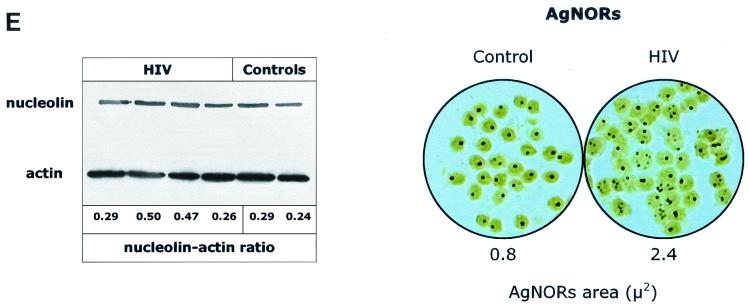
Effect of exogenous IL-2 administration on AgNOR (A) and nucleolin (B) staining of PBLs from HIV-infected patients and controls. Stainings were performed at 24 and 48 h after IL-2 stimulation, and total cell count, cell death (as indicated by trypan blue exclusion), and apoptosis (as indicated by a DNA content of <2n) were determined at the same times.
FIG. 5.
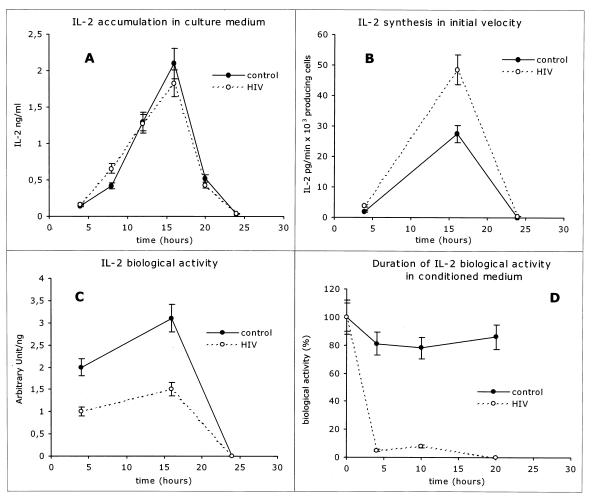
Lymphocytes from HIV-infected patients produce normal amounts of IL-2 with reduced and shortened biological activity. (A) IL-2 accumulation in culture medium from ConA-treated PBLs from HIV-infected patients (open symbols) and controls (solid symbols). IL-2 levels were measured by ELISA. IL-2 accumulation in the conditioned media was measured as follows. After 4, 8, 12, 16, 20, and 24 h of in vitro mitogen activation, cells were collected and spun and the supernatant was used to measure IL-2 levels. Pelleted cells were then reincubated with fresh medium at the original concentration on a 24-well plate. (B) Initial velocity of IL-2 production by ConA-treated PBLs from HIV-infected patients (open symbols) and controls (solid symbols). After 4, 16, and 24 h of culture cells were washed in complete medium and IL-2 production was determined at 1, 5, and 10 min. (C) IL-2 biological activity in conditioned media from PBLs from HIV-infected patients (open symbols) and controls (solid symbols) at 4, 16, and 24 h after ConA activation. IL-2 activity is measured as proliferation index of the IL-2-dependent CTLL cell line. (D) Duration of IL-2 biological activity in conditioned medium from ConA-activated PBLs from HIV-infected patients (open symbols) and controls (solid symbols). IL-2 biological activity of the conditioned media is expressed as a percentage of the post-ConA peak biological activity after 4, 16, and 24 h of 37°C incubation.
Exogenous IL-2 administration reverts the perturbation of intracellular kinetics of cell cycle-dependent proteins.
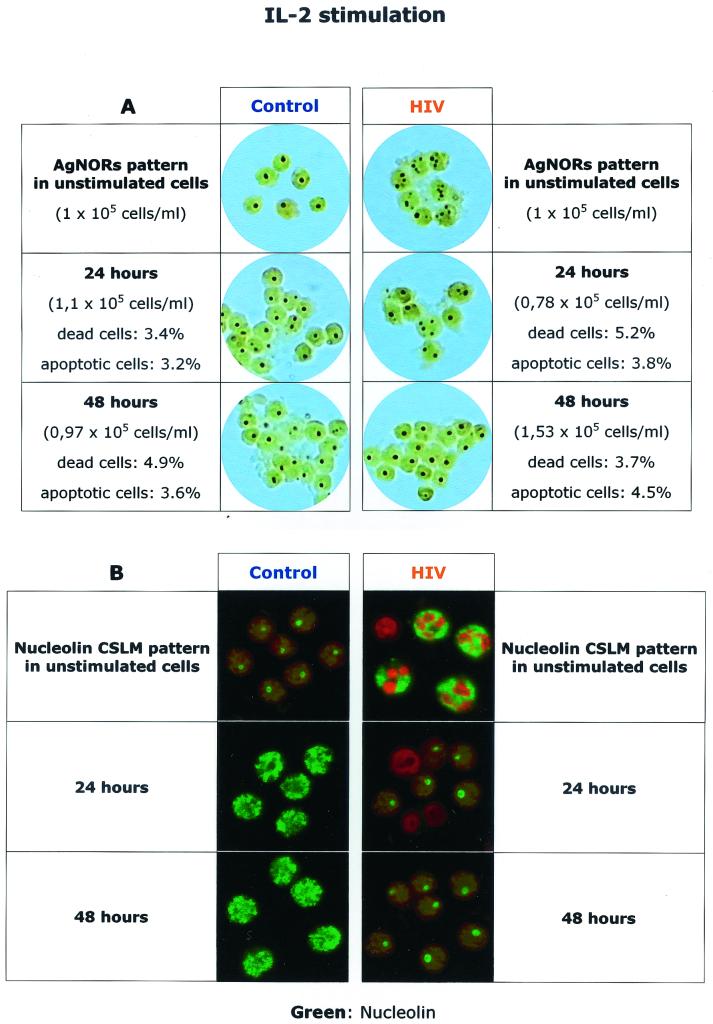
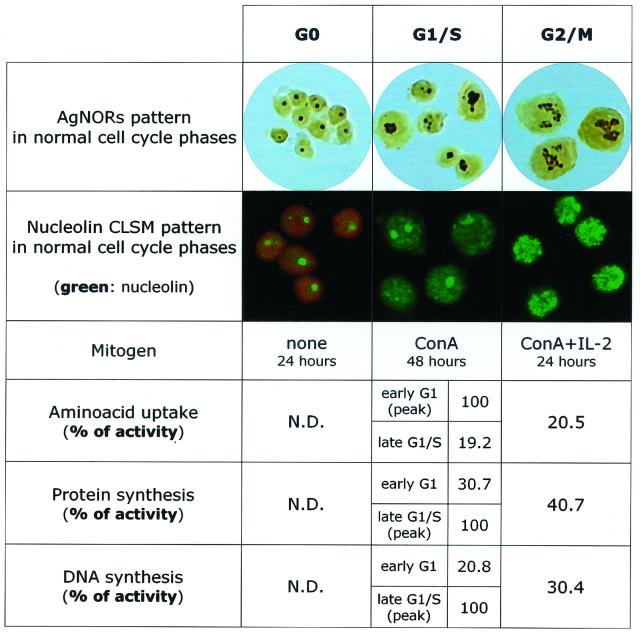
When PBLs from healthy individuals are activated in vitro with ConA and IL-2, they progress through the various phases of cell cycle. As shown in Fig. 2, unstimulated (G0) lymphocytes from healthy individuals show minimal metabolic activity, as indicated by the very low levels of transport of small metabolites, total protein synthesis, and DNA synthesis. When cells are in G0, AgNOR staining usually shows a single dot with an area between 0.5 and 1 μm2 and confocal microscopy shows a well-defined intranucleolar localization of nucleolin, a protein involved in the ribosomal biogenesis which represents a significant amount of the nucleolar structure (6, 10, 51–53). The transition to the G1/S phase was induced by a 48-h stimulation with ConA and is characterized by a rapid and sequential increase of transport of small metabolites, protein synthesis, and DNA synthesis. At this stage the AgNOR staining shows an enlarged nucleolar area, and the nucleolin stain also shows a larger area of distribution, which sometimes appears to be dividing into smaller areas. The transition to the G2/M phase, as induced by addition of IL-2 for 24 h, is characterized by AgNOR staining showing more complex forms with an increased number of dots per cell, while nucleolin staining shows increased diffusion of the protein, with the appearance of small peripheral aggregates. This phase is characterized by increased cell number in culture, and the rate of total cell death, as assessed by trypan blue staining, is about 7 to 10%, while the rate of apoptotic cell death, as assessed by cytofluorimetric measurement of DNA content, is about 4 to 8% (data not shown).
FIG. 2.
AgNOR and nucleolin staining in normal PBLs during phases of the cell cycle. G0 phase, unstimulated PBLs; G1/S, PBLs treated with ConA alone for 48 h (competence stimulus); G2/M, cells treated for 24 h with ConA followed by 24 h with IL-2 (competence and progression stimuli). Values of uptake of amino acids for system A (picomoles per minute per 106 cells), protein synthesis in initial velocity (nanomoles of [3H] leucine per 30 min per 106 cells), and DNA synthesis (counts per minute per 30 min per 106 cells) were normalized at 100 and expressed as percentages of peak activity. Values relative to those for unstimulated cells were described as not detectable (N.D.) since they were found to be consistently less than 0.5% of the peak activity.
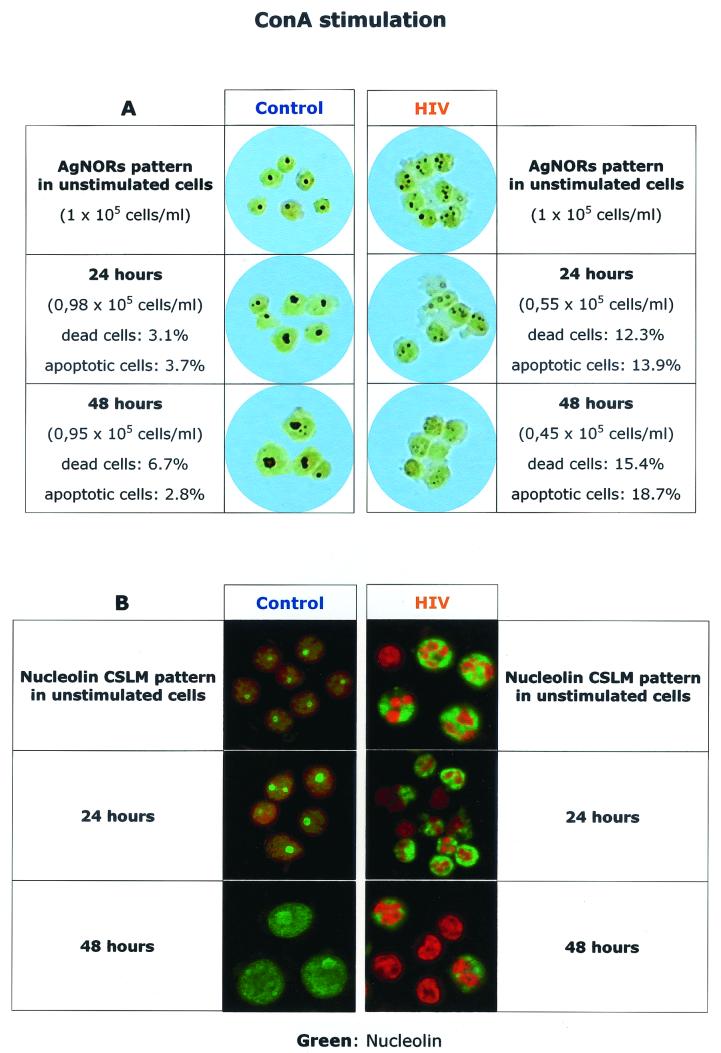
To assess the role of different mitogen stimuli, i.e., competence versus “progression,” we studied the pattern of expression of cycle-dependent proteins after lymphocyte in vitro activation with (i) a competence stimulus (ConA) alone and with (ii) a progression stimulus alone (IL-2). Figure 3 shows the effect of ConA stimulation of PBLs from HIV-infected patients and controls on the patterns of AgNOR and nucleolin staining and includes the number of cells in culture, as well as the rates of total and apoptotic cell death. Consistent with our previous observations (6), freshly isolated PBLs from HIV-infected individuals showed multiple AgNOR dots and an enlarged and irregular area of nucleolin distribution. The addition of ConA induced a frank dissolution of the AgNOR structure (the so-called empty nucleolus), which was associated with increased amounts of apoptotic cell death. Nucleolin staining showed an irregular area of distribution, which was eventually transformed into a more peripheral staining, which was also associated with increasing levels of apoptosis.
FIG. 3.
Effect of ConA stimulation on AgNOR (A) and nucleolin (B) staining of PBLs from HIV-infected patients and controls. Stainings were performed at 24 and 48 h after ConA stimulation, and total cell count, cell death (as indicated by trypan blue exclusion), and apoptosis (as indicated by a DNA content of <2n) were determined at the same times.
Interestingly, treatment of PBLs with IL-2 alone induced peculiar changes in the pattern of expression of AgNOR and nucleolin (Fig. 4). IL-2 treatment of PBLs from healthy uninfected individuals did not change the AgNOR morphology, consistent with the idea that “unprimed” lymphocytes are in general poorly responsive to IL-2. Accordingly, we have not found any change in total protein and DNA synthesis induced by IL-2 alone in PBLs from healthy controls (data not shown). Interestingly, IL-2 stimulation of the same cells induced a pattern of nucleolin staining which was similar to that observed during the G2/M transition. The coexistence of a nucleolin pattern typical of a G2/M phase transition with a metabolic profile typical of G0 suggests a synchronous arrest of cell cycle progression. This finding is not surprising if one considers that IL-2 treatment has a proapoptotic effect in unprimed lymphocytes (34). When added to PBLs from HIV-infected individuals (Fig. 4), IL-2 induced a profound normalizing effect on patterns of both AgNOR and nucleolin staining. Importantly, IL-2-mediated induction of AgNOR and nucleolin patterns similar to those observed in unstimulated lymphocytes from healthy controls was associated with only a mild increase in the rate of apoptotic cell death (predominantly observed in the first 24 h of culture) and a marked increase in the number of cells per well. These findings suggest that the most likely effect of IL-2 is to revert cells, on a per cell basis, to a G0 state upon completion of a round of cell cycling for which they were probably already primed in vivo.
Overall these results indicate that in vitro IL-2 treatment of PBLs from HIV-infected patients reverts the cell cycle perturbations and reestablishes a growth index and level of apoptosis comparable to those observed in normal PBLs.
PBLs from HIV-infected individuals show an intrinsic defect in endogenous IL-2 function.
The fact that IL-2 treatment corrects the perturbation of the intracellular turnover of cell cycle-dependent proteins in PBLs from HIV-infected patients is consistent with the possibility that an intrinsic defect in the endogenous IL-2 autocrine/paracrine function is present in these lymphocytes and contributes to the overall loss of proper cell cycle control.
To test this hypothesis, we studied ConA-induced in vitro IL-2 production by lymphocytes from HIV-infected patients and controls. As shown in Fig. 5A, the rates of IL-2 production, as measured by progressive accumulation of the cytokine in the medium, for HIV-infected patients and controls were similar and appeared mildly increased in PBLs from HIV-infected patients when measured as initial velocity of synthesis (Fig. 5B). However, when the biological activity of IL-2 was measured as [3H]thymidine incorporation on the IL-2-dependent CTLL cell line, PBLs from HIV-infected patients showed a two- to threefold decrease compared to lymphocytes from uninfected controls (Fig. 5C). Interestingly, the duration of the biological activity of IL-2 in the conditioned medium, measured as CTLL cell proliferation induced by cell-free conditioned media that were kept at 37°C for variable times, was also markedly decreased in lymphocytes from HIV-infected patients compared to that in lymphocytes from controls (Fig. 5D).
Overall these results indicate that, although the rates of IL-2 production are normal or slightly elevated, the biological activity of the IL-2 produced (measured in terms of both magnitude and duration) is markedly decreased in lymphocytes from HIV-infected patients. Normal rates of IL-2 production together with defective biological activity and a shorter half-life in the conditioned media suggest either (i) a molecular defect in the produced IL-2, which could in turn consist of secretion of a functionally abnormal IL-2 isoform and/or an abnormally glycosylated IL-2 or (ii) an increased “IL-2-neutralizing” activity of the media, possibly due to increased proteolytic activity (16, 31, 35). In experiments directed at testing the latter possibility we found that conditioned media from samples derived from HIV-infected patients tended to show increased serine protease activity and increased neutralization of exogenous IL-2 (data not shown). We are, however, cautious in the interpretation of these findings since extracellular proteolytic activity and IL-2-neutralizing ability are affected, respectively, by the rate of cell death and the density of cell culture, and both parameters were significantly different in lymphocyte cultures from HIV-infected patients and controls.
IL-2 reequilibration of cell cycle control is associated with normalization of the overall intracellular protein turnover.
The increased and unscheduled expression of cell cycle-dependent proteins observed in PBLs from HIV-infected patients suggests the presence of an abnormal intracellular protein turnover. This in turn can be related to either increased protein synthesis or impaired protein degradation. For cyclin B overexpression we have shown that increased half-life and lack of ubiquitination are commonly found, thus indicating that a defect in the ubiquitin-mediated pathway of protein degradation can be responsible for this abnormality (6).
To evaluate the effect of exogenous IL-2 administration on the overall intracellular protein turnover in lymphocytes from HIV-infected patients, we performed an integrated analysis of the effects of differential activation with either ConA or IL-2. When PBLs from both healthy controls and HIV-infected patients were cultured without mitogens, they showed low levels of protein synthesis, which were consistent with their resting state. When the same cells were activated with ConA, the rate of protein synthesis increased markedly (6, 47) but the amounts and species of newly synthesized proteins for HIV-infected patients and controls were similar (Fig. 6A). Remarkably, the half-life of the newly synthesized proteins in ConA-activated lymphocytes from HIV-infected patients was about one-third that observed in lymphocytes from healthy controls (Fig. 6B). Under the same experimental conditions, PBLs from HIV-infected individuals also displayed a marked decrease of the overall level of protein ubiquitination (Fig. 6C). Reduced level of total protein ubiquitination associated with the shorter half-life of newly synthesized proteins is suggestive of either a complex dysfunction of the protein degradation machinery with some degree of saturation of the ubiquitin/proteasome degradative pathway or, alternatively, a situation of decreased overall efficiency of the protein synthetic machinery with rapid ubiquitination and proteolysis of a large number of improperly assembled or folded proteins.
FIG. 6.
IL-2 restores the intracellular protein turnover in lymphocytes from HIV-infected patients. (A) Polyacrylamide gel electrophoresis PAGE and Coomassie staining (left) and PAGE and autoradiography of newly synthesized proteins (right), as indicated by [35S]methionine incorporation in ConA-activated PBLs from HIV-infected patients and controls. (B) Half-life of newly synthesized proteins after ConA or IL-2 activation of PBLs from HIV-infected patients and controls. Half-life was determined by pulse-chase experiment using [3H] leucine incorporation. (C) Autoradiography gel showing the level of protein ubiquitination in ConA-and IL-2-treated PBLs from HIV-infected patients and controls. The total pixel intensity relative to any individual lane is plotted on the right side of the panel (open bars, results from HIV-infected patients; solid bars, results from uninfected controls). (D) Intracellular concentration of the 19S regulator subunit 7 of the proteasome. Shown are a Western blot of freshly isolated PBLs from HIV-infected patients (open bars) and controls (solid bars) and a Western blot of the same samples after 24 h of IL-2 treatment.
Interestingly, when lymphocytes from HIV-infected individuals are treated with IL-2, the half-life of newly synthesized proteins becomes comparable to that observed in lymphocytes from healthy donors (Fig. 6B) and the level of total protein ubiquitination becomes comparable to that for ConA-treated lymphocytes from healthy donors (Fig. 6C). Consistent with this overall normalization of intracellular protein turnover, exogenous IL-2 administration also adjusted the expression of the 19S regulator subunit 7 of the proteasome in lymphocytes from HIV-infected patients to levels similar to those observed in uninfected controls (Fig. 6D).
Overall these results indicate that exogenous IL-2 administration reestablishes normal features of overall intracellular protein turnover following in vitro activation in PBLs from HIV-infected patients, thus providing a possible mechanistic explanation for the effect on intracellular kinetics of cell cycle-dependent proteins such as nucleolin and other AgNOR-related proteins (Fig. 4).
DISCUSSION
Although AIDS is commonly considered the result of HIV-induced progressive loss of CD4+ T cells, numerous functional abnormalities in the surviving lymphocytes from HIV-infected individuals have also been described (21, 41, 49, 50). These abnormalities include a defective proliferative response to antigens and mitogens, impaired cytokine production, exaggerated susceptibility to apoptosis, and abnormal regulation of cell cycle control (6, 20, 22, 23, 41, 47, 49, 50).
To find a biological link between the various lymphocyte defects observed during HIV infection, we focused our attention on the perturbation of cell cycle control. We have shown that lymphocytes isolated from HIV-infected patients consistently display abnormally high expression of cell cycle-dependent proteins and appear to have lost the proper control of cell cycle regulation. Interestingly, we were able to relate the perturbation of cell cycle control to the level of lymphocyte apoptosis that follows mitogen activation in vitro (6). We thus hypothesized that this perturbation is an important contributor to the “sick-lymphocyte” syndrome of AIDS patients, which is characterized by defective function and exaggerated propensity to apoptosis of bystander uninfected lymphocytes (20, 22, 23, 39, 41, 49, 50). We also hypothesized that this cell cycle dysregulation is related to the high level of in vivo lymphocyte activation that is associated with chronic HIV infection. Indeed, during HIV infection, normal regulation of T-cell activation and proliferation may be derailed for several reasons, including (i) high levels of background antigenic stimuli, (ii) disruption of normal lymphoid structures, (iii) an excess or imbalance of cytokines, and (iv) abnormal pressure on the regenerative capacity of T cells (8, 11, 24–28, 30, 38, 45, 55). All these mechanisms may contribute to the loss of proper cell cycle control in lymphocytes from HIV-infected patients.
When PBLs from HIV-infected patients are activated in vitro with mitogens, they show progressively abnormal patterns of both AgNOR distribution and nucleolin subcellular localization. The common prominent feature of these morphological patterns is the disruption of normal nucleolar structures, which is consistent with the finding that nucleolin, as detected by confocal microscopy, tends to localize mostly in the cytoplasm. At the same time, mitogen-activated PBLs from HIV-infected patients show a 2- to 3-fold decrease of the half-life of newly synthesized proteins and a 5- to 10-fold decrease in total protein ubiquitination. A possible explanation for this complex perturbation of intracellular protein turnover could be related to the presence of an abnormally active proteasome associated with functional saturation of the ubiquitination system. Consistent with this possibility is the abnormal expression of the 19S regulator subunit 7 of the proteasome in resting cells from HIV-infected patients. The functional overload of the proteasome may also explain the lack of degradation of phase-dependent proteins such as cyclin B (6). Indeed, an inappropriately active proteasome not only would decrease the half-life of newly synthesized proteins but also would induce a functional saturation of the ubiquitin system when its activity is required to precisely modulate the intracellular kinetics of phase-dependent proteins. This hypothesis would explain the coexistence of abnormally activated proteasomes in G0 cells and lack of proper cyclin B ubiquitination and degradation during cell cycle progression.
In the context of these complex abnormalities of both overall intracellular protein turnover and proper control of the sequential expression of phase-dependent proteins, administration of IL-2 seems to have a strikingly beneficial effect. In our experimental conditions exogenous administration of IL-2 is able to revert the overall cell cycle perturbation by restoring proper intracellular kinetics of cell cycle-dependent proteins such as nucleolin and other AgNOR-related proteins. Morphologically, this is demonstrated by the finding of patterns of both AgNOR and nucleolin staining that are similar to those observed in unstimulated lymphocytes from uninfected individuals. It is of note that in the same lymphocytes from controls the exogenous administration of IL-2 alone induces a change in the pattern of nucleolin staining characterized by an enlarged and “peripheral” nucleolin distribution which is reminiscent of the pattern found in G2/M cells. In this perspective it appears that IL-2 treatment exerts opposite effects on patients and controls. This effect consists of adding a “normalizing”, and previously lacking, progression stimulus to the lymphocytes from HIV-infected patients who were probably already primed by a competence stimulus in vivo. In contrast, administration of the progression stimulus, i.e., IL-2, to unprimed normal lymphocytes results in a dissociation between metabolic machinery (G0) and nucleolar structure (G2/M) of the cells that can ultimately lower the threshold of apoptosis. Importantly, IL-2 administration is also able to restore the normal intracellular protein turnover, as indicated by the half-life of newly synthesized proteins, overall level of protein ubiquitination, and expression of the 19S regulatory subunit of the proteasome. Ultimately, in vitro IL-2 administration induces a significant decline in the amount of apoptotic cell death which follows in vitro activation of lymphocytes from HIV-infected patients. Overall these observations support the hypothesis, originally proposed by Lenardo (34) and confirmed by Dooms and colleagues (14, 15), whereby signals that determine cell cycle progression of T cells also affect the cell death program. The fact that pro- or antiapoptotic activity of cytokines and their ability to drive T lymphocytes into the cell cycle are strictly related is consistent with the high level of integration between molecular mechanisms of cell cycle control and apoptosis (2, 17, 19). Interestingly, our observations indicating a prominent beneficial in vitro effect of exogenous IL-2 treatment are also consistent with several previous studies indicating the effects of IL-2 in reducing the exaggerated propensity for lymphocyte apoptosis observed during HIV disease (1, 9, 44, 48).
The beneficial effect of exogenous IL-2 administration is compatible with an abnormality of the endogenous IL-2 autocrine/paracrine function. Consistent with this hypothesis is the finding that lymphocytes from HIV-infected patients show a complex defect of activation-induced in vitro endogenous IL-2 function, consisting of production of normal amounts of IL-2 with reduced and shortened biological activity. In this context, the reduction of IL-2 activity suggests an intrinsic molecular defect of the produced IL-2, while the shortened half-life indicates an increased IL-2-neutralizing activity of the media. Experimental attempts to test these hypotheses have proved extremely challenging from the technical point of view, mainly because ConA-activated lymphocytes from HIV-infected patients show increased rates of cell death compared to those from uninfected controls. Increased rates of cell death may determine increased extracellular release of proteases, and if experiments are designed to adjust for the increased cell death by increasing the cell density of cultures from uninfected samples, exhaustion of the conditioned media would influence the determination of the biological activity of IL-2. Therefore, it is presently unclear to what extent the observed defect in IL-2 activity is related to the synthesis of a functionally abnormal protein as opposed to an accelerated degradation of an otherwise normal molecule. While the precise molecular nature of the defective endogenous IL-2 function is still unclear, it is tempting to speculate that this abnormality is related to the abnormal protein turnover and loss of proper cell cycle control. Indeed, given the specific up-regulation of IL-2 gene expression upon T-cell activation and commitment to the cell cycle, it is perhaps not surprising that the endogenous IL-2 autocrine and paracrine function can be impaired in in vitro-activated lymphocytes from HIV-infected patients.
Taken as a whole, our data suggest a revision in understanding the sick-lymphocyte syndrome of HIV-infected patients as a perturbation of the kinetics of cell cycle-dependent proteins associated with a relative defect of endogenous IL-2 function. According to this model, the well-described imbalance between metabolic profile and expression of phase-dependent proteins in lymphocytes from HIV-infected patients (6, 47) reflects the fact that these cells are exposed in vivo to an imbalanced combination of competence and progression stimuli. At this time we do not know whether this imbalance is caused by an excess of competence stimuli in vivo, perhaps as a consequence of the hyperimmune activation of AIDS, or, alternatively, is the result of a primary defect of IL-2 production by T lymphocytes. In any case, the in vitro administration of further competence stimuli such as ConA worsens the perturbation of the cell cycle machinery and ultimately results in significant levels of lymphocyte apoptosis. However, when exogenous IL-2 is administered, the balance among different activation stimuli is reestablished, with consequent normalization of cell cycle control and very low levels of activation-induced apoptosis. When considered in the in vivo situation, our model predicts that when a new antigenic challenge (i.e., competence signal) hits lymphocytes from HIV-infected patients, such as in the case of opportunistic infections, a further imbalance of cell cycle control follows, thus ultimately decreasing the threshold for apoptotic loss of lymphocytes. In this perspective, the HIV-associated sick-lymphocyte syndrome as defined above not only contributes to the explanation for the high levels of lymphocyte apoptosis observed in HIV-infected patients but also suggests a potential new rationale for the already-established use of IL-2 as a treatment for AIDS (12, 16, 32). Consistent with this model, therapeutic IL-2 administration would have a beneficial effect in normalizing in vivo the cell cycle abnormalities and the exaggerated propensity to apoptosis of HIV-infected patients. Interestingly, a recent report by Kovacs and colleagues indicates that IL-2 therapy also induces an increased expression of its receptor, CD25, on CD4+ T cells (32a), perhaps suggesting a role for exogenous IL-2 in increasing the T-cell responsiveness to suboptimal levels of endogenous cytokine. However, in the clinical setting it is difficult to assess if there is an additional in vivo antiapoptotic effect of IL-2 as opposed to highly active antiretroviral therapy (HAART) alone (2, 5, 13, 46), given the fact that HAART itself induces a striking reduction of the HIV-induced hyperimmune activation and apoptosis (4, 7, 29). It is therefore still difficult, at this time, to determine to what extent the intrinsic defect of the endogenous IL-2 autocrine and paracrine function is directly responsible in vivo for the observed cell cycle perturbation. This could be in fact related to the chronic high levels of immune activation, which may in turn cause the defect in IL-2 function through a yet-undefined mechanism. In this case the marked decrease of the chronic hyperimmune activation induced by HAART would be sufficient to normalize the cell cycle abnormalities, reduce the propensity to activation-induced cell death, and restore the intrinsic capacity for IL-2 production by ex vivo-isolated T cells.
The potential relation between cell cycle abnormalities and the chronic immune activation typical of HIV infection raises the question of whether other diseases with either acute (e.g., Epstein-Barr virus infection) or chronic (e.g., systemic lupus erythematosus) immune activation are consistently associated with similar cell cycle abnormalities. Although these studies are currently being proposed by our research group, at present we have data neither to support nor to exclude this possibility. Furthermore, to better test our pathogenic model of the cell cycle disease associated with HIV infection, an important point to investigate in further experiments is the presence of cell cycle perturbations in the fraction of T cells that is actively proliferating in vivo. Although technically challenging, this analysis may provide important insights into how the in vivo proliferative history of a given T lymphocyte relates to the abnormalities of cell cycle regulation observed after in vitro mitogen activation.
In conclusion, our results further underline the role of perturbation of cell cycle control in the pathogenesis of the lymphocyte dysfunction associated with HIV infection and define a beneficial role for IL-2 in reverting this perturbation, thus suggesting a new possible rationale for IL-2 therapy of AIDS patients.
ACKNOWLEDGMENTS
This work was supported by grants 30B.65 (to Giuseppe Piedimonte) and 30B52 (to Maria Montroni) from the Programma Nazionale di Ricerca sull'AIDS, Istituto Superiore di Sanitá, Rome, Italy.
We thank Mark B. Feinberg and Rebecca L. Elstrom for helpful discussions and Mary Wernett for technical assistance.
REFERENCES:
- 1.Adachi Y, Oyaizu N, Than S, McCloskey T W, Pahwa S. IL-2 rescues in vitro lymphocyte apoptosis in patients with HIV infection: correlation with its ability to block culture-induced down-modulation of bcl-2. J Immunol. 1996;157:4184–4193. [PubMed] [Google Scholar]
- 2.Amendola A, Poccia F, Martini F, Gioia C, Galati V, Pierdominici M, Marziali M, Pandolfi F, Colizzi V, Piacentini M, Girardi E, D'offizi G. Decreased CD95 expression on naive T cells from HIV-infected persons undergoing highly active anti-retroviral therapy (HAART) and the influence of IL-2 low dose administration. Irhan Group Study. Clin Exp Immunol. 2000;120:324–332. doi: 10.1046/j.1365-2249.2000.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994;79:927–930. doi: 10.1016/0092-8674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 4.Bohler T, Walcher J, Holzl-Wenig G, Geiss M, Buchholz B, Linde R, Debatin K M. Early effects of antiretroviral combination therapy on activation, apoptosis and regeneration of T cells in HIV-1-infected children and adolescents. AIDS. 1999;13:779–789. doi: 10.1097/00002030-199905070-00006. [DOI] [PubMed] [Google Scholar]
- 5.Caggiari L, Zanussi S, Bortolin M T, Dandrea M, Nasti G, Simonelli C, Tirelli U, DePaoli P. Effects of therapy with highly active anti-retroviral therapy (HAART) and IL-2 on CD4+ and CD8+ T lymphocyte apoptosis in HIV+ patients. Clin Exp Immunol. 2000;120:101–106. doi: 10.1046/j.1365-2249.2000.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannavó G, Palardini M, Galati D, Cervasi B, Montroni M, Guetard D, DeVico G, Lentile R, Picerno I, Magnani M, Silvestri G, Piedimonte G. Abnormal intracellular kinetics of cell cycle dependent proteins during HIV-infection: a novel biological link between immune activation, accelerated T cell turnover and high level of apoptosis. Blood. 2001;97:1756–1764. doi: 10.1182/blood.v97.6.1756. [DOI] [PubMed] [Google Scholar]
- 7.Chavan S J, Tamma S L, Kaplan M, Gersten M, Pahwa S G. Reduction in T cell apoptosis in patients with HIV disease following antiretroviral therapy. Clin Immunol. 1999;93:24–33. doi: 10.1006/clim.1999.4770. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Stuart J W, Hazenberg M D, Hamann D, Otto S A, Borleffs J C, Miedema F, Boucher C A, de Boer R J. The dominant source of CD4+ and CD8+ T cell activation in HIV infection is antigenic stimulation. J Acquir Immune Defic Syndr. 2000;25:203–211. doi: 10.1097/00126334-200011010-00001. [DOI] [PubMed] [Google Scholar]
- 9.Cordiali Fei P, Solmone M, Viora M, Vanacore P, Pugliese O, Giglio A, Caprilli F, Ameglio F. Apoptosis in HIV infection: protective role of IL-2. J Biol Regul Homeost Agents. 1994;8:60–64. [PubMed] [Google Scholar]
- 10.Crocker J. Nucleolar organizer regions. In: Underwood J C E, editor. Pathology of the nucleus. London, United Kingdom: Springer-Verlag; 1998. pp. 91–149. [Google Scholar]
- 11.Dalton D K, Haynes L, Chu C Q, Swain S L, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davey R T, Jr, Murphy R L, Graziano F M, Boswell S L, Pavia A T, Cancio M, Nadler J P, Chaitt D G, Dewar R L, Sahner D K, Duliege A M, Capra W B, Leong W P, Giedlin M A, Lane H C, Kahn J O. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: a randomized controlled trial. JAMA. 2000;284:183–189. doi: 10.1001/jama.284.2.183. [DOI] [PubMed] [Google Scholar]
- 13.De Paoli P, Zanussi S, Simonelli C, Bortolin M T, D'Andrea M, Crepaldi C, Talamini R, Comar M, Giacca M, Tirelli U. Effects of subcutaneous interleukin-2 therapy on CD4 subsets and in vitro cytokine production in HIV+ subjects. J Clin Immunol. 1997;100:2737–2743. doi: 10.1172/JCI119819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dooms H, Desmedt M, Vancaeneghem D, Rottiers P, Goossens V, Fiers W, Grooten J. Quiescence-inducing and antiapoptotic activities of IL-15 enhance secondary CD4+ T cell responsiveness to antigen. J Immunol. 1998;161:2141–2150. [PubMed] [Google Scholar]
- 15.Dooms H, Van Belle T, Desmedt M, Rottiers P, Grooten J. Interleukin-15 redirects the outcome of a tolerizing T-cell stimulus from apoptosis to anergy. Blood. 2000;96:1006–1012. [PubMed] [Google Scholar]
- 16.Eitan S, Schwartz M. A transglutaminase that converts interleukin-2 into a factor cytotoxic to oligodendrocytes. Science. 1993;261:106–108. doi: 10.1126/science.8100369. [DOI] [PubMed] [Google Scholar]
- 17.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1670. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 18.Emery S, Capra W B, Cooper D A, Mitsuyasu R T, Kovacs J A, Vig P, Smolskis M, Saravolatz L D, Lane H C, Fyfe G A, Curtin P T. Pooled analysis of 3 randomized, controlled trials of interleukin-2 therapy in adult human immunodeficiency virus type 1 disease. J Infect Dis. 2000;182:428–434. doi: 10.1086/315736. [DOI] [PubMed] [Google Scholar]
- 19.Enoch T, Nurse P. Coupling M phase and S phase: controls maintaining the dependence of mitosis on chromosome replication. Cell. 1991;65:921–923. doi: 10.1016/0092-8674(91)90542-7. [DOI] [PubMed] [Google Scholar]
- 20.Estaquier J, Idziorek T, de Bels F, Barre-Sinoussi F, Hurtrel B, Aubertin A M, Venet A, Mehtali M, Muchmore E, Michel P, Ameisen J C. Programmed cell death and AIDS: significance of T cell apoptosis in pathogenic and non pathogenic primate lentiviral infections. Proc Natl Acad Sci USA. 1994;91:9431–9435. doi: 10.1073/pnas.91.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fauci A S, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 23.Gougeon M L, Lecoeur H, Dulioust A, Enouf M G, Cruvoisier M, Goujard C, Debord T, Montagnier L. Programmed cell death in peripheral lymphocytes from HIV infected persons. Increased susceptibility to apoptosis of CD4 and CD8 cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 24.Haase A T. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 25.Hazenberg M D, Stuart J W, Otto S A, Borieffs J C, Boucher C A, de Boer R J, Miedema F, Hamann D. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–255. [PubMed] [Google Scholar]
- 26.Hazenberg M D, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–289. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 27.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan D, Sieg S. Role of the Fas/Fas ligand apoptotic pathway in human immunodeficiency virus type 1 disease. J Virol. 1998;72:6279–6282. doi: 10.1128/jvi.72.8.6279-6282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karrmochkine M, Parizot C, Calvez V, Coutellier A, Herson S, Debre P, Gorochov G. Susceptibility of peripheral blood mononuclear cells to apoptosis is correlated to plasma HIV load. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;15:419–423. doi: 10.1097/00042560-199804150-00006. [DOI] [PubMed] [Google Scholar]
- 30.Katsikis P D, Wunderlich E S, Smith C A, Herzenberg L A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kloth S, Flad H D, Brandt E. Detection of intracellular interleukin-2: evidence for novel immunologically related forms of the lymphokine. Cytokine. 1994;6:349–357. doi: 10.1016/1043-4666(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs J A, Vogel S, Albert J M, Falloon J, Davey R T, Jr, Walker R E, Polis M A, Spooner K, Metcalf J A, Baseler M, Fyfe G, Lane H C. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996;335:1350–1356. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 32a.Kovacs J A, Vogel S, Metcalf J A, Baseler M, Stevens R, Adelsberger J, Lempicki R, Hengel R L, Sereti I, Lambert L, Dewar R L, Davey R T, Jr, Walker R E, Falloon J, Polis M A, Masur H, Lane C H. Interleukin-2 induced immune effects in human immunodeficiency virus-infected patients receiving intermittent IL-2 immunotherapy. Eur J Immunol. 2001;31:1351–1360. doi: 10.1002/1521-4141(200105)31:5<1351::AID-IMMU1351>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Lanzavecchia A, Lezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 34.Lenardo M J. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 35.Lentsch A B, Nakagawa K, Yoshidome H, Gerassimides A, Miller F N, Edwards M J. Distinct biological activities of recombinant forms of human interleukin-2 in vivo. Cancer Immunol Immunother. 1997;43:331–336. doi: 10.1007/s002620050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis D E, Tang D S, Adu-Oppong A, Schober W, Rodgers J R. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J Immunol. 1994;153:412–420. [PubMed] [Google Scholar]
- 37.Liegler T J, Yonemoto W, Elbeik T, Vittinghoff E, Buchbinder S P, Greene W C. Diminished spontaneous apoptosis in lymphocytes from human immunodeficiency virus-infected long-term nonprogressors. J Infect Dis. 1998;178:669–679. doi: 10.1086/515378. [DOI] [PubMed] [Google Scholar]
- 38.McCune J M, Hanley M B, Cesar D, Halvorsen R, Hoh R, Schmidt D, Wieder E, Deeks S, Siler S, Neese R, Hellerstein M. Factors influencing T-cell turnover in HIV-1-seropositive patients. J Clin Investig. 2000;105:R1–R8. doi: 10.1172/JCI8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyaard L, Otto S A, Jonker R R, Mijnster M J, Keet R P, Miedema F. Programmed death of T cells in HIV infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- 40.Meyaard L, Otto S A, Keet I P, Roos M T, Miedema F. Programmed death of T cells in human immunodeficiency virus infection. No correlation with progression to disease. J Clin Investig. 1994;93:982–988. doi: 10.1172/JCI117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miedema F, Petit A J, Terpstra F G, Schattenkerk J K, deWolf F, Al B J, Roos M, Lange J M, Danner S A, Goudsmit J. Immunological abnormalities in HIV-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Investig. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muro-Cacho C A, Pantaleo G, Faucl A S. Analysis of apoptosis in lymph nodes of HIV infected persons. Intensity of apoptosis correlates with general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 43.Mustelin T, Pessa T, Lapinjoki S, Gynther J, Jarvinen T, Eloranta T, Andersonn L C. Two phases of omithine decarboxylase activation during lymphocyte mitogenesis. Adv Exp Med Biol. 1988;250:301–313. doi: 10.1007/978-1-4684-5637-0_27. [DOI] [PubMed] [Google Scholar]
- 44.Naora H, Gougeon M. Activation, survival and apoptosis of CD45RO+ and CD45RO− T cells of human immunodeficiency virus-infected individuals: effects of interleukin-15 and comparison with interleukin-2. Immunology. 1999;97:181–187. doi: 10.1046/j.1365-2567.1999.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Napolitano L, Grant R M, Deeks S G, Schmidt D, DeRosa S C, Herzenberg L A, Herndier B G, Andersson J, McCune J M. Increased production of IL-7 accompanies HIV-1 mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 46.Pandolfi F, Pierdominici M, Marziali M, Livia Bernardi M, Antonelli G, Galati V, D'Offizi G, Aiuti F. Low dose IL-2 reduces lymphocyte apoptosis and increases naive CD4 cells in HIV-infected patients treated with HAART. Clin Immunol. 2000;94:153–159. doi: 10.1006/clim.2000.4837. [DOI] [PubMed] [Google Scholar]
- 47.Piedimonte G, Corsi D, Paiardini M, Cannavó G, Ientile R, Picerno I, Montroni M, Silvestri G, Magnani M. Unscheduled cyclin B expression and p34 cdc2 activation in T lymphocytes from HIV-infected patients. AIDS. 1999;13:1159–1165. doi: 10.1097/00002030-199907090-00003. [DOI] [PubMed] [Google Scholar]
- 48.Regamey N, Harr T, Battegay M, Erb P. Downregulation of bcl-2, but not of Bax or Bcl-x, is associated with T lymphocyte apoptosis in HIV infection and restored by antiretroviral therapy or by interleukin-2. AIDS Res Hum Retroviruses. 1999;10:803–810. doi: 10.1089/088922299310700. [DOI] [PubMed] [Google Scholar]
- 49.Roos M T, Prins M, Koot M, deWolf F, Bakker M, Coutinho R A, Miedema F, Schellekens P T. Low T-cell responses to CD3 plus CD28 monoclonal antibodies are predictive of development of AIDS. AIDS. 1998;12:1745–1751. doi: 10.1097/00002030-199814000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Roos M T, Miedema F, Koot M, Tersmette M, Schaasberg W P, Coutinho R A, Schellekens P T. T cell function in vitro is an independent progression marker for AIDS in human immunodeficiency virus-infected asymptomatic subjects. J Infect Dis. 1995;171:531–536. doi: 10.1093/infdis/171.3.531. [DOI] [PubMed] [Google Scholar]
- 51.Sirri V, Roussel P, Gendron M C, Hernandez-Verdun D. Amount of the two major Ag-NOR proteins, nucleolin, and protein B23 is cell-cycle dependent. Cytometry. 1997;28:147–156. [PubMed] [Google Scholar]
- 52.Srivastava M, Pollard H B. Molecular dissection of nucleolin's role in growth and cell proliferation: new insights. FASEB J. 1999;13:1911–1922. [PubMed] [Google Scholar]
- 53.Tuteja R, Tuteja N. Nucleolin: a major nucleolar phosphoprotein. Crit Rev Biochem Mol Biol. 1998;33:407–436. doi: 10.1080/10409239891204260. [DOI] [PubMed] [Google Scholar]
- 54.Voges D, Zwickl P, Baumeister P. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–1168. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 55.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Kornbluth S. All aboard the cyclin train: subcellular trafficking of cyclins and their CDK partners. Trends Cell Biol. 1999;9:207–210. doi: 10.1016/s0962-8924(99)01577-9. [DOI] [PubMed] [Google Scholar]