Abstract
Large bowel obstructions (LBOs) often require urgent surgical intervention. Diagnosis relies on astute history and physical examination, as well as imaging with computed tomography (CT) scan for stable patients. Because of the high mortality associated with colonic perforation in patients with LBOs, decisive surgical decision-making is needed for optimal outcomes. This review seeks to provide an overview of the etiologies of LBO, diagnosis, and general management principles, as well as specific management for the most common etiologies, including colorectal cancer and strictures.
Keywords: large bowel obstruction, colorectal cancer, colonic stent
Workup and management of large bowel obstruction (LBO) is an important part of both general surgical and colorectal surgical practices. LBO can mandate urgent surgical intervention and carries a high risk of mortality at 9.8 to 20%. 1 2 LBOs represent 20 to 25% of all bowel obstructions and are more common in elderly individuals. 3 4 The etiology of LBO is varied and can include functional, benign, and malignant causes. The most common etiologies of LBO are adenocarcinoma of the colon or rectum, representing 50 to 60% of cases, diverticular strictures, and volvulus. 5 6 Management should be focused on the specific etiology, but this article will review broad surgical principles for management of both benign and malignant causes of LBO.
Etiology
The etiologies of LBO are varied but can be categorized into functional, benign, and malignant causes ( Table 1 ). Functional causes of LBO include acute colonic pseudo-obstruction (Ogilvie's syndrome) and constipation. Benign causes most commonly include volvulus, accounting for 10 to 17% of LBOs, and diverticular strictures, accounting for around 10% of LBOs. 7 Less common causes are Crohn's strictures, hernia, adhesions, intussusception, diverticular strictures, anastomotic strictures, endometriosis, retroperitoneal fibrosis, fecal impaction, and foreign bodies.
Table 1. Etiologies of large bowel obstruction.
| Functional | Benign | Malignant |
|---|---|---|
| ● Acute colonic pseudo-obstruction (Ogilvie's) ● Constipation/impaction |
● Volvulus ● Hernia ● Adhesions ● Intussusception ● Stricture (anastomotic, Crohn's, diverticular) ● Endometriosis ● Retroperitoneal fibrosis ● Foreign body |
● Colorectal cancer ● Metastatic cancer ● Pelvic or peritoneal tumors |
Malignancy is the most frequent etiology of LBO, accounting for over 50% of cases. 4 7 About 10 to 30% of colorectal cancers initially present with LBO. 2 4 8 Obstruction is more common in the descending colon due to its smaller lumen. 2 9 Metastatic disease or external compression from other intra-abdominal tumors (pancreatic cancer, ovarian cancer, and lymphoma) are also possible. 5
Diagnosis
Clinical Presentation, Physical Examination, and Laboratory Values
Patients with LBO often present with abdominal pain, distension, and obstipation. Compared with small bowel obstructions, patients with LBO are less likely to have nausea and vomiting unless they have delayed presentation or have an absent or incompetent ileocecal valve (e.g., prior ileocecal resection). The timing of symptom onset is helpful for distinguishing causes of LBO, as patients with rapid symptom onset are more likely to have volvulus, while gradual symptom onset may indicate a malignant or stricturing process. Prior episodes of left lower quadrant pain may indicate diverticular disease, while constitutional symptoms such as fatigue and weight loss may indicate malignancy. 10
Digital rectal examination can help identify distal masses or fecal impaction. In addition, physical examination findings and vital sign changes, such as peritonitis, fevers, tachycardia, and hypotension, may indicate perforation and mandate immediate surgical intervention. Laboratory values assessing leukocytosis and lactic acidosis can indicate the presence of ischemia. Preoperative albumin can give insight into patients' nutritional status. 11 12 Tumor markers, especially carcinoembryonic antigen (CEA), may also be obtained. Ultimately, imaging is required for definitive diagnosis.
Imaging
Plain abdominal radiographs are inexpensive, able to be obtained at the bedside, and can quickly identify pneumoperitoneum. Abdominal X-ray series have an 84% sensitivity and 72% specificity for identifying LBOs. 13 Abdominal X-ray is most helpful for volvulus, but less discerning for other causes of LBO. Contrast enemas can also be used in the diagnosis of LBO and can identify the level of obstruction with 96% sensitivity and 98% specificity. 13 However, obtaining a contrast enema in a timely fashion can be difficult in many hospitals and uncomfortable for patients. 8
Unless the patient is hemodynamically unstable or has findings of diffuse peritonitis, further evaluation with computed tomography (CT) scan is indicated. An example of CT showing LBO is shown in Fig. 1 . CT can provide information regarding the etiology and location of LBO. Classic findings on CT for LBO include air/fluid level within dilated colon and failure of oral contrast to pass distally with a change in the caliber of the lumen at the point of the obstruction. 6 CT findings such as wall thickening, mural hypoenhancement, pneumatosis, mesenteric stranding, and peritoneal fluid can indicate ischemia and impending perforation. 14 15 Additional findings such as wall thickening and hyperenhancement indicating inflammatory bowel disease or diverticulitis, or intraluminal masses indicating malignancy, can be helpful in identifying the etiology of the LBO. 6 The colon is considered dilated with diameter greater than 9 cm in the cecum and 6 cm in the left colon. At sizes greater than 12 cm of the cecum, there is an increased risk of perforation. 16 17 For these patients, early surgical intervention should be considered.
Fig. 1.
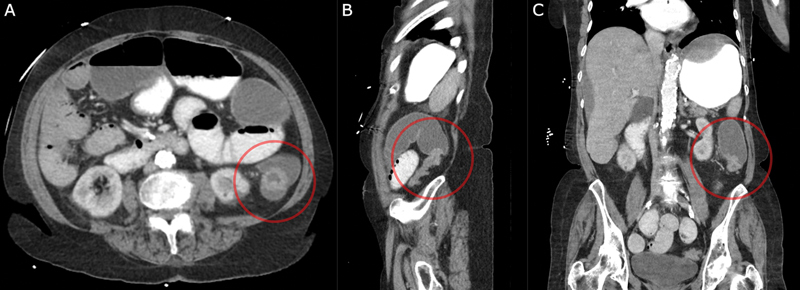
Computed tomography (CT) scan showing large bowel obstruction with dilated transverse colon and transition point in descending colon. ( A ) Axial views with a circle around the obstructing mass. ( B ) Sagittal views with a circle around the transition point. ( C ) Coronal views with a circle around the transition point.
Management
General Principles
The management of patients with LBO should be guided by the specific etiology, identified using diagnostic tools. However, some key principles of management are similar among different cases. First, patients with LBO often have intravascular volume depletion and electrolyte imbalances and require intravenous fluid resuscitation and repletion of electrolytes. For patients with signs of sepsis, ischemia, or perforation, antibiotics should be initiated. Patients with significant nausea and vomiting or evidence of gastric or small bowel distension on imaging should undergo gastric decompression. In contrast, patients without small bowel dilation, with a competent ileocecal valve, functionally have a closed loop obstruction, which can progress rapidly to cecal perforation.
For stable patients with malignant obstructions and no signs of ischemia or perforation, self-expanding metal stent (SEMS) placement may be considered. SEMS placement allows for decompression of the dilated colon. Obstruction secondary to colorectal cancer is the main indication for SEMS. In select cases, benign obstructions may be stented, although the complication rate is higher. 18 19 SEMS may be used in the palliative setting or as a bridge to surgery. In the latter, following stent placement, definitive resection may be performed in the elective setting, with a minimally invasive approach, more often with a primary anastomosis, and less often with stoma placement. 20 21 22 Because of these benefits, SEMS is the preferred approach in appropriate patients compared with immediate surgery. 23 SEMS placement has a success rate of approximately 70 to 80%, but is associated with several complications, including perforation (∼5%), stent migration, hemorrhage, and re-obstruction. 24 SEMS should not be considered a long-term solution because of the risk of stent erosion and re-obstruction over time. 24 They should also not be placed in areas of acute angulation (such as the flexures) due to higher risk of stent erosion or migration or in the rectum because it may result in pain and tenesmus. 25 SEMS followed by resection has shown equivalent long-term outcomes (3-year overall and disease-free survival) compared with immediate surgery. 24 26 27
For LBO of any etiology, ischemia, peritonitis, and perforation are associated with significantly higher mortality (up to 50%). 28 For this reason, decisive surgical intervention is needed to prevent these complications. Prior to proceeding to surgery, attention should be paid to resuscitation and antibiotic administration as described earlier. In addition, preoperative stoma marking, noting creases on the abdomen when the patient is sitting up, can decrease stoma complications and improve self-care postoperatively. 29 30 In the setting of LBO, preoperative mechanical bowel preparation is not possible and may precipitate perforation. Instead, on-table colonic lavage, performed by instilling 4 L of saline through the appendiceal orifice and collecting the effluent in anesthesia gas tubing secured to the cut end of the bowel, can reduce the colonic stool burden. 25 31 32 33
Surgical management of LBO involves the following key considerations: (1) resection versus proximal diversion only; (2) for patients who undergo resection, primary anastomosis versus ostomy creation; (3) for patients who undergo primary anastomosis, proximal diversion with a loop ostomy versus no diversion. Proximal diversion only is useful in the cases where immediate resection may carry a high risk of morbidity or mortality for malignant or benign etiologies not amenable to SEMS placement. 34 Similar to SEMS placement, proximal diversion followed by elective definitive resection is associated with higher likelihood of primary anastomosis and lower risk of permanent ostomy. 34 35 36 Of note, in the cases in which only proximal diversion is used, if the ileocecal valve is competent, a loop ileostomy will not relieve the obstruction and a loop colostomy or end colostomy with mucus fistula should be used instead. 10
If resection of the obstructing segment of the colon is pursued, the decision to perform primary anastomosis or to divert proximally should be carefully considered. 8 25 37 The decision to perform an anastomosis depends on the location of the obstruction, preoperative steroids or immunosuppression, the patient's nutrition and hemodynamic status, and the presence of perforation or fecal contamination. Right-sided anastomoses may have a lower risk of anastomotic leak (2.8–5.2%) and primary anastomosis is considered safer in the emergent setting. 31 38 39 In contrast, left-sided resection, especially low rectal anastomoses, has a higher risk of anastomotic leak (up to 36%) and proximal diversion should be considered in most cases. 40 Preoperative steroid use, immunosuppression, and preoperative weight loss are also known risk factors for anastomotic leak. 40 41 42 43 Intraoperative adjuncts, which have demonstrated utility in assessing risk of leak, include air leak test, evaluation with flexible endoscopy, and angiography with indocyanine green. 44 45 46 In higher risk cases, anastomosis with proximal diversion should be considered. Finally, in the cases with gross fecal contamination, septic shock, and large volume (>300 mL) of blood loss, or in the cases with severe dilation of the proximal colon with size mismatch, resection with diversion and possible mucus fistula (Hartmann's procedure) should likely be performed. 12 47 Depending on surgeon experience and the absence of small bowel dilation, a minimally invasive approach may be feasible and is associated with shorter length of stay. 48 In the cases in which the patient has profound hemodynamic instability and viability of the colon is marginal, a damage control approach is reasonable. 49
Colorectal Cancer
Resection of obstructing colorectal cancers should follow proper oncologic guidelines, including adequate margins and harvest of pericolic and mesenteric lymph nodes. 10 If not completed preoperatively, staging, CEA value, and colonoscopy should be completed postoperatively. 23 In stable patients with partial obstructions, definitive diagnosis, and evaluation for synchronous lesions with colonoscopy before surgery is ideal. Use of SEMS as a “bridge to surgery,” with improved postoperative outcomes has been discussed earlier. For patients with rectal cancer, proximal diversion for low cancers should be considered so that neoadjuvant therapy may be completed prior to definitive resection. 10 50 51 Patients with obstructing cancers have worse long-term outcomes (16 vs. 37% for 5-year survival), although this appears to be related to age and stage, rather than obstruction or emergency surgery in itself. 52 53 54 55 For elderly patients and those who have poor functional status preoperatively and/or have evidence of metastatic or peritoneal disease, goals of care discussions and consultation with palliative care services should be considered, as palliative surgery is associated with high rates of complications, readmission, and re-obstruction and limited life expectancy. 56 These patients may be better suited for palliative stent placement, unless they are receiving the antiangiogenesis agent bevacizumab, which increases risk of perforation. 51 57 58
Stricture
Strictures resulting in LBO may be caused by diverticulitis, ischemic colitis, Crohn's disease, or previous anastomoses. Diverticular strictures cause approximately 10 to 20% of LBO. 16 37 Surgical management of diverticular strictures includes considering the underlying etiology, with resection distally to the top of the rectum and proximally of gross diverticular disease to prevent recurrence of diverticulitis. 59 Stenting of diverticular strictures is associated with worse success rates and more frequent complications including perforations and fistulas. 19 60 Balloon dilation may be attempted for strictures secondary to Crohn's disease, especially if they are in the ileocolic location. The presence of active inflammation, need for ongoing medical therapy, smoking, and elevated C-reactive protein (CRP) are associated with failure of endoscopic balloon dilation and need for surgery. 61 62 Dilation of strictures with adjacent perforation or fistula is associated with increased risk of complications. 61 63
Conclusion
Patients with LBO need urgent intervention to prevent perforations and ensure optimal outcomes. CT scan is the most rapid and definitive diagnostic tool. Perforation and peritonitis are associated with worse outcomes, so decisive surgical intervention is needed for the highest risk patients. As alternatives to immediate surgery, SEMS for patients with colorectal cancer and balloon dilation for patients with Crohn's strictures may be considered. Careful consideration of patient and disease characteristics is needed for optimal surgical management.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Moghadamyeghaneh Z, Talus H, Ballantyne G, Stamos M J, Pigazzi A. Short-term outcomes of laparoscopic approach to colonic obstruction for colon cancer. Surg Endosc. 2021;35(06):2986–2996. doi: 10.1007/s00464-020-07743-w. [DOI] [PubMed] [Google Scholar]
- 2.Aslar A K, Özdemir S, Mahmoudi H, Kuzu M A. Analysis of 230 cases of emergent surgery for obstructing colon cancer: lessons learned. J Gastrointest Surg. 2011;15(01):110–119. doi: 10.1007/s11605-010-1360-2. [DOI] [PubMed] [Google Scholar]
- 3.Markogiannakis H, Messaris E, Dardamanis D et al. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13(03):432–437. doi: 10.3748/wjg.v13.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne J J. Large bowel obstruction. Am J Surg. 1960;99(02):168–178. doi: 10.1016/0002-9610(60)90111-2. [DOI] [PubMed] [Google Scholar]
- 5.Farkas N G, Welman T JP, Ross T, Brown S, Smith J J, Pawa N. Unusual causes of large bowel obstruction. Curr Probl Surg. 2019;56(02):49–90. doi: 10.1067/j.cpsurg.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe T, Thompson W M. Large-bowel obstruction in the adult: classic radiographic and CT findings, etiology, and mimics. Radiology. 2015;275(03):651–663. doi: 10.1148/radiol.2015140916. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Kostner F, Hool G R, Lavery I C. Management and causes of acute large-bowel obstruction. Surg Clin North Am. 1997;77(06):1265–1290. doi: 10.1016/s0039-6109(05)70617-4. [DOI] [PubMed] [Google Scholar]
- 8.Yeo H L, Lee S W. Colorectal emergencies: review and controversies in the management of large bowel obstruction. J Gastrointest Surg. 2013;17(11):2007–2012. doi: 10.1007/s11605-013-2343-x. [DOI] [PubMed] [Google Scholar]
- 9.Buechter K J, Boustany C, Caillouette R, Cohn I., JrSurgical management of the acutely obstructed colon. A review of 127 cases Am J Surg 1988156(3, Pt 1):163–168. [DOI] [PubMed] [Google Scholar]
- 10.Muldoon R L. Malignant Large Bowel Obstruction. Clin Colon Rectal Surg. 2021;34(04):251–261. doi: 10.1055/s-0041-1729922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telem D A, Chin E H, Nguyen S Q, Divino C M.Risk factors for anastomotic leak following colorectal surgery: a case-control study Arch Surg 201014504371–376., discussion 376 [DOI] [PubMed] [Google Scholar]
- 12.Parthasarathy M, Greensmith M, Bowers D, Groot-Wassink T. Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17 518 patients. Colorectal Dis. 2017;19(03):288–298. doi: 10.1111/codi.13476. [DOI] [PubMed] [Google Scholar]
- 13.Chapman A H, McNamara M, Porter G. The acute contrast enema in suspected large bowel obstruction: value and technique. Clin Radiol. 1992;46(04):273–278. doi: 10.1016/s0009-9260(05)80170-9. [DOI] [PubMed] [Google Scholar]
- 14.Frager D. Intestinal obstruction role of CT. Gastroenterol Clin North Am. 2002;31(03):777–799. doi: 10.1016/s0889-8553(02)00026-2. [DOI] [PubMed] [Google Scholar]
- 15.Frager D, Rovno H DS, Baer J W, Bashist B, Friedman M. Prospective evaluation of colonic obstruction with computed tomography. Abdom Imaging. 1998;23(02):141–146. doi: 10.1007/s002619900307. [DOI] [PubMed] [Google Scholar]
- 16.Ramanathan S, Ojili V, Vassa R, Nagar A. Large bowel obstruction in the emergency department: imaging spectrum of common and uncommon causes. J Clin Imaging Sci. 2017;7(01):15. doi: 10.4103/jcis.JCIS_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabbagh C, Siembida N, Yzet T et al. What are the predictive factors of caecal perforation in patients with obstructing distal colon cancer? Colorectal Dis. 2018;20(08):688–695. doi: 10.1111/codi.14056. [DOI] [PubMed] [Google Scholar]
- 18.Kwaan M R, Ren Y, Wu Y, Xirasagar S. Colonic stent use by indication and patient outcomes: a nationwide inpatient sample study. J Surg Res. 2021;265:168–179. doi: 10.1016/j.jss.2021.03.048. [DOI] [PubMed] [Google Scholar]
- 19.Meisner S, Hensler M, Knop F K, West F, Wille-Jørgensen P. Self-expanding metal stents for colonic obstruction: experiences from 104 procedures in a single center. Dis Colon Rectum. 2004;47(04):444–450. doi: 10.1007/s10350-003-0081-y. [DOI] [PubMed] [Google Scholar]
- 20.Tilney H S, Lovegrove R E, Purkayastha S et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc. 2007;21(02):225–233. doi: 10.1007/s00464-005-0644-1. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Lv B, Zhang S, Meng L. Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: a meta-analysis. J Gastrointest Surg. 2014;18(03):584–591. doi: 10.1007/s11605-013-2344-9. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Shi J, Shi B, Song C Y, Xie W F, Chen Y X. Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc. 2012;26(01):110–119. doi: 10.1007/s00464-011-1835-6. [DOI] [PubMed] [Google Scholar]
- 23.Vogel J D, Felder S I, Bhama A R et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of colon cancer. Dis Colon Rectum. 2022;65(02):148–177. doi: 10.1097/DCR.0000000000002323. [DOI] [PubMed] [Google Scholar]
- 24.Tan C J, Dasari B VM, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg. 2012;99(04):469–476. doi: 10.1002/bjs.8689. [DOI] [PubMed] [Google Scholar]
- 25.Schwartzberg D M, Valente M A. Surgical dilemmas associated with malignant large bowel obstructions. Clin Colon Rectal Surg. 2022;35(03):197–203. doi: 10.1055/s-0042-1742589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda A, Yamada T, Yokoyama Y, Takahashi G, Yoshida H. Long-term outcomes between self-expandable metallic stent and transanal decompression tube for malignant large bowel obstruction: a multicenter retrospective study and meta-analysis. Ann Gastroenterol Surg. 2023;7(04):583–593. doi: 10.1002/ags3.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skelton W P, IV, Franke A J, Iqbal A, George T J. Comprehensive literature review of randomized clinical trials examining novel treatment advances in patients with colon cancer. J Gastrointest Oncol. 2020;11(04):790–802. doi: 10.21037/jgo-20-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carraro P G, Segala M, Orlotti C, Tiberio G. Outcome of large-bowel perforation in patients with colorectal cancer. Dis Colon Rectum. 1998;41(11):1421–1426. doi: 10.1007/BF02237060. [DOI] [PubMed] [Google Scholar]
- 29.Nozawa H, Sasaki S, Hayashi Cet al. Preoperative stoma site marking reduces postoperative stoma-related complications in emergency surgery: a single center retrospective cohort study Scand J Surg 202314574969231186282 [DOI] [PubMed] [Google Scholar]
- 30.Yeo H, Park H. Benefits of a single-session, in-hospital preoperative education program for patients undergoing ostomy surgery: a randomized controlled trial. J Wound Ostomy Continence Nurs. 2023;50(04):313–318. doi: 10.1097/WON.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 31.Awotar G K, Guan G, Sun W et al. Reviewing the management of obstructive left colon cancer: assessing the feasibility of the one-stage resection and anastomosis after intraoperative colonic irrigation. Clin Colorectal Cancer. 2017;16(02):e89–e103. doi: 10.1016/j.clcc.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Hsu T C. Comparison of one-stage resection and anastomosis of acute complete obstruction of left and right colon. Am J Surg. 2005;189(04):384–387. doi: 10.1016/j.amjsurg.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 33.Allen-Mersh T G. Should primary anastomosis and on-table colonic lavage be standard treatment for left colon emergencies? Ann R Coll Surg Engl. 1993;75(03):195–198. [PMC free article] [PubMed] [Google Scholar]
- 34.Amelung F J, Mulder C LJ, Verheijen P M, Draaisma W A, Siersema P D, Consten E CJ. Acute resection versus bridge to surgery with diverting colostomy for patients with acute malignant left sided colonic obstruction: systematic review and meta-analysis. Surg Oncol. 2015;24(04):313–321. doi: 10.1016/j.suronc.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Amelung F J, Ter Borg F, Consten E CJ, Siersema P D, Draaisma W A. Deviating colostomy construction versus stent placement as bridge to surgery for malignant left-sided colonic obstruction. Surg Endosc. 2016;30(12):5345–5355. doi: 10.1007/s00464-016-4887-9. [DOI] [PubMed] [Google Scholar]
- 36.Amelung F J, Mulder C LJ, Broeders I AMJ, Consten E CJ, Draaisma W A. Efficacy of loop colostomy construction for acute left-sided colonic obstructions: a cohort analysis. Int J Colorectal Dis. 2017;32(03):383–390. doi: 10.1007/s00384-016-2695-2. [DOI] [PubMed] [Google Scholar]
- 37.Johnson W R, Hawkins A T. Large bowel obstruction. Clin Colon Rectal Surg. 2021;34(04):233–241. doi: 10.1055/s-0041-1729927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frago R, Biondo S, Millan M et al. Differences between proximal and distal obstructing colonic cancer after curative surgery. Colorectal Dis. 2011;13(06):e116–e122. doi: 10.1111/j.1463-1318.2010.02549.x. [DOI] [PubMed] [Google Scholar]
- 39.Lee Y M, Law W L, Chu K W, Poon R TP. Emergency surgery for obstructing colorectal cancers: a comparison between right-sided and left-sided lesions. J Am Coll Surg. 2001;192(06):719–725. doi: 10.1016/s1072-7515(01)00833-x. [DOI] [PubMed] [Google Scholar]
- 40.Caulfield H, Hyman N H. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg. 2013;148(02):177–182. doi: 10.1001/jamasurgery.2013.413. [DOI] [PubMed] [Google Scholar]
- 41.Midura E F, Hanseman D, Davis B R et al. Risk factors and consequences of anastomotic leak after colectomy: a national analysis. Dis Colon Rectum. 2015;58(03):333–338. doi: 10.1097/DCR.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 42.Nikolian V C, Kamdar N S, Regenbogen S E et al. Anastomotic leak after colorectal resection: a population-based study of risk factors and hospital variation. Surgery. 2017;161(06):1619–1627. doi: 10.1016/j.surg.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boccola M A, Buettner P G, Rozen W M et al. Risk factors and outcomes for anastomotic leakage in colorectal surgery: a single-institution analysis of 1576 patients. World J Surg. 2011;35(01):186–195. doi: 10.1007/s00268-010-0831-7. [DOI] [PubMed] [Google Scholar]
- 44.Kamal T, Pai A, Velchuru V R et al. Should anastomotic assessment with flexible sigmoidoscopy be routine following laparoscopic restorative left colorectal resection? Colorectal Dis. 2015;17(02):160–164. doi: 10.1111/codi.12809. [DOI] [PubMed] [Google Scholar]
- 45.Deidda S, Elmore U, Rosati R et al. Association of delayed surgery with oncologic long-term outcomes in patients with locally advanced rectal cancer not responding to preoperative chemoradiation. JAMA Surg. 2021;156(12):1141–1149. doi: 10.1001/jamasurg.2021.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allaix M E, Lena A, Degiuli M et al. Intraoperative air leak test reduces the rate of postoperative anastomotic leak: analysis of 777 laparoscopic left-sided colon resections. Surg Endosc. 2019;33(05):1592–1599. doi: 10.1007/s00464-018-6421-8. [DOI] [PubMed] [Google Scholar]
- 47.Leichtle S W, Mouawad N J, Welch K B, Lampman R M, Cleary R K. Risk factors for anastomotic leakage after colectomy. Dis Colon Rectum. 2012;55(05):569–575. doi: 10.1097/DCR.0b013e3182423c0d. [DOI] [PubMed] [Google Scholar]
- 48.Gash K, Chambers W, Ghosh A, Dixon A R. The role of laparoscopic surgery for the management of acute large bowel obstruction. Colorectal Dis. 2011;13(03):263–266. doi: 10.1111/j.1463-1318.2009.02123.x. [DOI] [PubMed] [Google Scholar]
- 49.Pisano M, Zorcolo L, Merli C et al. 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg. 2018;13:36. doi: 10.1186/s13017-018-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrelli F, Trevisan F, Cabiddu M et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(03):440–448. doi: 10.1097/SLA.0000000000003471. [DOI] [PubMed] [Google Scholar]
- 51.Malakorn S, Stein S L, Lee J H, You Y N. Urgent management of obstructing colorectal cancer: divert, stent, or resect? J Gastrointest Surg. 2019;23(02):425–432. doi: 10.1007/s11605-018-3990-8. [DOI] [PubMed] [Google Scholar]
- 52.Öhman U. Prognosis in patients with obstructing colorectal carcinoma. Am J Surg. 1982;143(06):742–747. doi: 10.1016/0002-9610(82)90050-2. [DOI] [PubMed] [Google Scholar]
- 53.Lavanchy J L, Vaisnora L, Haltmeier T et al. Oncologic long-term outcomes of emergency versus elective resection for colorectal cancer. Int J Colorectal Dis. 2019;34(12):2091–2099. doi: 10.1007/s00384-019-03426-8. [DOI] [PubMed] [Google Scholar]
- 54.AFC (French Surgical Association) Working Group . Manceau G, Voron T, Mege D et al. Prognostic factors and patterns of recurrence after emergency management for obstructing colon cancer: multivariate analysis from a series of 2120 patients. Langenbecks Arch Surg. 2019;404(06):717–729. doi: 10.1007/s00423-019-01819-5. [DOI] [PubMed] [Google Scholar]
- 55.Carraro P G, Segala M, Cesana B M, Tiberio G. Obstructing colonic cancer: failure and survival patterns over a ten-year follow-up after one-stage curative surgery. Dis Colon Rectum. 2001;44(02):243–250. doi: 10.1007/BF02234300. [DOI] [PubMed] [Google Scholar]
- 56.de Boer N L, Hagemans J AW, Schultze B TA et al. Acute malignant obstruction in patients with peritoneal carcinomatosis: the role of palliative surgery. Eur J Surg Oncol. 2019;45(03):389–393. doi: 10.1016/j.ejso.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 57.van Hooft J E, Veld J V, Arnold D et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) guideline—update 2020. Endoscopy. 2020;52(05):389–407. doi: 10.1055/a-1140-3017. [DOI] [PubMed] [Google Scholar]
- 58.Imbulgoda A, MacLean A, Heine J, Drolet S, Vickers M M. Colonic perforation with intraluminal stents and bevacizumab in advanced colorectal cancer: retrospective case series and literature review. Can J Surg. 2015;58(03):167–171. doi: 10.1503/cjs.013014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thaler K, Baig M K, Berho M et al. Determinants of recurrence after sigmoid resection for uncomplicated diverticulitis. Dis Colon Rectum. 2003;46(03):385–388. doi: 10.1007/s10350-004-6560-y. [DOI] [PubMed] [Google Scholar]
- 60.Paúl L, Pinto I, Gómez H, Fernández-Lobato R, Moyano E. Metallic stents in the treatment of benign diseases of the colon: preliminary experience in 10 cases. Radiology. 2002;223(03):715–722. doi: 10.1148/radiol.2233010866. [DOI] [PubMed] [Google Scholar]
- 61.Rieder F, Zimmermann E M, Remzi F H, Sandborn W J. Crohn's disease complicated by strictures: a systematic review. Gut. 2013;62(07):1072–1084. doi: 10.1136/gutjnl-2012-304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crohn's Stricture Study (CroSS) Group . Morar P S, Faiz O, Warusavitarne J et al. Systematic review with meta-analysis: endoscopic balloon dilatation for Crohn's disease strictures. Aliment Pharmacol Ther. 2015;42(10):1137–1148. doi: 10.1111/apt.13388. [DOI] [PubMed] [Google Scholar]
- 63.Lian L, Stocchi L, Remzi F H, Shen B. Comparison of endoscopic dilation vs surgery for anastomotic stricture in patients with Crohn's disease following ileocolonic resection. Clin Gastroenterol Hepatol. 2017;15(08):1226–1231. doi: 10.1016/j.cgh.2016.10.030. [DOI] [PubMed] [Google Scholar]


