Abstract
Diabetes leads to testicular damage and infertility. Mesenchymal stem cells and their secretory trophic factors have shown potential as regenerative therapies for diabetes and its associated complications. This study examined the effects of conditioned medium derived from Wharton's jelly mesenchymal stem cells (WJMSCs-CM) on sperm parameters, reproductive hormones, biochemical parameters, and histological changes in the testes of diabetic rats. Fifty-six male Sprague–Dawley rats (250–300 g) were assigned to eight groups: control, diabetes, and six diabetic groups receiving early or late treatments with WJMSCs-CM (D-CME, D-CML), insulin (D-INSE, D-INSL), or DMEM (D-DME, D-DML). In the early treatment groups, insulin (3 U/day, subcutaneously) and WJMSCs-CM (10 mg/week, intraperitoneally) were administered immediately after diabetes induction; in the late treatment groups, these interventions began 30 days postinduction. Blood glucose and insulin levels, along with sperm parameters, were assessed. Sex hormones, testicular antioxidant enzyme activity, malondialdehyde (MDA), and glutathione (GSH) concentrations were measured using colorimetric methods. Real-time PCR detected Bax, Bcl-2, and tumor necrosis factor-alpha (TNF-α) gene expression. Our results showed that diabetes increased blood glucose levels, decreased insulin and sex hormone levels, induced testicular oxidative stress and apoptosis, and reduced sperm parameters compared to the control. WJMSCs-CM significantly ameliorated hyperglycemia, increased insulin and sex hormone levels, and improved sperm quality. In WJMSCs-CM-treated diabetic rats, MDA levels were reduced, while GSH and antioxidant enzyme activity increased. Furthermore, WJMSCs-CM decreased the testicular Bax/Bcl-2 ratio and TNF-α expression, as well as enhanced spermatogenic, Sertoli, and Leydig cells. In conclusion, WJMSC-CM administration effectively mitigated diabetes-induced testicular damage by reducing oxidative stress, inflammation, and apoptosis. Early treatment with WJMSCs-CM was more effective than late treatment for diabetes-induced reproductive dysfunction.
1. Introduction
Diabetes mellitus is a prevalent chronic metabolic disorder that occurs worldwide. It is distinguished by hyperglycemia due to insufficient insulin production and/or insulin resistance. Chronic hyperglycemia has detrimental effects on various organs, resulting in problems such as neuropathy, cardiovascular dysfunction, nephropathy, and blindness. Additionally, it has important implications for male reproductive health [1, 2]. Diabetes is associated with an increased risk of reproductive problems in both humans and animals [3]. A few of its effects include structural and functional abnormalities in the testis and disturbances to the hypothalamic–pituitary–gonadal (HPG) axis, which leads to low levels of sex hormones like gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone, along with structural and functional abnormalities in the testis [2]. Testicular atrophy, seminiferous tubule disruption, and decreased spermatogenesis are all symptoms of diabetes-induced male reproductive dysfunction, which can result in infertility [2, 4]. Multiple clinical trials have also reported erectile and ejaculatory dysfunction, decreased semen volume, low sperm quality, and abnormal sperm morphology [5, 6]. One of the main reasons for these reproductive problems is oxidative stress that sperm experiences due to prolonged hyperglycemia. Prolonged hyperglycemia in diabetes increases reactive oxygen species (ROS) production and decreases antioxidant efficacy [2, 7, 8]. ROS induces the overexpression of genes involved in testicular inflammation, affecting cell membrane and DNA integrity [9, 10]. Conventional treatments for diabetes, while partially effective, often come with side effects and may not fully mitigate the risk of complications [11, 12, 13]. Mesenchymal stem cell (MSC) therapy, or the use of bioactive substances released by these cells, is one topic that has gained attention in recent years as a potential treatment for diabetes and its related problems.
MSCs are increasingly recognized as promising options for treating several disorders due to their regenerative properties and ability to secrete bioactive substances [14, 15]. Recently, there has been consideration of using MSCs or the bioactive chemicals produced by these cells as a therapy for diabetes and its associated effects. The secretomes of MSCs contain bioactive substances such as growth factors, cytokines, extracellular proteins, and exosomes. MSCs-derived secretomes can facilitate tissue repair and regeneration through paracrine activities [14, 16]. Conditioned medium obtained from MSCs (MSCs-CM) is a cell-free substance composed of MSCs released products. Because MSCs-CM has similar effects to MSCs, it can be used as a viable alternative treatment [17]. MSCs-CM has exhibited various biological properties such as tissue regenerating, antiapoptotic, antioxidant, and anti-inflammatory effects [18, 19, 20]. Multiple studies have shown the beneficial impact of MSCS-CM on various disorders [18, 21, 22].
This research used Wharton's jelly stem cells (WJMSCs) from the HUC to prepare MSCs-CM, which have advantages such as rapid proliferation, lower immunological rejection risk, and abundant collection sources [17, 23, 24]. Several animal studies and clinical trials have tested the efficacy of WJMSCs or their secretory bioactive factors in various diseases, including neurological disorders, cardiac disease, immunological diseases, cancer, liver disease, bone/cartilage disease, and diabetes and its complications [15, 17, 25, 26].
In this study, we aimed to investigate whether the substances released from WJMSCs in the culture medium (WJMSCs-CM) could have therapeutic effects on male reproductive disorders caused by diabetes. Specifically, our focus was on assessing the impact of WJMSCs-CM on sperm parameters, inflammatory markers, oxidative stress, and histological alterations in the testes of diabetic rats.
2. Materials and Methods
2.1. Ethical Approval
All animal experimentation procedures were assessed and approved by the Animal Care and Local Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.AEC.1401.045).
2.2. Animals and Experimental Design
The 12 weeks adult male Sprague–Dawley rats weighing 250–300 g (n = 56) were purchased from the Center of Comparative and Experimental Medicine of Shiraz University of Medical Sciences and used in this study. The rats were housed in a controlled laboratory environment with a 12-hr cycle of darkness and light, temperature 23 ± 2°C, humidity 35%–40%, and unrestricted access to normal rat food and water throughout the experiment.
Animals were given 2 weeks for acclimatization before any experiments began. Next, the rats were assigned at random to control (n = 7) and streptozotocin (STZ)-induced diabetic groups (n = 49). After diabetes induction with an intraperitoneal injection of STZ, the diabetic group was further randomly subdivided into an untreated diabetic group and early- and late-treated diabetic groups. In the early treatment groups, therapy was initiated immediately after the induction of diabetes and continued for 8 weeks, with diabetic rats receiving WJMSCs-CM (D-CME), insulin (D-INSE), and DMEM, Dulbecco's modified eagle medium (D-DME). In the late diabetic groups, treatment with WJMSCs-CM (D-CML), insulin (D-INSL), and DMEM (D-DML) began 30 days after the induction of diabetes and lasted for 4 weeks. In the treated diabetic groups, insulin (3 U/day) [18] was administered subcutaneously, while WJMSCs-CM (10 mg/week) and DMEM (10 mg/week) were given intraperitoneally [18].
2.3. Diabetic Induction
To induce diabetes, fasted (12 hr) rats were injected intraperitoneally with STZ (55 mg/kg; Solarbio Life Science) freshly dissolved in 0.1 M citrate buffer (pH 4.5). Seven days after STZ injection, blood glucose concentration was measured using a glucometer (Arkray, Japan), and animals with blood glucose levels above 250 mg/dL were accepted as diabetic and included in this study.
2.4. Procedure for Extracting and Culturing WJMSCs from the Umbilical Cord
Removed human umbilical cord (HUC) samples were randomly collected from healthy full-term neonates born at Shiraz University Hospital following institutional board permission and written informed consent. Within 4–24 hr of birth, umbilical cords were transported to our laboratory. After three rinses in phosphate-buffered saline (PBS) and vessel separation, the delicate Wharton's jelly tissue was extracted from the amniotic layer and chopped into small pieces. The WJMSCs were extracted by cutting the tissue and digesting it with collagenase and hyaluronidase enzymes. The separated cells were subsequently grown in T-25 culture flasks using Dulbecco's modified eagle medium (DMEM) supplemented with 15% fetal bovine serum and 1% penicillin/streptomycin (P/S) at 37°C, with media change occurring twice a week [18]. In our colleague's study, such cells were identified as MSCs by recognizing specific cell surface markers on these cells [27].
2.5. Preparation of WJMSCs Conditioned Medium (CM)
The study used WJMSCs from the fourth passage for CM collection. After 80% confluence, the supernatant was removed, and cells were washed with PBS. The cells were cultured in serum-free DMEM for 48 hr. The resulting CM was centrifuged to remove debris and filtered [18].
The supernatants were processed into a lyophilized powder in a freeze dryer (Christ Alpha1-2 LD Plus, Germany), concentrated 50 times, and quantified using the bicinchoninic acid (BCA) Protein Assay Kit (Rockford, IL) and for later use stored at −80°C. After preparing lyophilized CM powder, it was dissolved in distilled water and injected intraperitoneally at a concentration of 10 mg/mL for each rat once a week [28].
2.6. Sample Collection
At the end of the experiment, the animals were deeply anesthetized with a high dose of ketamine–xylazine (300/30 mg/kg), and blood samples were collected through cardiac puncture. After centrifuging of blood (3,000 g for 10 min), serum samples were collected and stored at –20°C for later measurements of serum glucose and hormone levels. After that, rats were euthanized by decapitation under deep anesthesia and testes were immediately removed and weighed. The left testis was stored at –80°C for real-time PCR investigation and oxidative stress evaluation, and the right testis was fixed in a 10% neutral buffered formalin solution for histological study.
2.7. Serum Glucose and Hormonal Analysis
The serum level of glucose was assessed by the glucose oxidase procedure (Pars Azmoon Co., Tehran, Iran). Serum concentrations of FSH, LH, and total testosterone were measured using the ELISA method following the manufacturer's instructions for commercial kits (Cusabio Biotech Company, Wuhan, China). The serum insulin levels were determined using a rat ELISA kit (Insulin, Mercodia, Uppsala, Sweden). All measurements were performed in duplicate.
2.8. Body and Testis Weight, Gonadosomatic Index
During the experiment, the body was recorded once a week. The testis weight and gonadosomatic index of each animal were measured. The gonadosomatic index was determined by multiplying the ratio of testicular weight to body weight by 100 and expressing it as a percentage.
2.9. Sperm Collection
To maintain the sample's integrity and quality, sperm collection was done under deep anesthesia before cardiac puncture. For the collection of viable sperm samples, the tail of epididymis was dissected and rapidly put in a small petri dish counting 5 mL PBS, and incubated at 37°C for 30 s to diffuse the semen. The semen solution was quietly vibrated to obtain uniform seminal fluid.
2.10. Sperm Analysis
2.10.1. Sperm Count
The seminal fluid was spread on a Neubauer hemocytometer and the sperm heads were counted manually by light microscope. About 200–300 head of sperms were counted in every rat and results were expressed as total sperm count per milliliter [29, 30].
2.10.2. Sperm Motility
The seminal fluid was loaded in preheated microscope slides. Ten microscopic fields at a final magnification of 400x randomly selected and 200–300 sperms per animal were evaluated. Sperm motility was classified as progressive motile, nonprogressive, and nonmotile according to the fifth edition of the World Health Organization guidelines [31]. The percentage of sperm motility was assessed by number of motile sperm × 100/total number of spermatozoa [29, 30].
2.10.3. Sperm Viability
For sperm viability, the smear of seminal fluid was stained with eosin and nigrosin dyes (Sigma–Aldrich) according to the World Health Organization guidelines. The heads of living sperms are not dyed, but the heads of dead sperms are dyed. The 200–300 stained and nonstained sperms per rat at a final magnification of ×400 in 10 random microscopic fields were evaluated. The percentage of live sperm was assessed by the number of live sperm × 100/sum of live and dead sperm [29, 30].
2.10.4. Sperm Morphology
To assess sperm morphology, 40 μL sperm suspension was gently mixed with 10 μL solution containing 1% eosin Y and nigrosin in a test tube. The sperm was incubated at room temperature for 60 min to facilitate staining. Subsequently, the sperm was resuspended using a pipette. A light microscope examined 200 spermatozoa on each slide (magnification: 400x). The abnormalities were classified as head and tail defects (double head, flattened head, bent tail, curved neck). The percentages of normal sperms were determined [32].
2.11. Evaluation of Oxidative Stress Markers
For assessment of the testicular oxidative stress parameters, 100 mg of the testis was weighed, then cut into small pieces and homogenized in cold PBS (pH = 7.4). Supernatants were isolated by centrifugation with 1 mM EDTA at 4,000 rpm for 15 min at 4°C and their supernatants were separated and used for lipid peroxidation, enzymatic activity, and protein quantitation studies. Malondialdehyde (MDA) content as a lipid peroxidation index was measured using the thiobarbituric acid reactive substance method [8]. Superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) activities, as well as glutathione concentration (GSH), were quantified with commercial test kits (Kiazist Life Sciences, Iran) using colorimetric methods. A BCA protein quantification kit (Pars Tous Biotech, Iran) was used to quantify the amount of protein in the tissues.
2.12. Total RNA Extraction and Real-Time PCR
First, the testis samples were sonicated with the help of ultrasound waves for 5 min with 100% power of the device on ice and in PBS solution. Then, the samples were centrifuged at 2,500 rpm and the supernatant was removed for the next step. The trizol/chloroform column extraction method was used to extract RNA from testis samples. For this purpose, Pars Tous company's RNA extraction kit was used. Then, using a cDNA Synthesis Kit (Fermentas et al.), the whole RNAs were reverse transcribed into cDNA according to the manufacturer's instructions.
After that, the testicular expression of B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), and tumor necrosis factor-alpha (TNF-ɑ) genes were conducted by real-time PCR on StepOne real-time PCR system using SYBR Green High ROX Master Mix (Amplicon, Brighton, UK) according to manufacturer's protocol. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was considered as an internal control. The relative expression level of genes was calculated by the 2−ΔΔCt formula. The sequences of primers used in this study are as follows:
For: Bax, F: 5′-GCTACAGGGTTTCATCCAGG-3′; R: 5′-TTGTTGTCCAGTTCATCGCC-3′;
For: Bcl2, F: 5′-CTGGTGGACAACATCGCTCT-3′; R: 5′-GCATGCTGGGGCCATATAGT-3′;
For: TNF-ɑ, F: 5'-TCAGCCTCTTCTCATTCCTGC-3′; R: 5'-TTGGTGGTTTGCTACGACGTG-3′;
For: GAPDH, F: 5′-AGTGCCAGCCTCGTCTCATA-3′; R: 5′-GAGAAGGCAGCCCTGGTAAC-3′.
2.13. Stereological Studies
2.13.1. Testis Tissue Collection and Preparation
After inducing deep anesthesia and sacrificing the rats, their testes were weighted, and primary testicular volume was measured with the immersion method in normal saline. To evaluate the stereological parameters such as tubule length, isotropic uniform random sections should be performed. So, the testis was cut according to the “orientator method” (Figures 1(a), 1(b), and 1(c)). The segments were maintained in 10% neutral buffered formalin for tissue processing. Afterward, the segments (8–12) were processed, embedded into blocks of paraffin, sectioned (5 and 25 µm thickness), and stained with Hematoxylin and Eosin (H&E). After that, 8–12 fields were selected from each microscopic slide according to systematic uniform random sampling, and stereological parameters were calculated [33].
Figure 1.
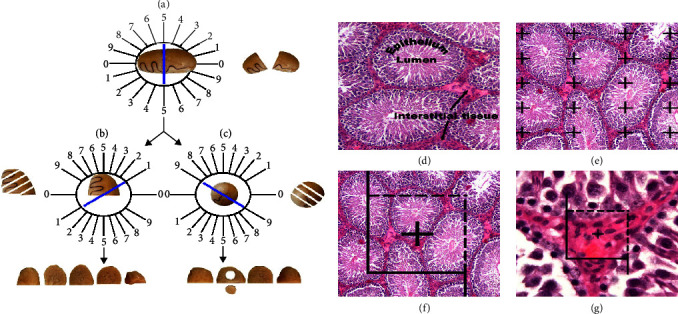
Application of stereological techniques: isotropic uniform random sections of testicular tissue were obtained using the random direction of the Φ and θ o'clock (a–c). The testis is located on Φ o'clock and a random number (here 5), the testis was divided into two parts (a). Then, the cutting surface of each part of the testicle is located in a 0–0 direction and the second cuts are made (here 1 and 9) (b and c). As a result, each testicle was cut in the form of parallel plates with the second cutting direction with a distance of 1 mm, and 8–12 slabs were prepared (b and c). The testicular tissue (seminiferous tubules, seminiferous epithelium, lumen, and interstitial tissue) is demonstrated in the histological sections (d). The point-counting technique was used to assess the volume density of the testicular tissue on the hematoxylin and eosin (H&E) stained tissue sections (e). An unbiased counting frame was employed to assess the seminiferous tubule length density (f). The disector method was employed for estimating the numerical density of the testicular tissue cells (g).
Usually, tissue processing results in a shrinkage of the testis. A simple way to evaluate the degree of shrinkage “d(shr)” is to punch a circular piece from the sections of the testis with a trocar (Figure 1(c)). Afterward, the areas of the circle after and before tissue processing were measured.
The degree of testicular shrinkage “d(shr)” was calculated by the subsequent formula:
| (1) |
where AA and AB are the circular parts of the testis after and before tissue processing, respectively. The secondary volume of the testis was estimated by multiplying the primary volume and the degree of shrinkage [33].
2.13.2. Estimation of the Seminiferous Tubules, Epithelium, and Interstitial Tissue Volumes
Five micrometers thick sections were used to estimate the volume of the seminiferous tubules, seminiferous epithelium, and interstitial tissue. The stereology tool was prepared from a Nikon E-200 microscope (Tokyo, Japan) with an oil objective lens (Plan Apo 60x, n/a: 1.4, Tokyo, Japan) and a Samsung video camera (SCB-2000P, Hanwha Techwin, South Korea) connected to a computer. The stereological probe was employed for the live image and the mentioned parameters were estimated with the “point-counting way,” with a stereology software system (Stereolite, SUMS, Shiraz, Iran) [33]. The volume density “Vv (structure/testis sections)” of the testis was assessed by the “point-counting method” (Figures 1(d) and 1(e)) and the after that formula:
| (2) |
where ∑P (structure) and ∑P (total) are the number of points that hit the seminiferous tubules, seminiferous epithelium, interstitial tissue, and the entire testicular tissue, respectively.
2.13.3. Estimation of Seminiferous Tubule Length
Five micrometers thick sections were used to evaluate the length density of seminiferous tubules with an unbiased counting frame. The unbiased counting frame has two prohibiting lines (the left and inferior sides and their extensions), and two acceptable lines (the right and superior sides) were superimposed on the image [33]. The seminiferous tubules are counted either completely or partially inside the unbiased counting frame and do not touch the outlet lines.
The length density (LV) of the seminiferous tubules is assessed by the next formula:
| (3) |
where ∑Q is the sum of counted seminiferous tubules, ∑P is the total points at the center of the unbiased counting frame that strikes the testicular tissue, and a/f is the area per counting frame [33] (Figure 1(f)). The total length of the seminiferous tubules was estimated by multiplying the length density and the secondary volume of the testis.
2.13.4. Stereological Estimation of the Cell Number
The numerical density “NV” of spermatogonia, spermatocytes, spermatids, Sertoli, and Leydig cells was estimated using the optical disector method on 25 µm thick sections.
The optical disector setting consisted of an Eclipse microscope (E200, Nikon, Tokyo, Japan) with a 40X oil immersion objective lens (Nikon, Japan, with a high numerical aperture (NA = 1.3)) which was connected to a video camera, that sent live microscopic image to a monitor and an electronic microcator (MT12, Heidenhain, Traunreut, Germany), for evaluating the movements in the Z direction.
An unbiased counting frame is used to count the number of spermatogenic, Sertoli, and Leydig cells that were explained. The guard's zones are above and below the surfaces of the histological sections, which are employed to prevent artifacts that occur during tissue processing in these sections.
The cells inside the guard's zones were not counted. The distance between the upper and lower guard's zones was equal to the height of the disector. All cells were identified, and each cell with a high level of focus in the frame, and completely or partially inside the counting frame without hitting the prohibited lines, was counted (Figure 1(g)).
The numerical density (NV) of the spermatogenic, Sertoli, and Leydig cells was estimated with the following formula:
| (4) |
where ΣQ− is the total number of counted cells within the sampling volume, ΣP is the total of counted frames in the entire microscopic fields, a(f) is the area of the frame, and h is the height of disector. The total number of cells was calculated by multiplying the numerical density (NV) and the secondary volume of the testis.
2.14. Statistical Analysis
The data are analyzed using GraphPad Prism (Version 8.4.3). The normality of the data was assessed using the Kolmogorov–Smirnov test (K–S test). All data except the BAX, Bcl2, and Bax/ Bcl2 variables had a normal distribution, and their comparisons were conducted using one-way ANOVA followed by the Tukey HSD test. The BAX, Bcl2, and Bax/Bcl2 variables were statistically analyzed with the Kruskal–Wallis nonparametric test. Parametric data are expressed as mean ± SEM; nonparametric data were reported as median with IQR. P < 0.05 is considered the level of significance for all analyses.
3. Results
3.1. Serum Glucose and Insulin Levels
Our findings showed that diabetic rats present high serum glucose and low serum insulin levels compared with control rats. Early and late treatments with WJMSCs-CM and insulin resulted in a statistically significant decrease in serum glucose compared to diabetic rats. Insulin levels in three groups of D-INSE, D-CME, and D-INSL were higher than those in the diabetic group. However, the serum glucose and insulin levels in early and late WJMSCs-CM-treated diabetic groups did not reach the control group. Serum insulin levels in D-INSE and D-INSL were significantly lower, and serum glucose levels in D-INSL were considerably higher than the control group (Figures 2(a) and 2(b)). Early and late administration of DMEM did not alter serum glucose and insulin levels compared to the diabetes group. The serum glucose level in early- and late-treated groups with WJMSCs-CM and insulin was lower than in D-DME. Serum insulin in two groups of D-CME and D-INSE was higher than D-DME. Late treatment with insulin showed a difference in insulin levels with D-DML. There were no differences in serum glucose and insulin levels between late and early administration insulin and CM.
Figure 2.
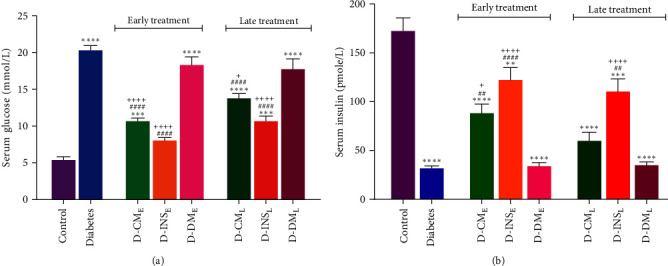
Serum levels of glucose (a) and insulin (b) in the control, diabetes, early and late insulin, CM, and DMEM-treated diabetic groups. Data were analyzed by one-way ANOVA (Tukey's HSD test) are presented as mean ± SEM (n = 7). ∗ ∗P < 0.01, ∗ ∗ ∗P < 0.001, ∗ ∗ ∗ ∗P < 0.0001 vs. the control group; ##P < 0.01, ####P < 0.001 vs. the diabetic group; and +P < 0.05, ++++P < 0.0001 vs. the D-DME and D-DML. D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; late, treatment was started 30 days after diabetes induction.
3.2. Body Weight Gain, Testis Weight, and Gonadosomatic Index
The body weight gain, testis weight, and gonadosomatic index of rats in all experimental groups are shown in Figures 3(a), 3(b), and 3(c). A significant weight loss was observed in the diabetic group. Early treatment with WJMSCs-CM or insulin and only late treatment with insulin significantly increased body weight gain. The testis weight was also reduced in diabetic rats compared to the control group. Although both early and late treatments with WJMSCs-CM and insulin increased testis weight in diabetic rats, it was only significant in D-INSE compared to the untreated diabetic group. There were no differences in the gonadosomatic index among all groups. The early and late DMEM-treated diabetic rats did not differ in body weight, testis weight, or gonadosomatic index with the diabetes group. Body weight gain in the D-INSE was higher than in the D-DME. There were no differences in three parameters between late and early administration of WJMSCs-CM and insulin.
Figure 3.
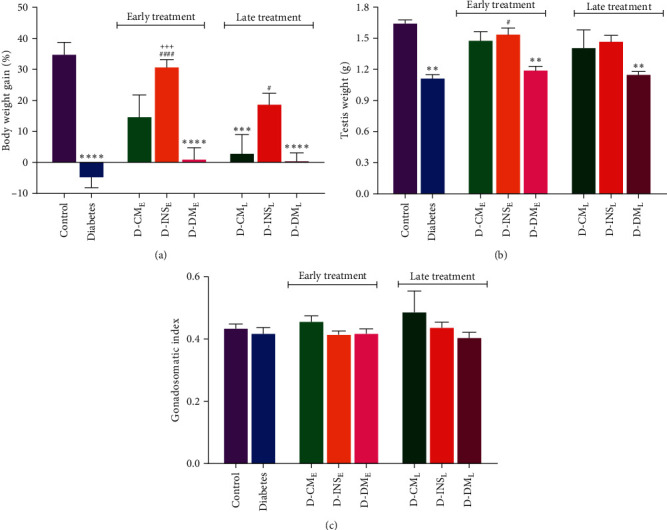
Body weight gain (a), testis weight (b), gonadosomatic index (c) in the control, diabetes, early and late insulin, CM, and DMEM-treated diabetic groups. Data were analyzed by one-way ANOVA (Tukey's HSD test) and presented as mean ± SEM (n = 7). ∗ ∗P < 0.01, ∗ ∗ ∗P < 0.001, ∗ ∗ ∗ ∗P < 0.0001 vs. the control group; #P < 0.05, ####P < 0.0001 vs. the diabetic group; and +++P < 0.001 vs. the D-DME. D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; late, treatment was started 30 days after diabetes induction.
3.3. Serum FSH, LH, and Testosterone Levels
Figures 4(a), 4(b), and 4(c) illustrate the serum concentrations of LH, FSH, and testosterone. Compared to the control group, the serum LH, FSH, and testosterone levels were significantly lower in diabetic rats (P < 0.0001). Early treatment with WJMSCs-CM and insulin led to a statistically significant increase in serum LH, FSH, and testosterone concentrations compared to diabetic rats. No statistically significant increment in serum levels of three hormones was observed in late WJMSCs-CM-treated rats. There were significant differences in serum levels of LH and testosterone between D-INSL and diabetic groups. Early and late administration of DMEM did not change FSH, LH, and testosterone concentrations compared to the diabetes group. The serum levels of all hormones in the D-CME group and FSH and testosterone in the D-INSE group were higher than in the D-DME. There were no differences in sex hormones between early and late WJMSCs-CM and insulin administration.
Figure 4.
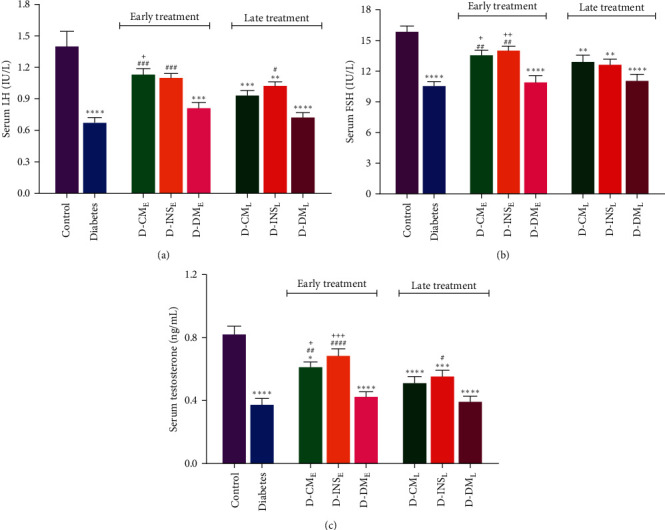
Serum LH (a), FSH (b), and testosterone (c), levels in the control, diabetes, early and late insulin, CM, and DMEM-treated diabetic groups. Data were analyzed by one-way ANOVA (Tukey's HSD test) and presented as mean ± SEM (n = 7). ∗P < 0.05, ∗∗P < 0.01, ∗ ∗ ∗P < 0.001, ∗ ∗ ∗ ∗P < 0.0001 vs. the control group; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs. the diabetic group; and +P < 0.05, ++P < 0.01, +++P < 0.001 vs. the D-DME group. D-CME, diabetic group receiving early WJMSCs-CM; D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; Late, treatment was started 30 days after diabetes induction.
3.4. Sperm Parameters
Statistical analysis of results showed that the total count (Figure 5(a)), motility (Figure 5(b)), viability (Figure 5(c)), and morphology (Figure 5(d)) of sperm were significantly lower in diabetic rats compared to the control group. Early treatment with WJMSCs-CM and insulin resulted in a statistically significant increase in three sperm parameters compared to the diabetic group. Although sperm count in the D-INSL group and sperm motility and viability were significantly increased in the D-CML group, they did not reach the control group. Statistically significant differences in these sperm parameters were observed between D-INSL and control groups. There were significant differences in sperm motility and viability between D-CME and D-CML, as well as between D-INSE and D-INSL in sperm viability. No differences in sperm parameters were found between early and late DMEM-treated diabetic rats and the diabetes group. Compared to the D-DME group, the D-INSE group showed significant differences in sperm count, motility, and viability. In contrast, significant differences in sperm motility and viability were observed in the D-CME group compared to the D-DME group. There were no differences in sperm morphology among all experimental groups (Figure 5(d)).
Figure 5.
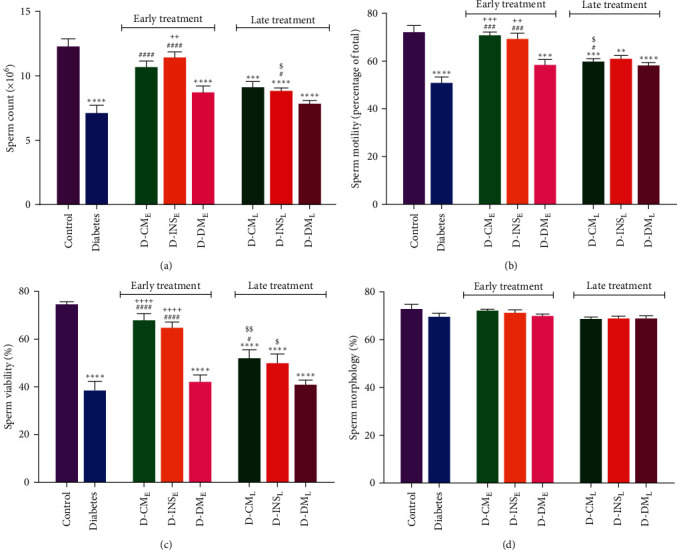
Count (a), motility (b), viability (c), and morphology (d) of sperm in the control, diabetes, early and late insulin, CM, and DMEM-treated diabetic groups. Data were analyzed by one-way ANOVA (Tukey's HSD test) and presented as mean ± SEM (n = 7). ∗ ∗P < 0.01, ∗ ∗ ∗P < 0.001, ∗ ∗ ∗ ∗P < 0.0001 vs. the control group; #P < 0.05, ###P < 0.001, ####P < 0.0001 vs. diabetic group; $P < 0.05, $$P < 0.01 vs. the early-treated diabetic group; and ++P < 0.01, +++P < 0.001, ++++P < 0.0001 vs. the D-DME group. D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; late, treatment was started 30 days after diabetes induction.
3.5. Testicular Oxidative Stress Parameters
Figures 6(a), 6(b), 6(c), 6(d), and 6(e) present testicular MDA and GSH levels and specific activity of SOD, GPx, and catalase in all of the experimental groups. The results of this study showed that the MDA level (Figure 6(a)) of testicular tissue in the diabetic group was significantly (P < 0.05) higher than that in the control group. The GSH concentration (Figure 6(b)) and specific activity of antioxidant enzymes of catalase (Figure 6(c)) and GPx (Figure 6(d)) were reduced in the diabetic group compared to the control group. Early and late treatments of WJMSCs-CM and insulin resulted in a significant decrease in the testicular MDA level compared to the control group. However, they did not reach the control group in both D-CML and D-INSL groups. Early- and late-treated rats with WJMSCs-CM and insulin showed significant differences in testicular MDA concentrations compared to early and late DMEM-treated rats. There were no significant differences in testicular MDA concentrations in early- and late-treated rats with WJMSCs-CM and insulin.
Figure 6.
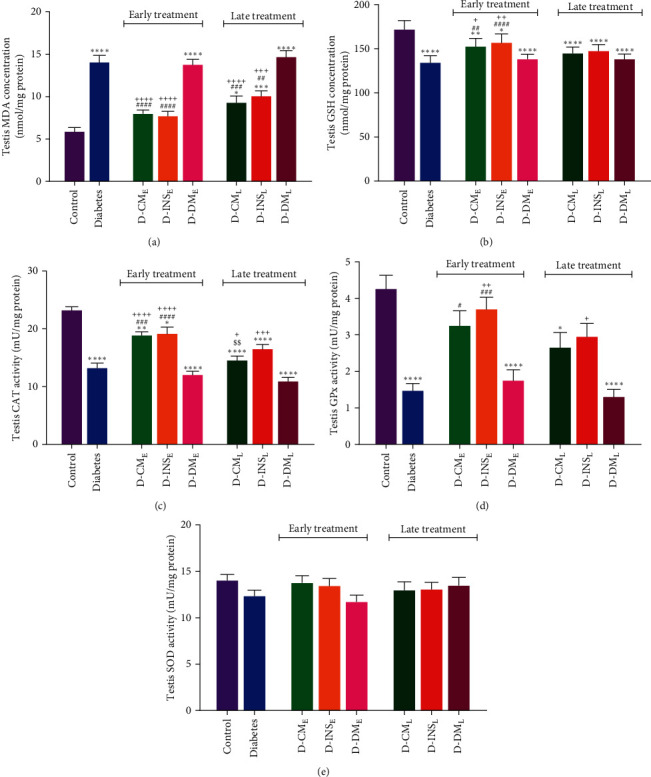
MDA (a), GSH (b) levels and specific activity of catalase (c), GPx (d), and SOD (e) of testis in the control, diabetes, early and late insulin, CM, and DMEM-treated diabetic groups. Data were analyzed by one-way ANOVA (Tukey's HSD test) and presented as mean ± SEM (n = 7). ∗P < 0.05, ∗ ∗P < 0.01, ∗ ∗ ∗P < 0.001, ∗ ∗ ∗ ∗P < 0.0001 vs. the control group; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs. the diabetic group; $$P < 0.01 vs. the early-treated diabetic group; +P < 0.05, ++P < 0.01, +++P < 0.001, ++++P < 0.0001 vs. the D-DME and D-DML groups. D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; late, treatment was started 30 days after diabetes induction.
After early treatment of diabetic rats with WJMSCs-CM and insulin, the testicular GSH concentration was significantly raised, but it was still considerably lower than the control group. Compared to diabetic rats, late treatment with WJMSCs-CM and insulin did not alter testicular GSH levels in D-CML and D-INSL groups (Figure 6(b)). Only early-treated rats with WJMSCs-CM and insulin showed a significant difference in testicular GSH level compared to early DMEM-treated rats. There was no significant difference in testicular GSH concentration in early- and late-treated rats with WJMSCs-CM and insulin.
A significant increase in catalase (CAT) and GPx activity was observed in the diabetic group after early treatment with WJMSCs-CM and insulin. However, catalase activity did not reach the levels of the control group (Figures 6(c) and 6(d)). Compared to diabetic rats, there was no significant alteration in testicular catalase and GPx activity in the D-CML and D-INSL groups. Early- and late-treated rats with WJMSCs-CM and insulin showed substantial differences in testicular catalase activity compared to early and late DMEM-treated rats. The testicular GPx activity in early and late insulin-treated rats was higher than in early and late DMEM-treated rats. Early and late administration of DMEM did not change oxidative stress parameters in the diabetes group. There was a significant difference in testicular catalase activity in early- and late-treated rats with WJMSCs-CM. There was no significant difference in testicular SOD activity among all groups (Figure 6(e)).
3.6. Testicular Bax, Bcl-2, and TNF-ɑ Gene Expression
The results depicted in Figures 7(a) and 7(b) demonstrate that the gene expression levels of Bax and Bcl-2 in testis were significantly elevated and reduced, respectively, in the untreated diabetes group compared to the control group (P < 0.01). Additionally, the testicular Bax/Bcl-2 ratio (Figure 7(c)) was significantly higher (P < 0.01) in the diabetes group compared to the control group. Although early and late treatments with WJMSCs-CM and insulin decreased the level of Bax expression in diabetic rats, the reduction was not significant (Figure 6(a)). On the other hand, the mRNA expression level of Bcl-2 in D-CME (P < 0.05), not in D-CML, was significantly higher than that in the diabetic group. There was no significant increase in Bcl-2 expression level in D-INSE and D-INSL compared to diabetic groups (Figure 6(b)). The testicular Bax/Bcl-2 ratio was decreased in diabetic groups with early WJMSCs-CM and insulin treatment compared to the untreated diabetic group. Although in comparison diabetic group, late treatment with WJMSCs-CM and insulin reduced the testicular Bax/Bcl-2 ratio in D-CML and D-INSL groups, this reduction was not significant (Figure 6(c)).
Figure 7.
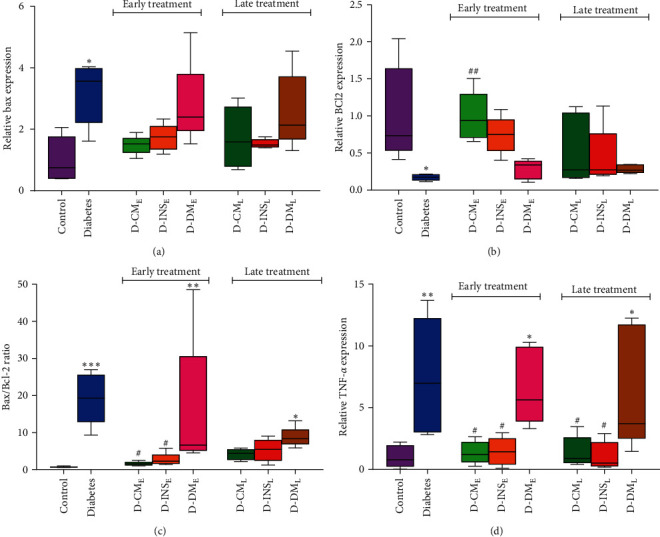
Changes in the testicular Bcl-2 (a), Bax (b), Bax/Bcl-2 ratio (c), and TNF-ɑ (d) gene expression in the control, diabetes, early and late insulin, CM, and DMEM-treated diabetic groups. The BAX, Bcl2, and Bax/Bcl2 data were statistically analyzed using the Kruskal–Wallis nonparametric test and reported as median with IQR. The variable of TNF-ɑ was analyzed by one-way ANOVA (Tukey's HSD test) and presented as mean ± SEM (n = 7). ∗P < 0.05, ∗ ∗P < 0.01, ∗ ∗ ∗P < 0.001 vs. the control group; #P < 0.05, ##P < 0.01 vs. the diabetic group. D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; late, treatment was started 30 days after diabetes induction.
Also, the mRNA expression level of TNF-ɑ in the diabetic group was significantly increased compared to the control group (P < 0.01). However, early and late treatments with WJMSCs-CM and insulin significantly decreased the expression level of TNF-ɑ mRNA compared to the untreated diabetes group (Figure 6(d)).
There were no significant differences in the gene expression levels of Bax, Bcl-2, and TNF-ɑ in early- and late-treated rats with WJMSCs-CM and insulin. Compared to the diabetes group, early and late DMEM administrations did not affect the gene expression levels of Bax, Bcl-2, and TNF-ɑ.
3.7. Histological Examination of the Testes
Results obtained from light microscopic examination of H&E-stained right testicular sections of all rats indicated that the seminiferous tubules in the control groups exhibited typical morphology of spermatogenic, Leydig, and Sertoli cells. In the diabetic group, atrophy of seminiferous tubules, loss of spermatogenic cells in the epithelium, decreased spermatozoa in the tubule lumen, and thinning of the seminiferous epithelium were observed. On the contrary, samples from early WJMSCs-CM and insulin-treated rats showed normal seminiferous tubules in both groups of D-CME and D-INSE. It appears that early treatment with WJMSCs-CM and insulin could prevent seminiferous tubule volume and spermatogenic cell loss. Late treatment with WJMSCs-CM and insulin slightly ameliorated diabetes-induced histological changes in the testis. A few irregular spermatogenic tubules covered with a few layers of spermatogenic cells were still observed in the D-CML and D-INSL groups. Like the untreated diabetes group, early and late DMEM-treated groups indicated marked tubular disorganization and a few layers of spermatogenic cells (Figure 8).
Figure 8.
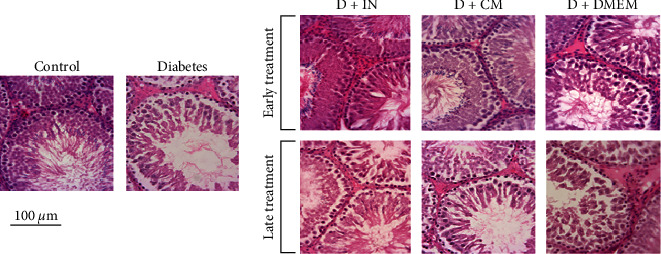
The microscopic images of seminiferous tubules of rat testis tissue in the control, diabetes, early and late insulin, WJMSCs-CM, and DMEM-treated diabetic groups. Normal appearance of spermatogenic, Leydig, and Sertoli cells was observed in the seminiferous tubules of control rats. Loss of spermatogenic cell populations in the epithelium, reduction of spermatozoa in the tubule lumen, and thinning of the seminiferous epithelium could be observed in the diabetic group. The histological changes in the epithelium and lumen of testis seminiferous tubules in the D-CME group and especially in the D-INSE group appear to be similar to normal histology. Photomicrograph of testicular tissue in late treated rats with insulin, WJMSCs-CM showed little disorganized seminiferous tubules lined by few layers of spermatogenic cells. The hematoxylin & eosin (H&E) stained testis sections. The scale bar is 100 μm. D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; late, treatment was started 30 days after diabetes induction.
3.8. Stereological Analyses of the Testes
3.8.1. Volume Estimation
As shown in Figures 9(a), 9(b), and 9(c), the total volumes of the testis, seminiferous tubules, and seminiferous epithelium in the diabetes group were significantly decreased compared to the control group. Contrarily, the total volume of interstitial tissue was increased in the diabetes group. Early treatment with WJMSCs-CM and insulin increased the total volumes of the testis in both D-CME and D-INSE groups, which was only significant in D-INSE (P < 0.05). Although the total volumes of seminiferous tubules in the D-CME and D-INSE groups were significantly higher than those in the diabetic group, they did not reach control. Early treatment with WJMSCs-CM and insulin increased epithelium volume and decreased interstitial tissue volume in the D-CME and D-INSE groups (P < 0.0001). Late treatment with WJMSCs-CM does not affect all the above parameters. In contrast, late treatment with insulin partially improved the total volumes of seminiferous tubules and interstitial tissue in the D-INSL group. No significant differences existed in the total volumes of the testis, seminiferous tubules, epithelium, and interstitial tissue between the early and late DMEM-treated groups and the diabetic group. Only early-treated rats with WJMSCs-CM and insulin showed substantial differences in epithelium and interstitial tissue volumes compared to early DMEM-treated rats. Significant differences existed in the total epithelium and interstitial tissue volumes between early- and late-treated rats with WJMSCs-CM and insulin.
Figure 9.
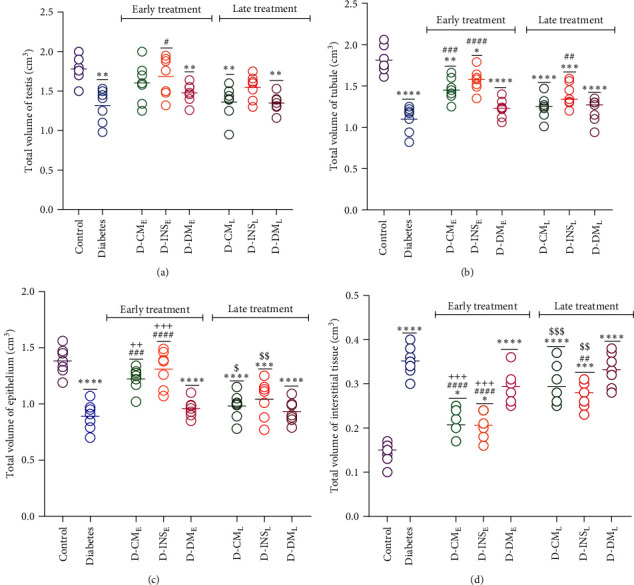
Effects of WJMSCs-CM and insulin on total volumes of the testis (a), seminiferous tubules (b), seminiferous epithelium (c), and interstitial tissue (d). Data were analyzed by one-way ANOVA (Tukey's HSD test) and presented as mean ± SEM (n = 7). ∗P < 0.05, ∗ ∗P < 0.01, ∗ ∗ ∗P < 0.001, ∗ ∗ ∗ ∗P < 0.0001 vs. the control group; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs. the diabetic group; $P < 0.05, $$P < 0.01, $$$P < 0.001 vs. the early-treated diabetic group; and ++P < 0.01, +++P < 0.001 vs. the D-DME. D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; late, treatment was started 30 days after diabetes induction.
3.8.2. The Length Assessment of Seminiferous Tubules
The length of seminiferous tubules in all experimental groups is shown in Figure 10. Diabetes caused a reduction in the length of tubules compared to control. Early treatment with WJMSCs-CM and insulin led to a significant increase in the length of tubules in both D-CME and D-INSE groups in comparison to the diabetic group. However, no difference in this parameter was observed between the late WJMSCs-CM and insulin-treated diabetic groups and the untreated diabetic group. There was no significant difference in the length of seminiferous tubules between the early and late DMEM-treated groups and the diabetic group. The length of seminiferous tubules in the D-CME and D-INSE groups was higher than in the D-CME group. Only a significant difference existed in the seminiferous tubule length between early- and late-treated rats with insulin.
Figure 10.
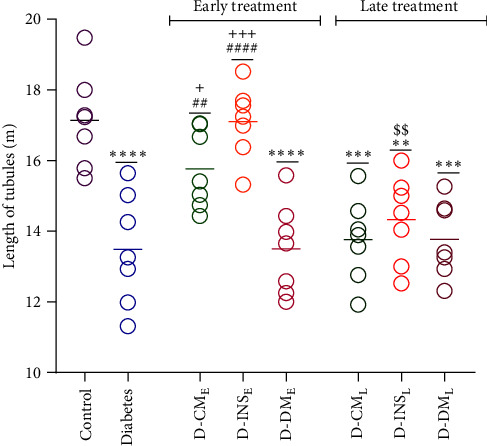
Effects of WJMSCs-CM and insulin on length of seminiferous tubules. Data were analyzed by one-way ANOVA (Tukey's HSD test) and presented as mean ± SEM (n = 7). ∗ ∗P < 0.01, ∗ ∗ ∗P < 0.001, ∗ ∗ ∗ ∗P < 0.0001 vs. the control group; ##P < 0.01, ####P < 0.0001 vs. the diabetic group; $$P < 0.01 vs. the early-treated diabetic group; and +P < 0.05, +++P < 0.001 vs. the D-DME. D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; late, treatment was started 30 days after diabetes induction.
3.8.3. Number of Cells
The total number of spermatogenic cells, Sertoli, and Leydig cells was reduced in the testes of diabetic rats in comparison to the control group ((Figures 11(a), 11(b), 11(c), 11(d), and 11(e)). Early treatment with WJMSCs-CM and insulin significantly increased the total number of all the above cells in the D-CME and D-INSE compared to the diabetic group so that the total number of spermatogonia, spermatid, and Sertoli in early WJMSCs-CM-treated diabetic groups, and all above cells in early insulin-treated diabetic groups reach control. In comparison to the diabetic group, a remarkable increase in the total number of spermatogenic cells, Sertoli, and Leydig cells was also observed in the D-INSL group in which only the total number of spermatogonia and Sertoli was near to control. There were also significant differences in the total number of spermatid and Leydig cells between the D-CML and diabetic groups, but they did not reach the control group. Early and late treatments with DMEM did not affect all the above parameters in diabetic groups. Two groups D-CME and D-INSE showed significant differences in all spermatogenic cells, Sertoli, and Leydig cells compared to D-DME. The total number of spermatid and Sertoli cells in the D-INSL and only the number of spermatid cells in the D-CML were higher than in the D-DML. The number of spermatocytes, spermatid, and Leydig cells differed between early and late groups treated with WJMSCs-CM and insulin.
Figure 11.
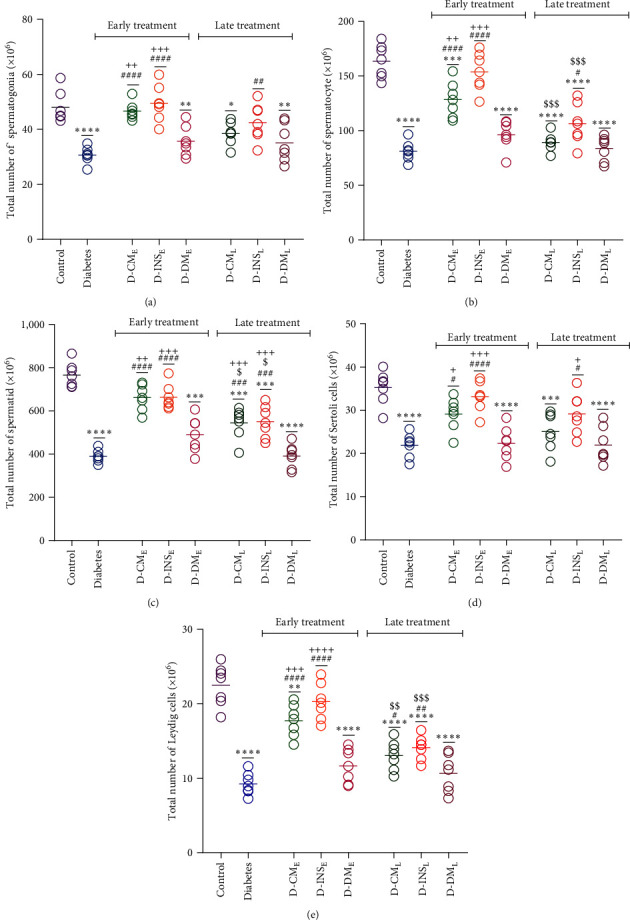
Effects of WJMSCs-CM and insulin on numbers of spermatogonia (a), spermatocyte (b), spermatid (c), Sertoli (d), and Leydig (e) cells of the testis. Data were analyzed by one-way ANOVA (Tukey's HSD test) and presented as mean ± SEM (n = 7). ∗P < 0.05, ∗ ∗P < 0.01, ∗ ∗ ∗P < 0.001, ∗ ∗ ∗ ∗P < 0.0001 vs. the control group; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs. the diabetic group; $P < 0.05, $$P < 0.01, $$$P < 0.001 vs. the early-treated diabetic group; and +P < 0.05, ++P < 0.01, +++P < 0.001, ++++P < 0.0001 vs. the D-DME. D-CME, diabetic group receiving early WJMSCs-CM; D-CML, diabetic group receiving late WJMSCs-CM; D-INSE, diabetic group receiving early insulin; D-INSL, diabetic group receiving late insulin; D-DME, diabetic group receiving early DMEM; D-DML, diabetic group receiving late DMEM; early, treatment was started immediately after diabetes induction; late, treatment was started 30 days after diabetes induction.
4. Discussion
Diabetes induces testicular injury and abnormalities in sperm. MSCs secretion-based CM has a repair function similar to MSCs but without tumor growth [34, 35]. In the present study, we investigated the early and late treatments of WJMSCs-CM on male reproductive health in the STZ-induced rat model of diabetes.
Our study found that STZ-induced diabetes leads to higher fasting blood glucose and lower serum insulin levels by destroying pancreatic islet cells through oxidative stress, metabolic disruption, and impaired mitochondrial function [36]. However, early and late administration of WJMSCs-CM to diabetic animals reduced hyperglycemia and enhanced insulin production. Previous research suggests that stem cell-derived CM regenerates pancreatic β cells for insulin production [18, 37].
In line with several studies, the present investigation demonstrated that STZ-induced diabetes lowered the body and testis weights in rats, likely due to protein wasting from insulin deficiency [38, 39, 40]. Both early and late insulin administration significantly increased body and testis weights, while MSCs-CM demonstrated partial improvements.
Our study showed that STZ-induced diabetes reduces male sex hormone levels, sperm count, motility, and viability, aligning with previous research [41, 42, 43, 44]. It is well-known that FSH and LH promote germ cell maturation and function in the gonads. Inadequate insulin in diabetes damages the HPG axis, decreasing GnRH, FSH, LH, and testosterone, leading to testicular atrophy, seminiferous tubules damage, and spermatogenic cell impairment [2]. In the current study, diabetic rats showed significantly lower serum testosterone levels, likely due to reduced insulin stimulation of Leydig cells and hyperglycemia-induced oxidative damage to these cells [2, 45]. Testosterone promotes protein synthesis in spermatogenic cells and reduced testosterone impairs protein synthesis in germ cells, causing germ cell degeneration, abnormalities in spermatogenesis, and low sperm count [46, 47].
MSCs primarily exert their therapeutic effects via paracrine signaling of their secretome, which includes growth factors, cytokines, chemokines, enzymes, and extracellular vesicles like exosomes and microvesicles [48, 49]. Important growth factors of WJMSCs-CM are hepatocyte growth factor (HGF), fibroblast growth factor-2 (FGF2), angiogenesis factors like vascular endothelial growth factor (VEGF), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and transforming growth factor β (TGFβ) [17, 50]. Some receptors for these growth factors are found in spermatozoa, indicating their role in improving sperm quality and male reproductive health [51]. Sharifian et al. [52] showed that CM derived from MSCs can improve sperm count and motility in the ischemia/reperfusion rat model. Several studies have also documented the important role of some secreted growth factors of CM, such as NGF, VEGF, IGF, and TGFβ, in the regulation of spermatogenesis, germ cell maturation, spermatozoa survival, steroidogenesis stimulation, and HPG axis activation [51, 53, 54].
The testis is vulnerable to oxidative stress due to its high concentration of unsaturated fatty acids and ROS-generating systems [55]. Hyperglycemia can adversely affect testicular tissue, including oxidative stress, inflammation, and apoptosis [8, 56]. The level of MDA in tissues is a crucial diagnostic indicator of oxidative stress, which can cause apoptosis through intra- and intermolecular cross-linkages. Increased ROS leads to lipid peroxidation in cell membranes, impacting the high mitotic activity of spermatogenic cells [57]. Studies have shown a reduction in antioxidative enzyme expression and an increase in the levels of MDA in the testis of rats with STZ-induced diabetes [8, 58, 59]. Our investigation revealed that diabetes dramatically enhanced MDA levels and decreased GSH concentration and the antioxidant enzyme activity of GPx and catalase. Additionally, hyperglycemia-induced oxidative stress triggers a pro-inflammatory and apoptotic signaling pathway, with ROS initiating inflammation [60, 61]. ROS stimulates the synthesis and release of various pro-inflammatory molecules, including leukotrienes and cytokines [62]. TNF-α is one of the important pro-inflammatory cytokines. Previous studies have indicated an increase in TNF-α expression in the testes of diabetic rats [56]. This research found that untreated diabetic rats showed inflammation in their testes, leading to a significant increase in TNF-α mRNA expression, triggering inflammatory, and apoptotic responses [56, 63].
The present study found that early and late administration of WJMSCs-CM and insulin reduced testicular lipid peroxidation and increased antioxidant enzyme activity in diabetic rats. Researchers discovered that major growth factors of conditioned media, such as HGF, FGF2, VEGF, NGF, and BDNF, have antioxidant properties and lower ROS in various tissues [51, 64, 65]. WJMSCs-CM and insulin also demonstrated anti-inflammatory actions, reducing TNF-α mRNA expression levels in diabetic rats' testis. Studies have indicated that the conditioned media derived from MSCs have anti-inflammatory properties, reducing the expression of pro-inflammatory molecules, such as TNF-α, IL-1, and IL-6 in various tissues [18, 66].
The present study found that early and late administration of WJMSCs-CM and insulin reduced testicular lipid peroxidation and increased antioxidant enzyme activity in diabetic rats. Researchers discovered that major growth factors of conditioned media, such as HGF, FGF2, VEGF, NGF, and BDNF, have antioxidant properties and lower ROS in various tissues.
On the other hand, apoptotic cell death is crucial for testicular injury in diabetic rats [67]. Consistent with previous research [56, 68], our study found a significant increase in mRNA expression of pro-apoptotic factor Bax and a decrease in antiapoptotic factor Bcl2 in the testis of untreated diabetic rats. The ratio of the apoptotic protein Bax to the antiapoptotic protein Bcl-2, which triggers the caspase cleavage cascade, is a critical factor in determining apoptosis [69]. Our findings indicated that early treatment with conditioned media derived by MSCs significantly decreased the Bax/Bcl2 ratio in the testicular tissue of MSCs-CM-treated diabetic rats. MSCs-CM contains secretory factors, including chemokines, cytokines, growth factors, lipids, and free nucleic acids that regulate cellular processes like proliferation, differentiation, immunomodulation, antiapoptotic mechanisms, and anti-inflammatory responses [35, 47]. Studies have shown that growth factors like HGF, BDNF, NGF, and VEGF, present in MSCs-CM, can protect sperm from apoptosis, improve sperm motility and viability, and promote testes recovery by upregulating mRNA and protein expression [47, 51].
This study investigates the impact of diabetes on the testes' histological structure. Consistent with numerous investigations [2, 70, 71], our research showed an increase in interstitial tissue, a decrease in the weight and volume of testes, and a reduction in seminiferous tubule length and volume in diabetic rats. It was found that the length and volume of the tubules are related to the height of the germinal epithelium [72]. Our finding revealed that, in diabetic rats, a reduction in volume seminiferous tubule volume was accompanied by a decrease in germinal epithelium volume, indicating that seminiferous tubules atrophied as diabetes progressed. Diabetes also negatively affects the population of spermatogonia, spermatocytes, spermatids, and Sertoli cells, reducing germinal epithelium volume. A study showed that MSCs-CM can reduce histological changes caused by ischemia and reperfusion [52]. Our study showed that treatment with WJMSCs-CM significantly improved the structural defects in the testis of diabetes rats. This improvement can be attributed to secreted substances by stem cells like growth factors, cytokines, and protective proteins.
5. Conclusion
Our study showed the therapeutic potential of WJMSCS-CM in treating testicular injury and sperm abnormalities caused by diabetes. The anti-inflammatory, antioxidant, and regenerative properties of MSC-derived secretome can effectively reduce diabetes's negative impact on reproductive health. It is noteworthy that growth factors, exosomes/microvesicles, and other biologically active substances of WJMSCs-CM can potentially cause these effects, and one of the limitations of our study was the lack of clarity about the specific bioactive molecules and their amount in the CM that mediate the therapeutic effects on testicular dysfunction caused by diabetes. Future research should focus on identifying these bioactive substances and their molecular mechanisms. In addition, to find the best treatment strategy and optimize its use, it is essential to test various dosages, frequencies, and durations of CM administration. It is recommended that additional studies be done on the combination of SMCs-CM with other methods of therapy for diabetes reproductive disorder.
Overall, the outcomes of our research can provide a basis for future clinical trials and the utilization of WJMSCS-CM as a therapeutic option. This study also demonstrated that early treatment with WJMSCs-CM was more effective than late treatment in ameliorating diabetic male reproductive dysfunction.
Acknowledgments
This study was financially supported by Shiraz University of Medical Sciences as a grant (grant no. 25377), Shiraz, Iran.
Data Availability
All data used to support the findings of this study were analyzed and included in the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest in association with the present study.
Authors' Contributions
Mojtaba Sargazi and Zohre Aghaei contributed to experimental investigation, data collection, and analysis. Narges Karbalaei, Saied Karbalay-Doust, and Sara Keshtgar contributed to the supervision, conception, design of the research, and interpretation of the findings. All authors have read and approved the final manuscript.
References
- 1.Kottaisamy C. P. D., Raj D. S., Kumar V. P., Sankaran U. Experimental animal models for diabetes and its related complications—a review. Laboratory Animal Research . 2021;37(1) doi: 10.1186/s42826-021-00101-4.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He Z., Yin G., Li Q. Q., Zeng Q., Duan J. Diabetes mellitus causes male reproductive dysfunction: a review of the evidence and mechanisms. In Vivo . 2021;35(5):2503–2511. doi: 10.21873/invivo.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maresch C. C., Stute D. C., Alves M. G., Oliveira P. F., de Kretser D. M., Linn T. Diabetes-induced hyperglycemia impairs male reproductive function: a systematic review. Human Reproduction Update . 2018;24(1):86–105. doi: 10.1093/humupd/dmx033. [DOI] [PubMed] [Google Scholar]
- 4.Shi G.-J., Li Z.-M., Zheng J., et al. Diabetes associated with male reproductive system damages: onset of presentation, pathophysiological mechanisms and drug intervention. Biomedicine & Pharmacotherapy . 2017;90:562–574. doi: 10.1016/j.biopha.2017.03.074. [DOI] [PubMed] [Google Scholar]
- 5.Mostafa T., Abdel-Hamid I. A. Ejaculatory dysfunction in men with diabetes mellitus. World Journal of Diabetes . 2021;12(7):954–974. doi: 10.4239/wjd.v12.i7.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali B. R., Alameri A. N., Rumaidh S. Al, Ethaib S. Correlation between reproductive hormones levels and semen quality in patients with diabetes. Journal of Medicine and Life . 2022;15(12):1507–1510. doi: 10.25122/jml-2022-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribas-Maynou J., Yeste M. Oxidative stress in male infertility: causes, effects in assisted reproductive techniques, and protective support of antioxidants. Biology . 2020;9(4) doi: 10.3390/biology9040077.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nna V. U., Abu Bakar A. B., Ahmad A., Eleazu C. O., Mohamed M. Oxidative stress, NF-κB-mediated inflammation and apoptosis in the testes of streptozotocin–induced diabetic rats: combined protective effects of Malaysian propolis and metformin. Antioxidants . 2019;8(10) doi: 10.3390/antiox8100465.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsawy H., Famurewa A. C., Sedky A. Resveratrol mitigates diabetic testicular dysfunction, endocrine deficits, and insulin resistance via suppression of sperm-endocrine aberrations and oxidative inflammation in rats. Andrologia . 2023;2023:10. doi: 10.1155/2023/6385767.6385767 [DOI] [Google Scholar]
- 10.Singh B., Kumar A., Singh H., et al. Zingerone produces antidiabetic effects and attenuates diabetic nephropathy by reducing oxidative stress and overexpression of NF-κB, TNF-α, and COX-2 proteins in rats. Journal of Functional Foods . 2020;74 doi: 10.1016/j.jff.2020.104199.104199 [DOI] [Google Scholar]
- 11.Päth G., Perakakis N., Mantzoros C. S., Seufert J. Stem cells in the treatment of diabetes mellitus—focus on mesenchymal stem cells. Metabolism-Clinical and Experimental . 2019;90:1–15. doi: 10.1016/j.metabol.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Yousef H. N., Sakr S. M., Sabry S. A. Mesenchymal stem cells ameliorate hyperglycemia in type I diabetic developing male rats. Stem Cells International . 2022;2022:13. doi: 10.1155/2022/7556278.7556278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhury A., Duvoor C., Dendi V. S. R., et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Frontiers in Endocrinology . 2017;8 doi: 10.3389/fendo.2017.00006.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nooshabadi V. T., Mardpour S., Yousefi-Ahmadipour A., et al. The extracellular vesicles-derived from mesenchymal stromal cells: a new therapeutic option in regenerative medicine. Journal of Cellular Biochemistry . 2018;119(10):8048–8073. doi: 10.1002/jcb.26726. [DOI] [PubMed] [Google Scholar]
- 15.Puig-Pijuan T., de Godoy M. A., Carvalho L. R. P., et al. Human Wharton’s jelly mesenchymal stem cells protect neural cells from oxidative stress through paracrine mechanisms. Future Science OA . 2020;6(9) doi: 10.2144/fsoa-2020-0036.FSO627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K.-S., Choi Y. K., Kim M. J., et al. Umbilical cord-mesenchymal stem cell-conditioned medium improves insulin resistance in C2C12 cell. Diabetes & Metabolism Journal . 2021;45(2):260–269. doi: 10.4093/dmj.2019.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamal M. M., Kassem D. H. Therapeutic potential of Wharton’s jelly mesenchymal stem cells for diabetes: achievements and challenges. Frontiers in Cell and Developmental Biology . 2020;8 doi: 10.3389/fcell.2020.00016.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aghaei Z., Karbalaei N., Namavar M. R., et al. Neuroprotective effect of Wharton’s jelly-derived mesenchymal stem cell-conditioned medium (WJMSC-CM) on diabetes-associated cognitive impairment by improving oxidative stress, neuroinflammation, and apoptosis. Stem Cells International . 2023;2023:18. doi: 10.1155/2023/7852394.7852394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleem R., Mohamed-Ahmed S., Elnour R., Berggreen E., Mustafa K., Al-Sharabi N. Conditioned medium from bone marrow mesenchymal stem cells restored oxidative stress-related impaired osteogenic differentiation. International Journal of Molecular Sciences . 2021;22(24) doi: 10.3390/ijms222413458.13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y., Mei X., Jiang W., et al. Mesenchymal stem cell-conditioned medium protects hippocampal neurons from radiation damage by suppressing oxidative stress and apoptosis. Dose-Response . 2021;19(1):1–10. doi: 10.1177/1559325820984944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J. Y., Zhang Y. F., Song X. J., Zhu J., Zhu Q. S. The healing effects of conditioned medium derived from mesenchymal stem cells on radiation-induced skin wounds in rats. Cell Transplantation . 2019;28(1):105–115. doi: 10.1177/0963689718807410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo S.-C., Chio C.-C., Yeh C.-H., et al. Mesenchymal stem cell-conditioned medium attenuates the retinal pathology in amyloid-β-induced rat model of Alzheimer’s disease: underlying mechanisms. Aging Cell . 2021;20(5) doi: 10.1111/acel.13340.e13340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acuto S., Iacono M. L., Baiamonte E., Re R. L., Maggio A., Cavalieri V. An optimized procedure for preparation of conditioned medium from Wharton’s jelly mesenchymal stromal cells isolated from umbilical cord. Frontiers in Molecular Biosciences . 2023;10 doi: 10.3389/fmolb.2023.1273814.1273814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian Q., Zhu N., Li W., et al. Human umbilical mesenchymal stem cells-derived microvesicles attenuate formation of hypertrophic scar through multiple mechanisms. Stem Cells International . 2023;2023:15. doi: 10.1155/2023/9125265.9125265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Can A., Celikkan F. T., Cinar O. Umbilical cord mesenchymal stromal cell transplantations: a systemic analysis of clinical trials. Cytotherapy . 2017;19(12):1351–1382. doi: 10.1016/j.jcyt.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Gaafar T., Attia W., Mahmoud S., et al. Cardioprotective effects of Wharton jelly derived mesenchymal stem cell transplantation in a rodent model of myocardial injury. International Journal of Stem Cells . 2017;10(1):48–59. doi: 10.15283/ijsc16063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayatollahi M., Talaei-Khozani T., Razmkhah M. Growth suppression effect of human mesenchymal stem cells from bone marrow, adipose tissue, and Wharton’s jelly of umbilical cord on PBMCs. Iranian Journal of Basic Medical Sciences . 2016;19(2):145–153. [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan Y., Shi M., Li L., et al. Mesenchymal stem cell-conditioned media ameliorate diabetic endothelial dysfunction by improving mitochondrial bioenergetics via the Sirt1/AMPK/PGC-1α pathway. Clinical Science . 2016;130(23):2181–2198. doi: 10.1042/CS20160235. [DOI] [PubMed] [Google Scholar]
- 29.O. World Health. WHO Laboratory Manual for the Examination and Processing of Human Semen . 6th. Geneva: World Health Organization; 2021. [Google Scholar]
- 30.Karbalay-Doust S., Noorafshan A. Ameliorative effects of curcumin on the spermatozoon tail length, count, motility and testosterone serum level in metronidazole-treated mice. Prague Medical Report . 2011;112(4):288–297. [PubMed] [Google Scholar]
- 31.O. World Health. WHO Laboratory Manual for the Examination and Processing of Human Semen . Geneva: World Health Organization; 2010. [Google Scholar]
- 32.Abbasi M., Alizadeh R., Abolhassani F., et al. Aminoguanidine improves epididymal sperm parameters in varicocelized rats. Urologia Internationalis . 2011;86(3):302–306. doi: 10.1159/000322154. [DOI] [PubMed] [Google Scholar]
- 33.Aminsharifi A., Monsef A., Noorafshan A., et al. Effects of intratesticular hematoma on testis microstructure, spermatogenesis, and testosterone production: defining a cutoff point for significant intratesticular hematoma. Urology . 2018;118:80–86. doi: 10.1016/j.urology.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Jing Y., Liang W., Zhang L., Tang J., Huang Z. The role of mesenchymal stem cells in the induction of cancer-stem cell phenotype. Frontiers in Oncology . 2022;12 doi: 10.3389/fonc.2022.817971.817971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng B.-Y., Dubey N. K., Mishra V. K., et al. Addressing stem cell therapeutic approaches in pathobiology of diabetes and its complications. Journal of Diabetes Research . 2018;2018:16. doi: 10.1155/2018/7806435.7806435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nahdi A. M. A. T., John A., Raza H. Elucidation of molecular mechanisms of streptozotocin-induced oxidative stress, apoptosis, and mitochondrial dysfunction in Rin-5F pancreatic β -cells. Oxidative Medicine and Cellular Longevity . 2017;2017:15. doi: 10.1155/2017/7054272.7054272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nugroho W. S., Kusindarta D. L., Susetya H., et al. The structural and functional recovery of pancreatic β-cells in type 1 diabetes mellitus induced mesenchymal stem cell-conditioned medium. Veterinary World . 2016;9(5) doi: 10.14202/vetworld.2016.535-539.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahangarpour A., Oroojan A. A., Heidari H., Ehsan G., Nooshabadi M. R. R. Effects of hydro-alcoholic extract of Rhus coriaria (Sumac) seeds on reproductive complications of nicotinamide-streptozotocin induced type-2 diabetes in male mice. The World Journal of Men’s Health . 2014;32(3):151–158. doi: 10.5534/wjmh.2014.32.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashid K., Sil P. C. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochimica et Biophysica Acta (BBA)–Molecular Basis of Disease . 2015;1852:70–82. doi: 10.1016/j.bbadis.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Zheng H., Hu Y., Shao M., Chen S., Qi S. Chromium picolinate protects against testicular damage in STZ-induced diabetic rats via anti-inflammation, anti-oxidation, inhibiting apoptosis, and regulating the TGF-β1/smad pathway. Molecules . 2023;28(22) doi: 10.3390/molecules28227669.7669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oskuye Z. Z., Bavil F. M., Hamidian G. R., et al. Troxerutin affects the male fertility in prepubertal type 1 diabetic male rats. Iranian Journal of Basic Medical Sciences . 2019;22(2):197–205. doi: 10.22038/ijbms.2018.32678.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gholizadeh F., Dastghaib S., Koohpeyma F., Bayat E., Mokarram P. The protective effect of Stevia rebaudiana Bertoni on serum hormone levels, key steroidogenesis enzymes, and testicular damage in testes of diabetic rats. Acta Histochemica . 2019;121(7):833–840. doi: 10.1016/j.acthis.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Khoei H. H., Fakhri S., Parvardeh S., et al. Testicular toxicity and reproductive performance of streptozotocin-induced diabetic male rats: the ameliorating role of silymarin as an antioxidant. Toxin Reviews . 2019;38(3):223–233. doi: 10.1080/15569543.2018.1444641. [DOI] [Google Scholar]
- 44.La Vignera S., Condorelli R., Vicari E., D’Agata R., Calogero A. E. Diabetes mellitus and sperm parameters. Journal of Andrology . 2012;33(2):145–153. doi: 10.2164/jandrol.111.013193. [DOI] [PubMed] [Google Scholar]
- 45.Andlib N., Sajad M., Kumar R., Thakur S. C. Abnormalities in sex hormones and sexual dysfunction in males with diabetes mellitus: a mechanistic insight. Acta Histochemica . 2023;125(1) doi: 10.1016/j.acthis.2022.151974.151974 [DOI] [PubMed] [Google Scholar]
- 46.Grande G., Barrachina F., Soler-Ventura A., et al. The role of testosterone in spermatogenesis: lessons from proteome profiling of human spermatozoa in testosterone deficiency. Frontiers in Endocrinology . 2022;13 doi: 10.3389/fendo.2022.852661.852661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammed S. S., Mansour M. F., Salem N. A. Therapeutic effect of stem cells on male infertility in a rat model: histological, molecular, biochemical, and functional study. Stem Cells International . 2021;2021:13. doi: 10.1155/2021/8450721.8450721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daneshmandi L., Shah S., Jafari T., et al. Emergence of the stem cell secretome in regenerative engineering. Trends in Biotechnology . 2020;38(12):1373–1384. doi: 10.1016/j.tibtech.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drobiova H., Sindhu S., Ahmad R., Haddad D., Al-Mulla F., Al Madhoun A. Wharton’s jelly mesenchymal stem cells: a concise review of their secretome and prospective clinical applications. Frontiers in Cell and Developmental Biology . 2023;11 doi: 10.3389/fcell.2023.1211217.1211217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sobolewski K., Małkowski A., Bańkowski E., Jaworski S. Wharton’s jelly as a reservoir of peptide growth factors. Placenta . 2005;26(10):747–752. doi: 10.1016/j.placenta.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Nafchi H. G., Azizi Y., Halvaei I. The role of growth factors in human sperm parameters: a review of in vitro studies. International Journal of Reproductive BioMedicine . 2022;20(10):807–818. doi: 10.18502/ijrm.v20i10.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharifian P., Yari S., Hasanein P., Nezhad Y. M. Conditioned medium of bone marrow mesenchymal stem cells improves sperm parameters and reduces histological alteration in rat testicular ischaemia/reperfusion model. Andrologia . 2022;54(11) doi: 10.1111/and.14624.e14624 [DOI] [PubMed] [Google Scholar]
- 53.Ferraguti G., Fanfarillo F., Tarani L., et al. NGF and the male reproductive system: potential clinical applications in infertility. International Journal of Molecular Sciences . 2022;23(21) doi: 10.3390/ijms232113127.13127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alkafagi A. S., Kadhum W. M., Abbas A. G. The impact of transforming growth factor-b1 on the men infertility. Journal of Physics: Conference Series . 2021;1999012029 [Google Scholar]
- 55.Aitken R. J., Roman S. D. Antioxidant systems and oxidative stress in the testes. Oxidative Medicine and Cellular Longevity . 2008;1(1):15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nna V. U., Bakar A. B. A., Ahmad A., Mohamed M. Diabetes-induced testicular oxidative stress, inflammation, and caspase-dependent apoptosis: the protective role of metformin. Archives of Physiology and Biochemistry . 2020;126(5):377–388. doi: 10.1080/13813455.2018.1543329. [DOI] [PubMed] [Google Scholar]
- 57.Zickri M. B., Moustafa M. H., Fasseh A. E.-E., Kamar S. S. Antioxidant and antiapoptotic paracrine effects of mesenchymal stem cells on spermatogenic arrest in oligospermia rat model. Annals of Anatomy–Anatomischer Anzeiger . 2021;237 doi: 10.1016/j.aanat.2021.151750.151750 [DOI] [PubMed] [Google Scholar]
- 58.Zha W., Bai Y., Xu L., et al. Curcumin attenuates testicular injury in rats with streptozotocin-induced diabetes. BioMed Research International . 2018;2018:10. doi: 10.1155/2018/7468019.7468019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghosh S., Chowdhury S., Das A. K., Sil P. C. Taurine ameliorates oxidative stress induced inflammation and ER stress mediated testicular damage in STZ-induced diabetic Wistar rats. Food and Chemical Toxicology . 2019;124:64–80. doi: 10.1016/j.fct.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 60.Forrester S. J., Kikuchi D. S., Hernandes M. S., Xu Q., Griendling K. K. Reactive oxygen species in metabolic and inflammatory signaling. Circulation Research . 2018;122(6):877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar P., Rao G. N., Pal B. B., Pal A. Hyperglycemia-induced oxidative stress induces apoptosis by inhibiting PI3-kinase/Akt and ERK1/2 MAPK mediated signaling pathway causing downregulation of 8-oxoG-DNA glycosylase levels in glial cells. The International Journal of Biochemistry & Cell Biology . 2014;53:302–319. doi: 10.1016/j.biocel.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 62.González P., Lozano P., Ros G., Solano F. Hyperglycemia and oxidative stress: an integral, updated and critical overview of their metabolic interconnections. International Journal of Molecular Sciences . 2023;24(11) doi: 10.3390/ijms24119352.9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischer R., Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxidative Medicine and Cellular Longevity . 2015;2015:18. doi: 10.1155/2015/610813.610813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim W.-S., Park B.-S., Kim H.-K., et al. Evidence supporting antioxidant action of adipose-derived stem cells: protection of human dermal fibroblasts from oxidative stress. Journal of Dermatological Science . 2008;49(2):133–142. doi: 10.1016/j.jdermsci.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Huang H. W., Yang C. M., Yang C. H. Fibroblast growth factor type 1 ameliorates high-glucose-induced oxidative stress and neuroinflammation in retinal pigment epithelial cells and a streptozotocin-induced diabetic rat model. International Journal of Molecular Sciences . 2021;22(13) doi: 10.3390/ijms22137233.7233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ibrahim M. A., Khalifa A. M., Mohamed A. A., et al. Bone-marrow-derived mesenchymal stem cells, their conditioned media, and olive leaf extract protect against cisplatin-induced toxicity by alleviating oxidative stress, inflammation, and apoptosis in rats. Toxics . 2022;10(9) doi: 10.3390/toxics10090526.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aly H. A. A. Mitochondria-mediated apoptosis induced testicular dysfunction in diabetic rats: ameliorative effect of resveratrol. Endocrinology . 2021;162(4) doi: 10.1210/endocr/bqab018.bqab018 [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y., Song W., Wang Z., et al. Resveratrol attenuates testicular apoptosis in type 1 diabetic mice: Role of Akt-mediated Nrf2 activation and p62-dependent Keap1 degradation. Redox Biology . 2018;14:609–617. doi: 10.1016/j.redox.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yuan J., Horvitz H. R. A first insight into the molecular mechanisms of apoptosis. Cell . 2004;116(2 Suppl):S53–S56. doi: 10.1016/S0092-8674(04)00028-5. [DOI] [PubMed] [Google Scholar]
- 70.Mardanshahi T., Rezaei N., Zare Z., Shafaroudi M. M., Mohammadi H. Effects of L-Carnitine on the sperm parameters disorders, apoptosis of spermatogenic cells and testis histopathology in diabetic Rats. International Journal of Reproductive BioMedicine . 2019;17(5):325–36. doi: 10.18502/ijrm.v17i5.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barsiah S., Behnam-Rassouli M., Shahabipour F., et al. Evaluation of testis hormonal and histopathological alterations in type I and type II diabetic rats. Journal of Cellular Biochemistry . 2019;120(10):16775–16785. doi: 10.1002/jcb.28936. [DOI] [PubMed] [Google Scholar]
- 72.Sadeghinezhad J., Yarmahmoudi F., Dehghan M. M., et al. Stereological study of testes following experimentally-induced unilateral cryptorchidism in rats. Clinical and Experimental Reproductive Medicine . 2023;50(3):160–169. doi: 10.5653/cerm.2023.06058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study were analyzed and included in the article.


