Abstract
Many patients with cancer experience cancer-related cognitive decline (CRCD). Previous studies have shown that elevated S100β, a calcium-binding protein commonly found in glial cells, can exhibit neurotoxic effects, including disruption of the blood-brain barrier (BBB). We studied changes in S100β levels in patients with breast cancer receiving chemotherapy, and the relationship to changes in cognitive function. A total of 505 women with breast cancer (mean (sd) age; 53.4 (53.6)) and 336 age-matched controls without cancer (52.8 (10.3)) were included from a nationwide study as part of the National Cancer Institute Community Oncology Research Program (NCORP). Both groups provided blood samples and completed neurocognitive assessments within 7 days before the patients with breast cancer received their first chemotherapy dose (pre-chemotherapy; T1) and within 1 month of their last chemotherapy administration (post-chemotherapy; T2). Utilizing a linear mixed model, multivariate linear regressions, and Spearman rank correlations (rs), we investigated longitudinal changes in serum S100β concentrations and their relationships to changes in neurocognitive outcomes over time. We observed an increase in S100β for patients with breast cancer (p = 0.002), but not for controls without cancer over time (p = 0.683). Additionally, we identified subtle relationships between increases in serum S100β and worsening in cognitive performance on the Backward Counting test (rs = 0.11, p = 0.041) and self-reported FACT-Cog Perceived Cognitive Abilities (rs = −0.10, p = 0.025). Regression analyses adjusted for age, race, body-mass index (BMI), education, menopausal status, anxiety, and depression revealed a trend remained for the relationship of S100β with Backward Counting. In conclusion, we found that patients with breast cancer experience a significant increase in concentration of serum S100β over the course of chemotherapy. This increase is correlated with worsening in some neurocognitive outcomes from pre-to post-chemotherapy, with trending results remaining following adjustment for covariates.
Keywords: S100, S100β, Cognition, Chemotherapy, Breast cancer, Cancer, Cognitive decline, Cancer-related cognitive decline, Blood-brain barrier integrity
Highlights
-
•
75% of patients experience deficits related to cancer-related cognitive decline.
-
•
S100β plays a role in various diseases associated with poor neurocognitive outcomes.
-
•
No studies examine S100β in the context of chemotherapy and cognition in humans.
-
•
Women with breast cancer experience a significant increase in serum S100β.
-
•
Increases in S100β illicit mild negative associations to neurocognitive outcomes.
1. Introduction
In 2023, the American Cancer Society anticipated 1.9 million new instances of cancer in the United States (Siegel et al., 2023), adding to the already 18.1 million cancer survivors nationwide in 2022 ('Statistics, Graphs and Definitions | Division of Cancer Control and Population Sciences (Statistics, Graphs and Definitions, 2022)). Cancer-related cognitive decline (CRCD), more commonly known as “chemo-brain” in the setting of chemotherapy, is an important clinical problem in which patients experience deficits in cognitive domains including memory, attention, and executive function (Yang and Hendrix 2018). A majority of studies on CRCD prevalence have reported rates ranging from 15% to 75% of patients during treatment, with anywhere from 15% to 35% of patients experiencing longer-lasting cognitive deficits post-treatment (Areklett et al., 2022). This is notable as the 15%–35% of cancer patients (across all ages) estimated to have long-term CRCD is higher than the 12% of older adults (65 and older) in the US diagnosed with memory-related disorders (MRD) such as dementia and Alzheimer's Disease, for example (Qian et al., 2021).
The biological mechanisms underlying CRCD are not clearly understood, yet identifying these mechanisms would help inform the development of novel interventions to alleviate symptoms and provide possible biomarkers to help identify patients at risk of cognitive decline. We previously found that serum biomarkers of inflammation were significantly associated with worse cognitive performance in women with breast cancer in our cohort (Janelsins et al., 2022; Belcher et al., 2022). It is not clear if this inflammatory response contributes to a central nervous system (CNS)-mediated response, either directly or indirectly. Recently, our group investigated molecular signatures associated with CRCD to assess the impact of cancer and chemotherapy on the CNS in a pre-clinical model. We found neuroinflammation following systemic chemotherapy, suggesting a possible role for chemotherapy in CNS neurotoxicity (Netherby-Winslow et al., 2023). We hypothesize that one mechanism that may contribute to this finding is weakening of the blood-brain barrier (BBB).
S100β found in serum in patients is a promising biomarker of potential neural injury. Specifically, S100β is a calcium-binding protein found primarily in glial cells (astroglia and Schwann cells) that aids in the development of the CNS and recovery after neuronal injury (Yardan et al., 2011). Although the family of S100 proteins maintains several purposes, the function of S100β varies depending on its concentration. At nanomolar concentrations, S100β has regulatory purposes and promotes growth and survival of neurons in the cortex during CNS development (Yardan et al., 2011; Astrand et al., 2013). At micromolar concentrations, S100β can promote expression of inflammatory cytokines – such as Interleukin 6 (IL-6) – which can induce apoptosis of neurons in the CNS (Yardan et al., 2011; Astrand et al., 2013).
S100β has previously been studied in cancer, but few studies have focused on chemotherapy for breast cancer. In patients with cancers such as melanoma and lung cancer, serum S100β has been shown to be associated with poorer prognoses and increased likelihood of relapse and metastasis, including brain metastasis (Tarhini et al., 2009; Janka et al., 2021; Chen et al., 2019; Choi et al., 2016). Yen et al. found a role of tissue cultured S100β as a marker for degree of metastasis in breast cancer (Yen et al., 2018). A small study conducted on lymphoma found that patients treated with hyperosmotic BBB disruption and chemotherapy also had elevated levels of serum S100β, further suggesting its role as a marker for BBB function (Kanner et al., 2003). Although S100β normally has low expression in serum, it may serve as a biomarker in CRCD due to its associations with BBB integrity following glial response to injury (Kleindienst and Bullock 2006; Kadry et al., 2020; Oris et al., 2021). Herein we investigate S100β as a potential biomarker of CRCD symptoms due to its associations with BBB disruption.
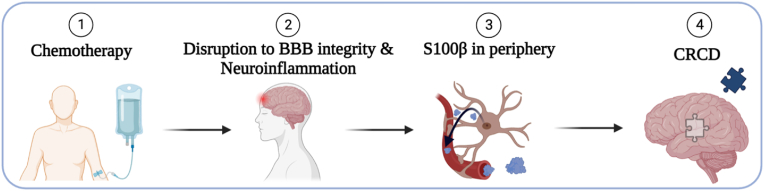
Elevated levels of S100β have been shown to be related to worse cognitive performance in patients with dementia and Alzheimer's disease (Chaves et al., 2010), ischemic stroke (Li et al., 2023), chronic stress-induced exhaustion disorder (Wallensten et al., 2022), and type-2 diabetes (Yu et al., 2020), amongst other disorders (Chen et al., 2017; Jin et al., 2023; Polyakova et al., 2022; Prohl et al., 2007; Park et al., 2020). Currently, however, little information is available correlating biomarker concentrations with patient outcomes, specifically in the context of CRCD. We hypothesized that S100β levels would increase in patients with breast cancer from pre-to post-chemotherapy, and that a greater increase in serum S100β levels would be correlated to worse cognitive functioning (Fig. 1). We assessed longitudinal changes in serum S100β levels in a nationwide cohort of breast cancer survivors receiving chemotherapy compared to individuals without cancer serving as controls assessed at equivalent times. We also report the relationship between serum S100β concentrations with changes in cognitive outcomes from pre-chemotherapy to post-chemotherapy in breast cancer patients and at equivalent times for controls without cancer.
Fig. 1.
Proposed mechanism of cancer-related cognitive decline (CRCD).
2. Methods
2.1. Participants and study design
Patients with breast cancer and age-matched individuals without cancer serving as controls were recruited from 22 National Cancer Institute Community Oncology Research Program (NCORP) locations nationwide for a prospective longitudinal cohort study with the primary objective of assessing CRCD (URCC10055) (Janelsins et al., 2018). The methods of this study have been previously described by prior work from our group (Janelsins et al., 2018). Briefly, eligibility for patients with breast cancer included women with a confirmed non-metastatic breast cancer diagnosis Stage I-IIIC who were scheduled to begin chemotherapy, chemotherapy naïve, age ≥21 years, no CNS disease, no neurodegenerative disease, no recent major psychiatric illness leading to hospitalization, and no plan to receive concurrent radiation therapy from pre-to post-chemotherapy. Similarly, age-matched (within 5-years) female control participants without cancer met all eligibility criteria except the first two (Janelsins et al., 2018).
This study was approved by the University of Rochester institutional review board (IRB) and the IRB of each NCORP; all participants provided informed consent. Both groups provided blood samples and completed neurocognitive assessments within 7 days before the patients with breast cancer received their first chemotherapy administration (pre-chemotherapy; T1) and within 1 month of their last chemotherapy administration (post-chemotherapy; T2) (Janelsins et al., 2018). Participants self-reported demographic information via on-study forms, and relevant clinical information was abstracted from the participants’ medical records.
964 patients were originally consented from previous research by our group (Janelsins et al., 2018), 21 of whom were removed before baseline analysis due to being overwhelmed or having a medical issue. Of the 943 patients used for analysis, we included 580 patients with breast cancer and 363 controls. 505 and 336, respectively, provided a serum sample at both timepoints and are included in the analysis of this current investigation (Fig. 2).
Fig. 2.
Consort diagram of study participants used for analysis. 964 patients were originally consented for an investigation previously done by our group (Janelsins et al., 2018), but were omitted from analysis for being overwhelmed (n = 16) or due to a medical issue (n = 5).
2.2. Serum sample collection & analysis
Serum was stored at -80°C until analysis. Serum S100β concentrations (pg/mL) were analyzed using Millipore Sigma enzyme-linked immunosorbent assays (ELISA) kits (EZHS100Β−33K) at the Cancer Control and Psychoneuroimmunology Lab. Serum available at T1 and T2 from the same patient were included on the same 96-well plate and run in duplicate for each sample. We reported the average concentration of duplicate samples. Samples with coefficients of variability (CV) of over 20% were assayed a second time to get appropriate serum S100β concentrations if possible, or excluded from analyses if the CV was >20%. Final serum S100β concentrations used for analysis had a CV below the 20% threshold and no values were below the lower limit of detection (LLOD).
2.3. Neurocognitive assessments
Participants completed a standard neurocognitive battery including computerized, paper, and phone measures of memory, attention, and executive function, as previously reported (Janelsins et al., 2018). For the current exploratory analyses, to minimize multiple comparisons, we included neurocognitive outcomes where patients performed significantly worse than controls pre-to post-chemotherapy (T1 to T2) in our previous research (Janelsins et al., 2018, Janelsins et al., 2022). Computerized cognitive assessments included items from the Cambridge Neuropsychological Test Battery (CANTAB) (Vardy et al., 2015; Russo et al., 2003; Fray and Robbins, 1996; Robbins et al., 1994; Sahakian and Owen, 1992): the Verbal Recognition Memory (VRM) task (Verbal Recognition Memory (VRM), n.d.) and the One Touch Stockings of Cambridge (OTS) (One Touch Stockings of Cambridge (OTS), n.d.). Paper-based cognitive assessments included the Hopkins Verbal Learning Test-Revised (HVLT-R) for both immediate and delayed recall (Benedict et al., 1991; Brandt and Benedict, 2001), the Trail Making Test – Part A (TMT-A) (Ciolek and Lee 2020; Bowie and Harvey, 2006; Reitan, 1958), and the Controlled Oral Word Association test (COWA) (Kemenoff et al., 2002; Benton and Sivan, 1978; Ruff et al., 1996; Lezak et al., 2004). Telephone-based cognitive assessments included the Brief Test of Adult Cognition by Telephone (BTACT); namely Backward Counting, Digits Backward, and Category Fluency tests (Lachman et al., 2014; Tun and Lachman, 2006).
2.4. Patient-reported outcomes
On a Likert scale (0–10), participants self-reported severity of individual symptoms via single-items on a symptom inventory, including remembering things, concentration, paying attention, ability to multitask, and ability to plan things, among other items. Additionally, we used four sub-scales of the self-reported Functional Assessment of Cancer Therapy – Cognitive Function (FACT-Cog) questionnaire: Perceived Cognitive Impairments (PCI), Comments from Others, Perceived Cognitive Abilities (PCA), and Impact on Quality of Life (QOL). Subsequently, we calculated a cumulative FACT-Cog Total score by summing all four sub-scales for each patient (FACT-Cog. Functional Assessment of Cancer Therapy – Cognitive Function, n.d.).
Participants also self-reported levels of anxiety via the State-Trait Anxiety Inventory for Adults (STAI) (Spielberger and Gorsuch 1983). Additionally, we collected participants’ self-reported ratings of depression via Question 21 (“I feel depressed”) of the Multidimensional Fatigue Symptom Inventory – Short Form (MFSI-SF) (Stein et al. 1998, 2004).
2.5. Statistical analysis
Baseline differences in demographic characteristics between patients with breast cancer and controls without cancer were compared using chi-squared tests (Table 1). Because the distribution of serum S100β concentrations was skewed, the serum S100β concentration transformed by the natural logarithm values were used for any statistical modeling or hypothesis testing. Tabulated and reported means and standard deviations of serum S100β concentrations are based on original values before transformation.
Table 1.
Demographic and clinical characteristics of URCC 10055 nationwide longitudinal cohort participants.
| Characteristic | Count (%) |
a p-value | |
|---|---|---|---|
| Patients |
Controls |
||
| n = 505 | n = 336 | ||
| RACE | 0.009 | ||
| White | 454 (89.9) | 319 (94.9) | |
| Non-White | 51 (10.1) | 17 (5.1) | |
| AGE | |||
| ≥65 | 81 (16.0) | 44 (13.1) | 0.240 |
| <65 | 424 (84.0) | 292 (86.9) | |
| Mean ± SD | 53.4 ± 10.6 | 52.8 ± 10.3 | |
| MENOPAUSAL STATUS | 0.043 | ||
| Premenopausal | 157 (31.1) | 93 (27.7) | |
| Perimenopausal | 35 (6.9) | 42 (12.5) | |
| Postmenopausal | 268 (53.1) | 168 (50.0) | |
| Medically Induced | 45 (8.9) | 33 (9.8) | |
| MARITAL STATUS | 0.300 | ||
| Single | 37 (7.3) | 28 (8.3) | |
| Widowed | 26 (5.1) | 15 (4.5) | |
| Divorced/Separated | 74 (14.7) | 35 (10.4) | |
| Married/Living as Married | 368 (72.9) | 258 (76.8) | |
| EDUCATION | <0.001 | ||
| High School GED or Lower | 123 (24.4) | 39 (11.6) | |
| Partial College/University | 171 (33.9) | 144 (42.9) | |
| College/University | 119 (23.6) | 104 (31.0) | |
| Graduate School | 92 (18.2) | 49 (14.6) | |
| BODY-MASS INDEX (BMI) | 0.005 | ||
| Underweight/Healthy | 142 (28.1) | 105 (31.3) | |
| Overweight | 124 (24.6) | 109 (32.4) | |
| Obese | 239 (47.3) | 121 (36.0) | |
| Unknown | 0 (0.0) | 1 (0.3) | |
| ANXIETY (STAI) | <0.001 | ||
| Mean ± SD | 35.6 ± 12.1 | 28.1 ± 9.2 | |
| DEPRESSION (Q21, MFSI-SF) | <0.001 | ||
| Mean ± SD | 0.6 ± 0.9 | 0.4 ± 0.8 | |
| STAGE | |||
| 1 | 134 (26.5) | ||
| 2 | 255 (50.5) | ||
| 3 | 95 (18.8) | ||
| Unknown | 21 (4.2) | ||
| REGIMEN | |||
| Anthracycline | 242 (47.9) | ||
| Non-Anthracycline | 261 (51.7) | ||
| Unknown | 2 (0.4) | ||
| SMOKING STATUS | |||
| Non-Smoker | 43 (8.5) | ||
| Former Smoker | 108 (21.4) | ||
| Current Smoker | 63 (12.5) | ||
| Unknown | 291 (57.6) | ||
NOTE: Stage, treatment regimen, and smoking status data were unavailable for controls without cancer.
P-values were calculated using Chi-Squared test.
Differences in baseline serum S100β concentrations across demographic characteristics (at T1) for patients with breast cancer were compared using linear regression models, where characteristic data served as the predictor of the outcome for serum S100β concentration at baseline. Linear mixed models were used to evaluate group differences between patients and controls in log-transformed serum S100β concentrations at T1 and T2. For our change analysis, we calculated ΔS100β by subtracted participant serum S100β concentration at T2 by their serum S100β concentration at T1. A linear mixed model was then used to evaluate whether there were group differences between patients and controls in how much serum S100β concentrations changed from T1 to T2. As this is an analysis of a prospective observational study of parallel cohorts of patients with breast cancer undergoing chemotherapy compared to individuals without cancer, a linear mixed model of log transformed S100β was used to analyze differences of biomarker concentration over time from T1 to T2 within each group separately.
Spearman Rank Correlations were used to assess the strength and direction of associations between changes in serum S100β concentrations and neurocognitive outcomes. Multivariate linear regression models were conducted to estimate the magnitude of effect for changes in cognitive outcomes associated with changes in serum S100β concentration while adjusting for covariates including age, race, body mass index (BMI), education, menopausal status, anxiety, and depression, as these are related to S100β concentrations (Oris et al., 2021; Gannon et al., 2020; Steiner et al., 2010; Arora et al., 2019; Schroeter et al., 2013; Navinés et al., 2022; Arolt et al., 2003) and/or were characteristics that exhibited statistically significant differences between groups at baseline (Table 1).
This is an exploratory analysis focused on identifying whether indicators of blood-brain barrier damage may be associated with CRCD. Analyses were considered hypothesis generating. Therefore, we did not adjust for multiple comparisons, and any associations noted should be interpreted cautiously. Additionally, a complete-case analysis was used to handle missing neurocognitive data. All statistical data analyses were performed in R Statistical Software (v.4.2.2; R Core Team, 2022) and RStudio (v.2023.6.1.524; RStudio Team, 2023).
3. Results
Characteristics of The Study Population. Retention of participants was 87.1% for patients with breast cancer and 92.6% for controls without cancer. All analyses contained all available data. Missing data were not common, except with the Brief Test of Adult Cognition by Telephone (BTACT), as a result of inability to contact participants by telephone; we had 132 participants without available phone-based cognitive assessment data for at least one timepoint.
Baseline Characteristics. Baseline characteristics at T1 showed that there were more non-white patients with breast cancer compared with controls (p = 0.009, Table 1). Controls without cancer had more participants who were perimenopausal (p = 0.043, Table 1). Also, we recruited more high school-educated patients with cancer (p <0.001, Table 1). Finally, based on BMI, more patients with breast cancer were considered obese compared to their control counterparts (p = 0.005, Table 1). Participant characteristics were not different – where groups showed no statistically significant differences – in respect to age (mean age at T1 (sd); Patients with breast cancer: 53.4 (10.6); Controls without cancer: 52.8 (10.3)) and marital status (Table 1). This was expected as the controls without cancer were age-matched to the first 363 patients with breast cancer recruited and consented to the study. However, another 217 patients with breast cancer were recruited after this age-matching process. For further analysis of the baseline participant demographic characteristics, refer to Table 1. Of the 580 patients with breast cancer, we retained 505 subjects who provided serum at both timepoints; leaving 75 patients that were not included in our analyses. Supplemental Table 1 provides a comparison of demographic and clinic characteristics of patients with breast cancer that were retained in our analyses compared to those that were not retained. Of note, more patients with breast cancer that were not retained in our analyses were missing information regarding their treatment regimen (p <0.001, Supplemental Table 1). There were differences between those retained compared to those not retained in our analyses in regard to the stage of their cancer at baseline, however this did not reach the threshold of significance (p = 0.051, Supplemental Table 1). Otherwise, all characteristics between the two groups were similar.
Baseline Serum S100β Concentrations by Patient Characteristics. Baseline S100β concentration was elevated in non-white patients with breast cancer (p <0.001, Table 2) and also in patients categorized as obese (BMI ≥30 kg/m2)(p = 0.043, Table 2). There were no differences in serum S100β concentrations across categories of age, menopausal status, marital status, education, stage, treatment regimen, or smoking status in patients with breast cancer.
Table 2.
S100β concentration by patient with breast cancer characteristics at T1.
| Characteristic | Mean S100β (pg/mL) (SD) | ap-value |
|---|---|---|
| RACE | <0.001 | |
| White | 41.0 (39.3) | |
| Non-White | 64.4 (39.3) | |
| AGE | 0.936 | |
| ≥65 | 40.8 (24.7) | |
| <65 | 43.9 (42.2) | |
| MENOPAUSAL STATUS | 0.203 | |
| Premenopausal | 38.1 (23.3) | |
| Perimenopausal | 44.3 (29.8) | |
| Postmenopausal | 44.1 (31.8) | |
| Medically Induced | 56.9 (96.2) | |
| MARITAL STATUS | 0.099 | |
| Single | 46.4 (27.9) | |
| Widowed | 68.1 (115.1) | |
| Divorced/Separated | 44.5 (30.2) | |
| Married/Living as Married | 41.1 (31.3) | |
| EDUCATION | 0.828 | |
| High School GED or Lower | 41.6 (26.7) | |
| Partial College/University | 46.6 (53.8) | |
| College/University | 43.0 (37.0) | |
| Graduate School | 40.2 (25.6) | |
| BODY-MASS INDEX (BMI) | 0.043 | |
| Underweight/Healthy | 36.6 (17.9) | |
| Overweight | 42.6 (40.5) | |
| Obese | 47.8 (47.9) | |
| STAGE | 0.907 | |
| 1 | 45.8 (59.1) | |
| 2 | 43.0 (32.0) | |
| 3 | 42.4 (27.2) | |
| Unknown | 36.9 (19.8) | |
| REGIMEN | 0.907 | |
| Anthracycline | 42.0 (31.2) | |
| Non-Anthracycline | 44.7 (46.7) | |
| Unknown | 32.3 (19.5) | |
| SMOKING STATUS | 0.977 | |
| Non-Smoker | 46.8 (43.6) | |
| Former Smoker | 39.5 (26.9) | |
| Current Smoker | 46.1 (44.2) | |
| Unknown | 43.7 (42.5) |
p-values are assessing if serum concentration of S100β at timepoint 1 (T1) differ by patient characteristics (e.g., age). Derived from univariate linear regression models with log-transformed concentration of S100β at T1 as the dependent variable (outcome) and each characteristic the independent variable (predictor).
Changes in Serum S100β concentrations in Patients and Controls. There were no statistically significant differences in serum S100β concentrations between patients and controls at either T1 (p = 0.078, Table 3) or T2 (p = 0.917, Table 3). However, patients with breast cancer had a statistically significant increase in serum S100β concentration over time (mean T1 to T2 change in S100β (SE) pg/mL: 3.5 (2.12), p = 0.002, Table 3), while no increase was observed for controls without cancer (-2.4 (2.60), p = 0.683, Table 3) resulting in a significant difference between patients and controls (p = 0.022, Table 3) in change from T1 to T2.
Table 3.
Comparison of S100β concentrations in patients with breast cancer and non-cancer controls.
| Timepoint | Mean S100β (pg/mL) (SE) |
||||||
|---|---|---|---|---|---|---|---|
| S100β |
ln(S100β) |
||||||
| Patients (n = 505) | Controls (n = 336) | Patients – Controls | Patients (n = 505) | Controls (n = 336) | Patients – Controls | p-valuea | |
| Pre-Chemotherapy (T1) | 43.4 (2.01) | 46.2 (2.46) | -2.85 (3.18) | 3.56 (0.027) | 3.63 (0.033) | -0.076 (0.043) | 0.078 |
| Post-Chemotherapy (T2) | 46.9 (2.01) | 43.8 (2.46) | 3.07 (3.18) | 3.63 (0.027) | 3.62 (0.033) | 0.004 (0.043) | 0.917 |
| ΔS100β (T2 – T1) | 3.5 (2.12) | -2.4 (2.60) | 5.92 (3.36) | 0.07 (0.022) | -0.01 (0.027) | 0.080 (0.035) | 0.022 |
| p-valueb | 0.002 | 0.683 | |||||
Between Group linear mixed model of log-transformed S100β.
Within Group linear mixed model of log-transformed S100β.
Changes in Cognitive Function Associated with Changes in Serum S100β concentration in Patients. Using Spearman rank correlations, we found small but significant relationships between changes in serum S100β and changes in some neurocognitive outcomes (Table 4). Increases in S100β concentration over time were associated with worse performance over time on the Backward Counting task (r = 0.110, p = 0.041, Table 4) and FACT-Cog PCA (r = -0.100, p = 0.025, Table 4). After adjusting for age, race, BMI, education, menopausal status, anxiety, and depression in linear regression models, the relationship in changes in serum S100β concentration from T1 to T2 with changes in neurocognitive outcomes were no longer statistically significant; however, association of increased serum S100β concentrations with a worsened backward counting (β = 0.0116, t(373) = 1.839, p = 0.067, Table 4) retained trend in the linear regression model.
Table 4.
Spearman correlation and linear regression model for S100β & neurocognitive outcomes for patients with breast cancer.
| Domain & Test | Better Score | n | Pre- to Post-Chemotherapy (T2 – T1) |
|||
|---|---|---|---|---|---|---|
| Spearman Rank Correlation |
Linear Regression |
|||||
| r | p-value | β [95% CI] | p-value | |||
| Memory | ||||||
| Computer | ||||||
| CANTAB VRM | Higher (+) | 496 | -0.078 | 0.084 | 2.20E-04 [-1.79E-03, 2.23E-03] | 0.830 |
| Paper | ||||||
| HVLT-R (immediate recall) | Higher (+) | 498 | 0.036 | 0.417 | 1.69E-03 [-4.02E-04, 3.77E-03] | 0.113 |
| HVLT-R (delayed recall) | Higher (+) | 489 | 0.026 | 0.560 | -7.72E-06 [-2.97E-03, 2.96E-03] | 0.996 |
| Self-Reported Level of Difficulty | ||||||
| Sing Item Memory | Lower (-) | 497 | 0.062 | 0.170 | 9.50E-04 [-2.82E-03, 4.72E-03] | 0.621 |
| Attention | ||||||
| Paper | ||||||
| TMT A (CTMT 1) | Lower (-) | 496 | 0.018 | 0.697 | 1.27E-02 [-8.57E-03, 3.40E-02] | 0.241 |
| Telephone | ||||||
| Backward Counting | Lower (-) | 373 | 0.110 | 0.041 | 1.16E-02 [-8.06E-04, 2.40E-02] | 0.067 |
| Self-Reported Level of Difficulty | ||||||
| Single Item Attention | Lower (-) | 496 | 0.066 | 0.144 | 8.03E-04 [-3.11E-03, 4.72E-03] | 0.687 |
| Executive Function | ||||||
| Computer | ||||||
| CANTAB OTS of Cambridge | Lower (-) | 494 | 0.019 | 0.680 | 9.32E-05 [-3.15E-04, 5.01E-04] | 0.654 |
| Paper | ||||||
| COWA | Higher (+) | 497 | -0.072 | 0.108 | -2.64E-03 [-6.69E-03, 1.41E-03] | 0.201 |
| Telephone | ||||||
| Digits Backward | Higher (+) | 373 | -0.015 | 0.775 | 3.59E-05 [-2.48E-03, 2.55E-03] | 0.978 |
| Category Fluency | Higher (+) | 373 | 0.006 | 0.916 | 6.74E-04 [-5.53E-03, 6.87E-03] | 0.831 |
| Self-Reported Level of Difficulty | ||||||
| Single Item Executive Function | Lower (-) | 496 | 0.077 | 0.088 | 9.70E-04 [-3.37E-03, 5.31E-03] | 0.661 |
| Self-Reported (FACT-Cog) | ||||||
| Perceived Cognitive Impairments | Higher (+) | 499 | -0.038 | 0.397 | -2.04E-02 [-5.16E-02, 1.08E-02] | 0.200 |
| Comments from Others | Higher (+) | 498 | -0.042 | 0.350 | -3.06E-03 [-7.13E-03, 1.01E-03] | 0.140 |
| Perceived Cognitive Abilities | Higher (+) | 499 | -0.100 | 0.025 | -1.01E-02 [-2.51E-02, 4.92E-03] | 0.187 |
| Impact on Quality of Life | Higher (+) | 498 | -0.001 | 0.979 | -1.25E-03 [-1.20E-02, 9.51E-03] | 0.820 |
| Total | Higher (+) | 497 | -0.059 | 0.190 | -3.46E-02 [-8.59E-02, 1.67E-02] | 0.186 |
NOTE: Spearman Rank Correlation estimated relationships between changes in serum S100β and changes neurocognitive assessments. Linear regression estimated association of changes in cognitive outcomes with ΔS100β after adjusting for age, race, BMI, education, menopausal status, anxiety, and depression. β (95% CI) & p-values reported from S100β effect.
Abbreviations: 'CANTAB VRM' = Cambridge Neuropsychological Test Automated Battery, Verbal Recognition Memory; 'HVLT-R' = Hopkins Verbal Learning Test-Revised; 'TMT-A' = Trail Making Test (Part A); 'CANTAB OTS' = Cambridge Neuropsychological Test Automated Battery, One Touch Stockings of Cambridge; 'COWA' = Controlled Oral Word Association Test; 'FACT-Cog' = Functional Assessment of Cancer Therapy - Cognitive Function.
4. Discussion
The goal of this study was to assess longitudinal changes in S100β concentration, a marker of neural injury and neuroinflammation, and its correlation with neurocognitive outcomes among patients with breast cancer over time. This is an exploratory hypothesis-generating study, and our findings lend insight into a potential mechanism underlying CRCD experienced by patients with breast cancer. We report that patients with breast cancer experienced a statistically significant increase in concentration of serum S100β during chemotherapy, while controls without cancer did not experience significant change in S100β over the same period of time. This increase in serum S100β for patients with breast cancer had negative associations with some of the neurocognitive outcomes as shown in the spearman rank correlations. However, once adjusted for relevant covariates such as age, race, BMI, education, menopausal status, anxiety, and depression, serum S100β concentration was no longer a statistically significant predictor of cognitive assessment performance. Further investigation of S100β may guide our understanding of its role in CRCD and has the potential to contribute to the development of interventions for various CRCD phenotypes sustained by patients.
We observed significant correlations between S100β and each a backward counting test and patient-reported perceived cognitive abilities, but not for other neurocognitive assessments used in this investigation. However, these associations were no longer statistically significant once we adjusted for covariates in a linear regression model, revealing a trend in the anticipated direction of increased S100β associated with poor neurocognitive outcomes. The goal of the BTACT backward counting task we used is to see how quickly participants can count backwards from 100 by 1s without omitting any numbers in the sequence. Backward counting measures aspects of processing speed, attention, and working memory. Further validation studies are needed to confirm these associations and multiple timepoints are needed to assess the changes of S100β in the context of CRCD.
One possible explanation for the lack of statistical significance in our models is that S100β may be an acute biomarker for CRCD in patients with breast cancer. This is a challenge when interpreting findings in existing literature since there is no optimally identified time window for assessing peripheral levels of S100β which may vary based on the insult studied. For example, Bouvier et al., investigated S100β′s utility for diagnosing concussions in rugby players measuring serum samples at 2 and 36 h after a match. Increases of S100β correctly identified concussion with 100% sensitivity and 81% specificity (Bouvier et al., 2017), respectively. The use of these acute timepoints support the notion that serum S100β may be highest after acute injury. To address this hypothesis, we could study S100β during chemotherapy, just prior and following each chemotherapy infusion.
We focused our investigation on the calcium-binding protein S100β due to its role in various systemic processes once it has entered peripheral blood following CNS injury. Although we did not look at further downstream targets of these metabolic pathways, we acknowledge these may be important factors to study in relation to the role of S100β in CRCD. In the setting of brain injury, disease, or inflammation, extracellular S100β, secreted from astrocytes or released from damaged astrocytes and neurons, can interact with receptor for advanced glycation end products (RAGE): a pathway that regulates the assembly of tight junction proteins of the BBB (Yardan et al., 2011; Krishnan et al., 2020). The resultant hyperpermeability of the BBB can facilitate neurotoxic effects via interaction of multiple biological pathways that can result in deficits characteristic of a number of neurodegenerative diseases, and, for the purposes of this study, CRCD in cancer patients.
The subsequent S100β/RAGE binding can elicit downstream effects of mitogen-activated protein kinase (MAPK) pathways. The MAPK-dependent kinase cascade then promotes further downstream effects such as promoted expression of inflammatory cytokines, chemokines, and reactive oxygen species (ROS) (Garcia et al., 2022). The upregulation of these biomolecules may promote endogenous neuroinflammation and ultimately microglial damage or death. These pathways are similar to preclinical model reports of increased neuroinflammation and oxidative stress (Zou et al., 2022; Bialowas-McGoey et al., 2008). Overall, it is a biomarker of interest due to its low expression in serum, unless in the setting of possible BBB disruption.
The strengths of this study include a large, nationwide longitudinal cohort from 22 participating NCORP facilities. Our sample was recruited from nationwide community oncology sites that were not in academic medical centers, increasing generalizability; this patient population is representative of where the majority of breast cancer patients are treated. Additionally, the use of multiple cognitive outcomes for memory, attention, and executive function at pre- and post-chemotherapy timepoints expands the scope of focus for the questions proposed. Cognitive outcomes of age-matched, controls without cancer of the same sex were measured at the same times in parallel to patients with breast cancer, strengthening the reliability and validity of our group comparisons. This study had excellent retention for analysis of participants who were consented. Additionally, being a nationwide study, the data is geographically expansive.
There are also limitations to this work, as well as opportunities for future research. As with many clinical investigations, we found that while the cohort had high retention and was diverse with respect to educational attainment and geography, the enrollment of racial and ethnic minority populations was low, despite being a nationwide study. Although non-white patients comprised less than 15% of our enrollment, racial and ethnic diversity can be enhanced to better represent the population. We also acknowledge that social and environmental factors may have been more limited. We recognize factors such as depression, anxiety, and fatigue may affect cognitive performance in the context of cancer and CRCD. Relationships between these factors, S100β, and cognitive function should continue to be addressed in future studies. Already, S100β has known associations with depression and anxiety (Arora et al., 2019; Schroeter et al., 2013; Navinés et al., 2022; Arolt et al., 2003), although few have assessed this association in the specific context of cancer as we have in our current investigation. The exploratory analysis is hypothesis generating, and we did not control for the family-wise error to minimize Type II error. This study focused on patients with breast cancer, although, we are currently accruing a lymphoma cohort with a similar study design that will be used to compare findings between men and women and between tumor types. Additionally, we may have been underpowered to detect some associations due to the small effect sizes and missing data for some cognitive test. As mentioned above, we are currently investigating the impact of individual chemotherapy cycles to increase S100β, to enable relative comparisons with the pre-to post-chemotherapy changes presented herein.
In summary, we have conducted a nationwide study that identified correlations of increased S100β concentration in serum with poorer outcomes on some cognitive assessments from pre-to post-chemotherapy. S100β is likely only part of the mechanism leading to CRCD. There is the possibility that potential drivers of CRCD include a combination of astrocyte activation, neuronal injury, leaky BBB, neuroinflammation, and other factors that contribute to cognitive decline experienced by patients with breast cancer who have undergone chemotherapy; these should be considered in future research. Our findings altogether aid the investigation of the possible mechanisms underlying CRCD symptoms experienced by patients with breast cancer.
CRediT authorship contribution statement
Aaron N. Huynh: Writing – review & editing, Writing – original draft, Methodology, Formal analysis. AnnaLynn M. Williams: Writing – review & editing, Writing – original draft, Supervision, Conceptualization. Elizabeth K. Belcher: Writing – review & editing, Methodology, Data curation, Conceptualization. Paige Van Haute: Writing – review & editing, Data curation. Louis T. Lotta: Writing – review & editing, Supervision, Data curation. Bryan Thompson: Writing – review & editing, Data curation. Colleen Netherby-Winslow: Writing – review & editing, Supervision, Data curation. Amarinthia Curtis: Writing – review & editing, Project administration, Data curation. Benjamin T. Esparaz: Writing – review & editing, Project administration, Data curation. Carla Jorgensen: Writing – review & editing, Project administration, Data curation. Sara Alberti: Writing – review & editing, Supervision, Data curation. Emma Bentley: Writing – review & editing, Data curation. Hongying Sun: Conceptualization, Formal analysis, Writing – review & editing. Eva Culakova: Writing – review & editing, Writing – original draft, Supervision, Formal analysis, Data curation. Michelle C. Janelsins: Writing – review & editing, Writing – original draft, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
None.
Acknowledgements
We thank the Cancer Control Psychoneuroimmunology Lab (CCPL) and the Human Biophysiology Shared Resource (HBSR) at Wilmot Cancer institute for assistance with ELISAs, providing biobanking support, and data interpretation and for guidance in study design and protocol development. This research is supported by R01CA231014 and DP2CA195765 (Dr. Michelle C. Janelsins) and the URCC NCORP Research Base CA120025 (Dr. Karen Mustian and Dr. Gary Morrow).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2024.100860.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Areklett Elisabeth Wang, Fagereng Elisabeth, Bruheim Kjersti, Andersson Stein, Lindemann Kristina. Self-reported cognitive impairment in cervical cancer survivors: a cross-sectional study. Psycho Oncol. 2022;31:298–305. doi: 10.1002/pon.5818. [DOI] [PubMed] [Google Scholar]
- Arolt V., Peters M., Erfurth A., Wiesmann M., Missler U., Rudolf S., Kirchner H., Rothermundt M. S100B and response to treatment in major depression: a pilot study. Eur. Neuropsychopharmacol. 2003;13:235–239. doi: 10.1016/s0924-977x(03)00016-6. [DOI] [PubMed] [Google Scholar]
- Arora P., Sagar R., Mehta M., Pallavi P., Sharma S., Mukhopadhyay A.K. Serum S100B levels in patients with depression. Indian J. Psychiatr. 2019;61:70–76. doi: 10.4103/psychiatry.IndianJPsychiatry_391_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrand R., Unden J., Romner B. Clinical use of the calcium-binding S100B protein. Methods Mol. Biol. 2013;963:373–384. doi: 10.1007/978-1-62703-230-8_23. [DOI] [PubMed] [Google Scholar]
- Belcher E.K., Culakova E., Gilmore N.J., Hardy S.J., Kleckner A.S., Kleckner I.R., Lei L., Heckler C., Sohn M.B., Thompson B.D., Lotta L.T., Werner Z.A., Geer J., Hopkins J.O., Corso S.W., Rich D.Q., van Wijngaarden E., Janelsins M.C. Inflammation, attention, and processing speed in patients with breast cancer before and after chemotherapy. J. Natl. Cancer Inst. 2022;114:712–721. doi: 10.1093/jnci/djac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R.H.B., Schretlen D., Groninger L., Brandt J. Hopkins Verbal Learning Test--Revised (HVLT-R) [Database record] APA PsycTests. 1991 doi: 10.1037/t10851-000. [DOI] [Google Scholar]
- Benton A.L., Sivan A.B. Controlled Oral Word Association Multilingual Aphasia Examination Professional Manual. Psychological Assessment Resources; 1978. [Google Scholar]
- Bialowas-McGoey L.A., Lesicka A., Whitaker-Azmitia P.M. Vitamin E increases S100B-mediated microglial activation in an S100B-overexpressing mouse model of pathological aging. Glia. 2008;56:1780–1790. doi: 10.1002/glia.20727. [DOI] [PubMed] [Google Scholar]
- Bouvier D., Duret T., Abbot M., Stiernon T., Pereira B., Coste A., Chazal J., Sapin V. Utility of S100B serum level for the Determination of concussion in male rugby players. Sports Med. 2017;47:781–789. doi: 10.1007/s40279-016-0579-9. [DOI] [PubMed] [Google Scholar]
- Bowie C.R., Harvey P.D. Administration and interpretation of the trail making test. Nat Protoc. 2006;1(5):2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- Brandt J., Benedict R.H. Hopkins Verbal LearningTest-Revised Professional Manual. Psychological Assessment Resources. 2001. [Google Scholar]
- Chaves M.L., Camozzato A.L., Ferreira E.D., Piazenski I., Kochhann R., Dall'Igna O., Mazzini G.S., Souza D.O., Portela L.V. Serum levels of S100B and NSE proteins in Alzheimer's disease patients. J. Neuroinflammation. 2010;7:6. doi: 10.1186/1742-2094-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Tian L., Chen N., Xiu M., Wang Z., Yang G., Wang C., Yang F., Tan Y. Cognitive dysfunction correlates with elevated serum S100B concentration in drug-free acutely relapsed patients with schizophrenia. Psychiatr. Res. 2017;247:6–11. doi: 10.1016/j.psychres.2016.09.029. [DOI] [PubMed] [Google Scholar]
- Chen L., Hu X., Wu H., Jia Y., Liu J., Mu X., Wu H., Zhao Y. Over-expression of S100B protein as a serum marker of brain metastasis in non-small cell lung cancer and its prognostic value. Pathol. Res. Pract. 2019;215:427–432. doi: 10.1016/j.prp.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Choi H., Puvenna V., Brennan C., Mahmoud S., Wang X.F., Phillips M., Janigro D., Mazzone P. S100B and S100B autoantibody as biomarkers for early detection of brain metastases in lung cancer. Transl. Lung Cancer Res. 2016;5:413–419. doi: 10.21037/tlcr.2016.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolek C.H., Lee S.Y. In: Guccione's Geriatric Physical Therapy. fourth ed. Avers D., Won R.A., editors. Mosby; St. Louis, MO, United States: 2020. Chapter 19 - cognitive issues in the older adult. [Google Scholar]
- FACT-Cog Functional Assessment of Cancer Therapy – Cognitive Function. FACIT group, n.d. https://www.facit.org/measures/FACT-Cog
- Fray P.J., Robbins T.W. CANTAB battery: Proposed utility in neurotoxicology. Neurotoxicol Teratol. 1996;18:499–504. doi: 10.1016/0892-0362(96)00027-x. [DOI] [PubMed] [Google Scholar]
- Gannon J.M., Kelly D.L., Besch A., Thakur T., Khurana N., Shurin M.R., Shurin G.V., Brar J.S., Cihakova D., Talor M.V., Chengappa K.N.R. Racial differences in S100b levels in persons with schizophrenia. Psychiatr. Q. 2020;91:137–145. doi: 10.1007/s11126-019-09687-4. [DOI] [PubMed] [Google Scholar]
- Garcia Velia, Perera Yasiru Randika, Chazin Walter Jacob. A structural perspective on calprotectin as a ligand of receptors mediating inflammation and potential drug target. Biomolecules. 2022;12:519. doi: 10.3390/biom12040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins M.C., Heckler C.E., Peppone L.J., Ahles T.A., Mohile S.G., Mustian K.M., Palesh O., O'Mara A.M., Minasian L.M., Williams A.M., Magnuson A., Geer J., Dakhil S.R., Hopkins J.O., Morrow G.R. Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J. Clin. Oncol. 2018;36 doi: 10.1200/JCO.2018.78.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins M.C., Lei L., Netherby-Winslow C., Kleckner A.S., Kerns S., Gilmore N., Belcher E., Thompson B.D., Werner Z.A., Hopkins J.O., Long J., Cole S., Culakova E. Relationships between cytokines and cognitive function from pre- to post-chemotherapy in patients with breast cancer. J. Neuroimmunol. 2022;362 doi: 10.1016/j.jneuroim.2021.577769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janka E.A., Varvolgyi T., Sipos Z., Soos A., Hegyi P., Kiss S., Dembrovszky F., Csupor D., Keringer P., Pecsi D., Solymar M., Emri G. Predictive performance of serum S100B versus LDH in melanoma patients: a systematic review and meta-analysis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.772165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Guan, Yang Yanhao, Bi Feng, Yang Mingyan, Ma Yinhua. 5-HT and S100β values in evaluating severity of cognitive impairment after traumatic brain injury. Folia Neuropathol. 2023;61:47–52. doi: 10.5114/fn.2023.125119. [DOI] [PubMed] [Google Scholar]
- Kadry H., Noorani B., Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:69. doi: 10.1186/s12987-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner A.A., Marchi N., Fazio V., Mayberg M.R., Koltz M.T., Siomin V., Stevens G.H., Masaryk T., Aumayr B., Vogelbaum M.A., Barnett G.H., Janigro D. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806–2813. doi: 10.1002/cncr.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenoff L.A., Miller B.L., Kramer J.H. In: Encyclopedia of the Human Brain. Ramachandran V.S., editor. Academic Press; New York, United States: 2002. Frontal lobe. [Google Scholar]
- Kleindienst A., Ross Bullock M. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J. Neurotrauma. 2006;23:1185–1200. doi: 10.1089/neu.2006.23.1185. [DOI] [PubMed] [Google Scholar]
- Krishnan Anuradha, Wu Hao, Venkataraman Venkat. 2020. Astrocytic S100B, Blood-Brain Barrier and Neurodegenerative Diseases. (IntechOpen) [Google Scholar]
- Lachman M.E., Agrigoroaei S., Tun P.A., Weaver S.L. Monitoring cognitive functioning: psychometric properties of the brief test of adult cognition by telephone. Assessment. 2014;21:404–417. doi: 10.1177/1073191113508807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M., Howieson D.B., Loring D.W., et al. Neuropsychological Assessment. 4th. Oxford University Press; Oxford, United Kingdom: 2004. [Google Scholar]
- Li Y., Chen X., Zhou R., Xu W., Wang X., Chao W., Xue S. Correlation between cognitive impairment and homocysteine and S100B protein in patients with progressive ischemic stroke. Neuropsychiatric Dis. Treat. 2023;19:209–217. doi: 10.2147/NDT.S393624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navinés R., Oriolo G., Horrillo I., Cavero M., Aouizerate B., Schaefer M., Capuron L., Meana J.J., Martin-Santos R. High S100B levels predict antidepressant response in patients with major depression even when considering inflammatory and metabolic markers. Int. J. Neuropsychopharmacol. 2022;25:468–478. doi: 10.1093/ijnp/pyac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherby-Winslow C., Thompson B., Lotta L., Gallagher M., Van Haute P., Yang R., Hott D., Hasan H., Bachmann K., Bautista J., Gerber S., Cory-Slechta D.A., Janelsins M. Effects of mammary cancer and chemotherapy on neuroimmunological markers and memory function in a preclinical mouse model. Brain Behav Immun Health. 2023;34 doi: 10.1016/j.bbih.2023.100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- One Touch Stockings of Cambridge (OTS). Cambridge Cognition. https://www.cambridgecognition.com/cantab/cognitive-tests/executive-function/one-touch-stockings-of-cambridge-ots/
- Oris C., Bouillon-Minois J.B., Pinguet J., Kahouadji S., Durif J., Meslé V., Pereira B., Schmidt J., Sapin V., Bouvier D. Predictive performance of blood S100B in the management of patients over 65 Years old with mild traumatic brain injury. J Gerontol A Biol Sci Med Sci. 2021;76:1471–1479. doi: 10.1093/gerona/glab055. [DOI] [PubMed] [Google Scholar]
- Park Bong Soo, Lee Hae, Yoo Jeonghyun, Park Si Hyung, Kim Yang, Kim Si, Kim Il, Park Jin, Park Kang Min. Serum S100B represents a biomarker for cognitive impairment in patients with end-stage renal disease. Clin. Neurol. Neurosurg. 2020;195 doi: 10.1016/j.clineuro.2020.105902. [DOI] [PubMed] [Google Scholar]
- Polyakova M., Mueller K., Arelin K., Lampe L., Rodriguez F.S., Luck T., Kratzsch J., Hoffmann K.T., Riedel-Heller S., Villringer A., Schoenknecht P., Schroeter M.L. Increased serum NSE and S100B indicate neuronal and glial alterations in subjects under 71 Years with mild neurocognitive disorder/mild cognitive impairment. Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.788150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohl J., Röther J., Kluge S., de Heer G., Liepert J., Bodenburg S., Pawlik K., Kreymann G. Prediction of short-term and long-term outcomes after cardiac arrest: a prospective multivariate approach combining biochemical, clinical, electrophysiological, and neuropsychological investigations. Crit. Care Med. 2007;35:1230–1237. doi: 10.1097/01.CCM.0000261892.10559.85. [DOI] [PubMed] [Google Scholar]
- Qian Y., Chen X., Tang D., Kelley A.S., Li J. Prevalence of memory-related diagnoses among U.S. Older adults with early symptoms of cognitive impairment. J Gerontol A Biol Sci Med Sci. 2021;76:1846–1853. doi: 10.1093/gerona/glab043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2022. https://www.R-project.org/ [Google Scholar]
- Reitan R.M. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8(3):271–276. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- Robbins T.W., James M., Owen A.M., et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- RStudio Team . RStudio: Integrated Development for R. RStudio. PBC; Boston, MA: 2023. http://www.rstudio.com/ [Google Scholar]
- Ruff R.M., Light R.H., Parker S.B., Levin H.S. Benton controlled oral word association test: reliability and updated norms. Arch. Clin. Neuropsych. 1996;11(4):329–338. doi: 10.1016/0887-6177(95)00033-X. [DOI] [PubMed] [Google Scholar]
- Russo S., Nielen M.M, Boon J.C., et al. Neuropsychological investigation into the carcinoid syndrome. Psychopharmacology (Berl) 2003;168:324–328. doi: 10.1007/s00213-003-1455-5. [DOI] [PubMed] [Google Scholar]
- Sahakian B.J., Owen A.M. Computerized assessment in neuropsychiatry using CANTAB: Discussion paper. J. R Soc. Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Schroeter M.L., Sacher J., Steiner J., Schoenknecht P., Mueller K. Serum S100B represents a new biomarker for mood disorders. Curr. Drug Targets. 2013;14:1237–1248. doi: 10.2174/13894501113149990014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- Spielberger Charles Donald, Gorsuch Richard L. Consulting Psychologists Press, Inc.; Palo Alto, CA: 1983. Manual for the State-Trait Anxiety Inventory (Form Y) : ("self-Evaluation Questionnaire") [Google Scholar]
- Stein K.D., Martin S.C., Hann D.M., Jacobsen P.B. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- Statistics, Graphs and Definitions . Division of Cancer Control and Population Sciences (DCCPS) National Cancer Institute, Division of Cancer Control & Population Sciences; 2022. https://cancercontrol.cancer.gov/ocs/statistics [Google Scholar]
- Stein K.D., Jacobsen P.B., Blanchard C.M., Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J. Pain Symptom Manag. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J., Schiltz K., Walter M., Wunderlich M.T., Keilhoff G., Brisch R., Bielau H., Bernstein H.G., Bogerts B., Schroeter M.L., Westphal S. S100B serum levels are closely correlated with body mass index: an important caveat in neuropsychiatric research. Psychoneuroendocrinology. 2010;35:321–324. doi: 10.1016/j.psyneuen.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Tarhini A.A., Stuckert J., Lee S., Sander C., Kirkwood J.M. Prognostic significance of serum S100B protein in high-risk surgically resected melanoma patients participating in Intergroup Trial ECOG 1694. J. Clin. Oncol. 2009;27:38–44. doi: 10.1200/JCO.2008.17.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun P.A., Lachman M.E. Telephone assessment of cognitive function in adulthood: the brief test of adult cognition by telephone. Age Ageing. 2006;35:629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Verbal Recognition Memory (VRM). Cambridge Cognition. https://www.cambridgecognition.com/cantab/cognitive-tests/memory/verbal-recognition-memory-vrm/
- Vardy J.L., Dhillon H.M., Pond G.R., et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J. Clin. Oncol. 2015;33:4085–4092. doi: 10.1200/JCO.2015.63.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallensten J., Mobarrez F., Asberg M., Borg K., Beser A., Wilczek A., Nager A. Plasma levels of S100B and neurofilament light chain protein in stress-related mental disorders. Sci. Rep. 2022;12:8339. doi: 10.1038/s41598-022-12287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Hendrix C.C. Cancer-related cognitive impairment in breast cancer patients: influences of psychological variables. Asia Pac J Oncol Nurs. 2018;5:296–306. doi: 10.4103/apjon.apjon_16_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardan T., Erenler A.K., Baydin A., Aydin K., Cokluk C. Usefulness of S100B protein in neurological disorders. J. Pakistan Med. Assoc. 2011;61:276–281. [PubMed] [Google Scholar]
- Yen M.C., Huang Y.C., Kan J.Y., Kuo P.L., Hou M.F., Hsu Y.L. S100B expression in breast cancer as a predictive marker for cancer metastasis. Int. J. Oncol. 2018;52:433–440. doi: 10.3892/ijo.2017.4226. [DOI] [PubMed] [Google Scholar]
- Yu H., Li H., Liu X., Du X., Deng B. Levels of serum S100B are associated with cognitive dysfunction in patients with type 2 diabetes. Aging (Albany NY) 2020;12:4193–4203. doi: 10.18632/aging.102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z., Li L., Li Q., Zhao P., Zhang K., Liu C., Cai D., Maegele M., Gu Z., Huang Q. The role of S100B/RAGE-enhanced ADAM17 activation in endothelial glycocalyx shedding after traumatic brain injury. J. Neuroinflammation. 2022;19:46. doi: 10.1186/s12974-022-02412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.