Summary
Background
Obesity drives metabolic disease development. Preventing weight gain during early adulthood could mitigate later-life chronic disease risk. Increased dietary fibre intake, leading to enhanced colonic microbial fermentation and short-chain fatty acid (SCFA) production, is associated with lower body weight. Despite national food policy recommendations to consume 30 g of dietary fibre daily, only 9% of adults achieve this target. Inulin-propionate ester (IPE) selectively increases the production of the SCFA propionate in the colon. In previous studies, IPE has prevented weight gain in middle-aged adults over 6 months, compared with the inulin control. IPE is a novel food ingredient that can be added to various commonly consumed foods with a potential health benefit. This 12-month study aimed to determine whether using IPE to increase colonic propionate prevents further weight gain in overweight younger adults.
Methods
This multi-centre randomised-controlled, double-blind trial was conducted in London and Glasgow, UK. Recruited participants were individuals at risk of weight gain, aged between 20 and 40 years and had an overweight body mass index. Sealed Envelope Software was used to randomise participants to consume 10 g of IPE or inulin (control), once per day for 12 months. The primary outcome was the weight gained from baseline to 12 months, analysed by an ‘Intention to Treat’ strategy. The safety profile and tolerability of IPE were monitored through adverse events and compliance. This study is registered with the International Standard Randomised Controlled Trials (ISRCT) Database (ISRCT number: 16299902).
Findings
Participants (n = 135 per study arm) were recruited from July 2019 to October 2021. At 12 months, there was no significant difference in baseline-adjusted mean weight gain for IPE compared with control (1.02 kg, 95% CI: −0.37 to 2.41; p = 0.15; n = 226). Neither the IPE (+1.22 kg) nor the control arm (+0.07 kg) unadjusted mean gains in body weight reached the expected 2 kg threshold. In the IPE arm, fat-free mass was greater by 1.07 kg (95% CI: 0.21–1.93), and blood glucose elevated by 0.11 mmol/L (95% CI: 0.01–0.21). Compliance, determined by intake of ≥50% sachets, was reached by 63% of IPE participants. There were no unexpected adverse events or safety concerns.
Interpretation
Our study indicates that at 12 months, IPE did not differentially affect weight gain, compared with the inulin control, in adults between 20 and 40 years of age, at risk of obesity.
Funding
NIHR EME Programme (15/185/16).
Keywords: Obesity, Prevention, Short-chain fatty acids, Dietary fibre, Gut microbiota
Research in context.
Evidence before this study
Previous studies have demonstrated that 10 g/day IPE for 24 weeks in overweight middle-aged adults (40–65 years) significantly reduced body weight gain, correlated with decreased abdominal visceral adipose tissue and improved β-cell function compared to the control group. However, the effect of IPE has not been explored in a cohort of young adults at highest risk of weight gain.
Added value of this study
This is the first study to explore the specific role of the SCFA propionate in appetite regulation and body weight gain in young adults. Neither group gained significant weight over 12 months and there was a non-significant baseline-adjusted mean difference in weight gain of 1.02 (95% CI: −0.37 to 2.41) kg for IPE versus inulin control.
Implications of all the available evidence
The effects of IPE and inulin on weight gain prevention were not significantly different. Further research is required to understand whether there are age-related differences in appetite regulation and energy balance in response to SCFAs.
Introduction
Approximately 2.8 billion people are overweight or obese worldwide.1 Obesity-related diseases cost the NHS £11.4 billion in 2021.2 In adults, weight gain occurs at the fastest rate between the ages of 20 and 35 at 0.8–2.2 kg per year, creating conditions for later-life obesity.3,4 Lifestyle changes during this stage of life contribute to a small but persistent positive energy balance of 50–100 kcal per day, promoting incremental weight gain.5 After becoming obese, a return to a body mass index (BMI) of ≤25 kg/m2 is achieved by one in 210 men and one in 124 women.6 Substantial adipose tissue deposition during this period increases metabolic disease risk and sets the weight gain trajectory for later life. Increased fat mass during young adulthood can lead to elevated triglycerides, fasting glucose, blood pressure, total cholesterol and low-density lipoprotein (LDL) cholesterol in older age.7 Preventing the incremental increase in body weight observed in young adults will lead to better metabolic health throughout the lifespan.
High-fibre diets are associated with lower weight gain.8 Dietary fibres are largely indigestible in the small intestine and reach the colon where gut bacteria ferment them to produce short-chain fatty acids (SCFAs): acetate, propionate, and butyrate.9 In humans, SCFAs are natural ligands for free fatty acid receptor (FFARs) activation in the gastrointestinal tract and appear to play a role in modulating appetite and energy balance.10,11 Despite public health recommendations, less than 10% of the population meet the recommended daily intake of 30 g of dietary fibre12 and only one-third of adults consume five portions of fruit and vegetables per day.13
Rodent faecal donor studies indicate that lower body weight and adiposity are associated with increased propionate.14 Additionally, propionate is an end product of fermentation15 and has the highest affinity of all SCFAs to FFARs.16 We produced inulin-propionate ester (IPE), a novel food ingredient, to specifically enhance the production of propionate in the colon in quantities equivalent to the fermentation of 60 g of fibre17 contained within one 10 g dose. Our previous work demonstrated that middle-aged, overweight participants consuming IPE daily experienced less weight gain compared with the inulin-consuming group over a six-month intervention.17 IPE has the potential to be added to a wide variety of foods without affecting organoleptic properties.
The aim of this multi-centre, randomised-controlled, double-blind trial was to investigate the effect of IPE on weight gain in a younger, overweight population (20–40 years) at risk of further weight gain over one year.
Methods
Study design
The iPREVENT study was a multi-site, double-blind, parallel, randomised-controlled trial conducted between July 2019 and October 2022. The study sites were Imperial College London and The University of Glasgow, UK. The trial was registered to the International Standard Randomised Controlled Trial (ISRCT) registry (ISRCT No: 16299902, March 2018). The trial protocol and participant-facing documents were approved by the London Hampstead Research Ethics Committee (REC) on 29th January 2019 (REC reference no: 19/LO/0095). Study recruitment commenced in July 2019 and the final participant was enrolled in October 2021. Recruitment was paused from March 2020 to September 2020 due to the COVID-19 pandemic. The protocol for this study was published in October 2022.18 In the current paper, we report the primary and secondary outcomes of the main study only.
Participants
The 270 participants were recruited through GP practices and NHS trusts, newspaper and social media (Facebook/Instagram) adverts, pop-up events and posters. Participants in the study underwent screening at the sites’ clinical research facilities to ensure they met specific inclusion criteria. These criteria included being within the age range of 20–40 years, having a BMI of 23–27 kg/m2 for individuals of South Asian descent or 25–30 kg/m2 for those of other ethnicities, being on stable medication for over 3 months at the time of screening, and exhibiting an elevated risk of weight gain. This elevated risk was defined by at least one of the following self-reported factors: low physical activity levels (determined by the international physical activity questionnaire score at screening), weight gain of 2 kg or more in the past year, consumption of more than one sugar-sweetened beverage per day or of less than two portions of fruit and vegetables per day. In January 2020, the participant age range was extended from 20 to 35, to 20–40 years. This and all other amendments are documented in the online trial protocol.18
Participants who met any exclusion criteria were not enrolled in the study. Participants were excluded due to the presence of chronic diseases such as type 1 and 2 diabetes, cancer, renal failure, heart disease, and organic acidaemia. Additionally, individuals with gastrointestinal conditions such as coeliac disease, inflammatory bowel disease, and irritable bowel syndrome were excluded, along with those who had undergone previous bowel reconstruction surgery or had untreated vitamin B12 deficiency. Participants reporting a gastrointestinal upset in the last two weeks, experiencing weight loss of 3 kg or more in the past 3 months, who were pregnant or lactating, using antibiotics, or currently participating in a weight loss program or consuming weight loss products were also excluded from the study.
Randomisation and masking
Randomisation was undertaken using Sealed Envelope Software (Open-source software, www.sealedenvelope.com), by the method of minimisation with a random element to balance the arms by research centre, sex, BMI within ethnicity (South Asians: 24.00–25.49 kg/m2 and 25.50–27.00 kg/m2; non-South Asians: 25.00–27.49 kg/m2 and 27.50–30.00 kg/m2) and whether they participated in the mechanistic sub-study. Participants were identified with a unique trial identification (ID). IPE or control sachets were identified with a unique treatment code linked to the allocation and trial ID. The treatment code was not broken as there were no medical emergencies or need to report unexpected and related Severe Adverse Events (SAE) to the REC.
Interventions
Participants received blinded, organoleptically identical trial interventions of IPE or inulin control in 10 g pre-packed, foil-backed sachets. IPE and inulin are both white powders. Participants were instructed to consume one sachet per day orally, mixed in a cool drink or water, at any time with their normal diet. Participants were asked to attend screening, baseline (randomisation), 2-, 6-, and 12-month study visits at the Clinical Research Facility (CRF) of each participating site. Participants were given an initial trial supply at the baseline visit and subsequent supplies were dispensed at 2 and 6 months. Throughout the study, participants were encouraged to maintain their current diet and exercise routines.
IPE was produced at a pilot-plant by Moorepark Technology Limited (Fermoy, Ireland) using a previously described protocol.19 Safety of the products was assessed by microbiological and heavy metal testing by an external accredited laboratory.
Study procedures
At the screening visit, the study rationale and procedures were explained. Participants then provided informed consent. Body weight and body composition (body water, fat mass, fat-free mass) were measured to the nearest 0.1 kg using the Tanita BC-418MA scales (Tanita Corporation, Japan). Participants were asked to remove heavy items of clothing, shoes, and accessories. They were also asked to void their bladder before the measurements were taken. A blood sample was taken for a full blood count to rule out the risk of vitamin B12 deficiency or anaemia. Information regarding medical history, current medications, alcohol intake, smoking or vaping, physical activity and recreational drug use was collected. Blood pressure and waist/hip circumference were also measured. At the baseline, 2-, 6- and 12-month visits, these measurements were repeated. Fasted blood samples for glucose and lipid profile were taken at baseline, 6- and 12-months. Participants attended these visits in the morning, after fasting for at least 8 h and were asked to abstain from alcohol and intense physical activity the day before the visit. At 2-, 6- and 12-months, compliance was measured by counting used and unused inulin or IPE sachets returned by participants and the occurrence of adverse events (AEs) or SAEs was documented. To assess dietary changes, participants were asked to complete a seven-day food diary at baseline, 2-, 6- and 12-month visits. Further information on study methodology can be found in the Supplementary Material (Supplementary information, Supplementary Methods).
Patient and public involvement
Public involvement was an intrinsic part of this clinical trial. A Study Advisory Group (SAG) was formed, consisting of four members of the public with similar characteristics to the trial population. The SAG advised on recruitment, retention, engagement and dissemination strategies. One member of the SAG also joined the Trial Steering Committee and Trial Management Group.
The SAG met three times before the trial began and provided feedback on the participant information sheet to ensure it was accurate and readily understood by a lay audience. The SAG identified aspects of the trial protocol which might be impractical for participants and highlighted potential compliance challenges. The SAG suggested ways to recruit participants, including via social media adverts, texts from GP surgeries and engaging people from ethnic minority backgrounds via community centres. One SAG member produced a short animation video to share on social media. Not all suggestions could be implemented, mostly due to budget constraints. When issues like this arose, they were discussed openly with the SAG.
The SAG provided a novel perspective during the trial, offering reasons why participants might be reluctant to attend study visits and suggesting ways in which attendance could be incentivised. For example, the SAG commented that COVID-19 had caused some people to worry about being identified as overweight and highlighted inequalities in healthcare outcomes among people from ethnic minority backgrounds in the UK.
At the dissemination stage, the SAG discussed possible dissemination strategies and two members of the SAG were involved in writing the funder's report and this research paper.
Outcomes
The Primary outcome is weight gain from baseline to 12 months.
Secondary outcomes, change from baseline to 12 months (unless otherwise indicated), were:
-
•
Weight gain from baseline to 2 and 6 months
-
•
Fasting biochemistry:
-
•
Glucose
-
•
Insulin
-
•
Triglycerides
-
•
Total cholesterol
-
•
Low-density lipoprotein (LDL) cholesterol
-
•
High-density lipoprotein (HDL) cholesterol
-
•
Blood pressure
-
•
Waist and hip circumference, body composition measurements, BMI
-
•
Compliance with intervention (sachet percentage)
-
•
Occurrence of AEs and SAEs
-
•
Changes in physical activity from baseline to 2, 6 and 12 months
-
•
Changes in diet from baseline, to 2, 6 and 12 months
-
•
Changes in other lifestyle factors (drinking, smoking, vaping, recreational drugs) from baseline to 2, 6 and 12 months
Statistical analysis
The sample size was calculated from the randomised proof of concept trial where the difference between arms in the change in body weight over 24 weeks was 1.4 kg (95% CI (Confidence Interval): −0.3 to 3.1), p = 0.099.17 Using a Bayesian method recommended for preliminary trials in which evidence in the 95% CI is translated into probabilities,20 there was a 95% posterior probability of an underlying, positive, between-arm difference favouring the intervention. The posterior probabilities of intervention-favouring differences greater than 1 kg, 1.5 kg, and 2 kg were 69%, 47% and 25%, respectively, based on 24-week intervention. The difference increased in magnitude through successive eight-week, 16-week, and 24-week time points. By 24 weeks there were significant reductions in the proportion of intervention participants gaining 3%, and 5% of body weight from a mean baseline of 90 kg. A 2 kg between-arm 12-month effect size was therefore chosen. This agreed with a weight gain prevention trial over nine months in young adults21 which aimed to detect a 2 kg effect and achieved 4.3 kg, with a pooled standard deviation (SD) for body weight change of 4.35 kg and 81% retention.
On this basis, a sample size of 270 randomised participants (135 per arm) was chosen to provide 90% power to detect a 2 kg difference between arms in mean body weight change over 12 months using a two-sided 5% level significance test, assuming a 4.35 kg SD and with 25% dropout allowance (68 participants). The sample size was calculated using R Project for Statistical Computing (RRID: SCR_001905).
This trial aimed to understand whether intake of IPE prevented further weight gain, compared with the control. In addition, an explanatory element of the trial aimed to understand the mechanisms of the causal pathway of such body weight change and any limitations from compliance. Therefore, analyses were primarily conducted in the ‘Intention to Treat’ population. The analysis of the primary endpoint incorporated the earlier correlated interim measurements of body weight in a linear mixed effects model, using an unstructured correlation structure, and adjusting for baseline continuous body weight and stratifiers. Additionally, due to COVID-19 restrictions participant self-measured weights were permitted, and a covariate was included to indicate whether the measured weight was provided in the clinic or directly by the participant at each time point. The implicit ‘missing at random’ assumption was challenged through a set of sensitivity analyses involving all randomised participants.
Further to this we conducted a complier average causal effect (CACE) analysis, as outlined by Dunn et al., estimating the effect of IPE versus Inulin control on the primary outcome in a more highly compliant population, whilst respecting randomisation.22 The CACE estimate is the ratio of the estimated treatment effect to the proportion compliant.23 The pre-defined compliance level was ≥50% and secondarily, ≥80% of daily sachets taken over one year.
Where possible, continuous secondary endpoints were adjusted for their baseline to improve the precision of estimated intervention effects. Repeated measures were analysed using linear mixed-effects models adjusting also for randomisation stratifiers. Comparisons between arms for binary outcomes were summarised as differences in proportions and estimated 95% confidence intervals were used to make inferences about effect sizes. The Wilson score-based method with no continuity correction was used to obtain these when there were fewer than five events in either of the arms.24 Between-group findings were presented with 95% confidence intervals. An interval excluding zero, for the analysis of a difference in means or proportions, was labelled as tentatively statistically significant due to multiple secondary outcome tests. p-values arising from hypothesis testing were reserved for the powered analysis of the primary outcome.
IBM SPSS Statistics (RRID: SCR_019096), version 28.0.1, was used for all statistical analyses. Further details are provided in the Statistical Analysis Plan (SAP) (available at: https://data.mendeley.com/datasets/n33kky5dww/2), which was approved by the TSC. The final approved SAP was based on the protocol and took precedence for undertaking the analysis of the main trial.
Role of the funding source
The study funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
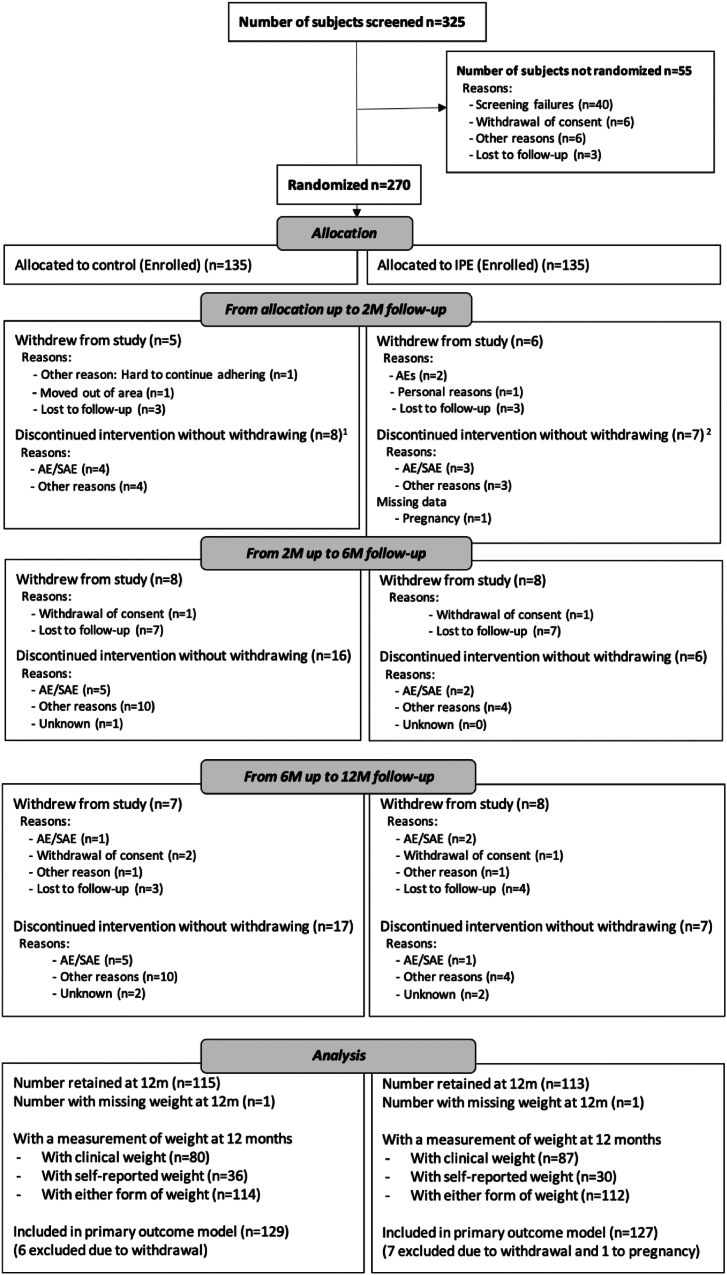
Between July 2019 and October 2021, 325 individuals were screened for this study and 270 participants were randomly allocated to the inulin control (n = 135) or IPE (n = 135) group. The mean (SD) age was 30.2 (5.4) years and BMI was 27.5 (1.5) kg/m2. Baseline characteristics were well-balanced between the two study arms (Table 1). Participant withdrawal rates (discontinued the study) were lower than the 25% threshold at 16% (42/270) overall. There were no apparent differences between those withdrawing from the study and those completing the trial. Of those withdrawing, baseline weight was lower, and there were more females in the IPE arm than the control arm (Supplementary Information, Supplementary Table S1). Overall, 45% (61/135) of participants in the control group and 31% (42/135) in the IPE group discontinued supplementation, of whom 15% (20/135) and 16% (22/135), respectively, withdrew from the study entirely. The Consolidated Standards of Reporting Trials (CONSORT) diagram describes the flow of participants through the study stages (Fig. 1).
Table 1.
Socio-demographic and clinical characteristics by arm at Baseline.
| IPE N = 135 N (%) |
Inulin N = 135 N (%) |
Total N = 270 N (%) |
|
|---|---|---|---|
| Age Mean (SD) | 30.3 (5.2) | 30.1 (5.7) | 30.2 (5.4) |
| Sex: Female | 86 (64%) | 86 (64%) | 172 (64%) |
| Sex: Male | 49 (36%) | 49 (36%) | 98 (36%) |
| Ethnicity | |||
| White | 90 (67%) | 95 (70%) | 185 (68%) |
| Asian | 14 (10%) | 20 (15%) | 34 (13%) |
| Mixed | 7 (5%) | 6 (4%) | 13 (5%) |
| Black | 8 (6%) | 4 (3%) | 12 (4%) |
| Any other ethnic group | 16 (12%) | 10 (7%) | 26 (10%) |
| Weight (kg) Mean (SD) | 79.6 (10.9) | 79.1 (10.6) | 79.3 (10.7) |
| Height (cm) Mean (SD) | 169.6 (9.5) | 169.5 (9.3) | 169.6 (9.3) |
| Waist (cm) Mean (SD) | 94.0 (8.7) | 93.2 (8.2) | 93.6 (8.5) |
| BMI (Kg/m2) Mean (SD) | 27.5 (1.6) | 27.4 (1.5) | 27.5 (1.5) |
| Smoking status | |||
| Current | 25 (19%) | 17 (13%) | 42 (16%) |
| Ex-smoker | 25 (19%) | 28 (21%) | 53 (20%) |
| Never | 85 (63%) | 90 (67%) | 175 (65%) |
| Vaping status | |||
| Current | 8 (6%) | 11 (8%) | 19 (7%) |
| Ex-vaper | 4 (3%) | 3 (2%) | 7 (3%) |
| Never | 123 (91%) | 121 (90%) | 244 (90%) |
| Drinking statusa | |||
| Current | 106 (79%) | 110 (81%) | 216 (80%) |
| Ex-drinker | 12 (9%) | 9 (7%) | 21 (8%) |
| Never | 16 (12%) | 16 (12%) | 32 (12%) |
| Recreational drugs taken in the past year | 7 (5%) | 6 (4%) | 13 (5%) |
One participant in the IPE arm failed to report their drinking status.
Fig. 1.
CONSORT Study Flow Diagram. 1One of these participants withdrew later in the 2 M–6 M period. 2Two of these participants withdrew later in the 6 M–12 M period.
There was no significant difference in the primary outcome of body weight between the IPE and inulin control groups at the 12-month study visit, adjusted difference in means (95% Confidence intervals [CI]) was 1.02 (−0.37 to 2.41) kg, p = 0.15 (Table 2). This included 256 (95%) participants with at least one follow-up weight measurement in the primary outcome model (Table 2). The conclusion was not changed in the sensitivity analysis for missing data (data not shown).
Table 2.
Results for primary and secondary outcomes.
| IPE |
Inulin |
Adjustede difference in means (IPE—Inulin) (95% CI) | |||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | ||
| Weight (kg) | |||||
| Baseline | 135 | 79.6 (10.9) | 135 | 79.1 (10.6) | |
| 2 ma | 125 | 80.5 (10.8) | 121 | 79.5 (11.2) | 0.20 (−0.41 to 0.81) |
| 6 mb | 108 | 80.9 (10.9) | 106 | 78.9 (10.9) | 0.19 (−0.90 to 1.28) |
| 12 mc (Primary outcome) | 112 | 81.4 (11.9) | 114 | 78.9 (11.8) | 1.02 (−0.37 to 2.41) |
| BMI (Kg/m2) | |||||
| Baseline | 135 | 27.5 (1.6) | 135 | 27.4 (1.5) | |
| 12 md | 87 | 27.9 (2.4) | 80 | 27.6 (2.2) | 0.31 (−0.23 to 0.85) |
| Waist/hip ratio | |||||
| Baseline | 135 | 0.87 (0.08) | 135 | 0.86 (0.07) | |
| 12 m | 85 | 0.90 (0.10) | 78 | 0.86 (0.07) | −0.01 (−0.02 to +0.01) |
| Body fat (kg) | |||||
| Baseline | 134 | 24.8 (5.7) | 135 | 24.2 (6.2) | |
| 12 m | 86 | 24.9 (6.6) | 78 | 24.4 (7.0) | 0.07 (−1.07 to +1.21) |
| Fat-free mass (kg) | |||||
| Baseline | 134 | 54.8 (11.1) | 135 | 55.0 (10.9) | |
| 12 m | 86 | 56.1 (10.7) | 77 | 55.2 (10.5) | 1.07 (0.21–1.93) |
| Water mass (kg) | |||||
| Baseline | 134 | 39.5 (7.9) | 135 | 39.7 (7.8) | |
| 12 m | 86 | 40.5 (7.6) | 78 | 40.0 (7.5) | 0.72 (0.10–1.33) |
| Diastolic BP (mm/Hg) | |||||
| Baseline | 135 | 72.1 (9.8) | 135 | 70.3 (9.7) | |
| 12 m | 86 | 71.2 (9.7) | 79 | 71.4 (8.9) | −0.64 (−2.71 to 1.44) |
| Systolic BP (mm/Hg) | |||||
| Baseline | 135 | 116.2 (11.3) | 135 | 113.2 (10.5) | |
| 12 m | 86 | 115.7 (11.6) | 79 | 115.8 (10.6) | −1.30 (−3.84 to 1.24) |
| Glucose (mmol/L) | |||||
| Baseline | 127 | 4.59 (0.40) | 125 | 4.65 (0.42) | |
| 12 m | 85 | 4.74 (0.39) | 80 | 4.66 (0.39) | 0.11 (0.01–0.21) |
| Ln Insulin (pmol/L) | |||||
| Baseline | 130 | 4.50 (0.97) | 131 | 4.52 (0.88) | |
| 12 m | 84 | 4.47 (0.86) | 75 | 4.60 (0.80) | 0.03 (−0.13 to 0.20) |
| Total cholesterol (mmol/L) | |||||
| Baseline | 134 | 4.70 (0.94) | 133 | 4.58 (0.74) | |
| 12 m | 86 | 4.66 (0.85) | 80 | 4.69 (0.93) | −0.12 (−0.32 to 0.09) |
| LDL cholesterol (mmol/L) | |||||
| Baseline | 131 | 2.90 (0.83) | 132 | 2.72 (0.67) | |
| 12 m | 86 | 2.82 (0.73) | 79 | 2.78 (0.79) | −0.12 (−0.30 to 0.05) |
| HDL cholesterol (mmol/L) | |||||
| Baseline | 133 | 1.35 (0.31) | 133 | 1.37 (0.36) | |
| 12 m | 86 | 1.37 (0.30) | 80 | 1.40 (0.38) | −0.00 (−0.07 to 0.07) |
| Ln Triglycerides (mmol/L) | |||||
| Baseline | 132 | −0.10 (0.46) | 133 | −0.07 (0.52) | |
| 12 m | 86 | −0.11 (0.46) | 80 | −0.07 (0.58) | 0.01 (−0.11 to 0.13)f |
BMI, body mass index; BP Blood pressure; CI, confidence intervals; HDL high-density lipoprotein; LDL, low-density lipoprotein; Ln, natural log.
This included 30 participants in the IPE arm and 32 in the inulin arm whose measured weights were self-reported. The mean (SD) clinical weight in the IPE arm was 80.1 (10.6) and 80.3 (11.1) in the inulin arm at 6-months follow-up.
This included 25 participants in the IPE arm and 32 in the inulin arm whose measured weights were self-reported. The mean (SD) clinical weight in the IPE arm was 80.7 (11.0) and 80.3 (11.5) in the inulin arm at 2-months follow-up.
This included 25 participants in the IPE arm and 34 in the inulin arm whose measured weights were self-reported. The mean (SD) clinical weight in the IPE arm was 80.8 (11.1) and 79.8 (10.5) in the inulin arm at 12-months follow-up.
In a post hoc analysis computing BMI using the height given at baseline and the weight used for primary outcome, there were 112 participants with a mean (SD) BMI of 28.0 (2.5) in the IPE arm at 12 months, and 114 participants with BMI of 27.4 (2.3) in the Inulin arm. The adjusted mean difference was 0.39 (−0.10 to 0.87).
Adjusted at each timepoint for the baseline of the measurement, age, sex, BMI by ethnicity (except for BMI, which was only adjusted for ethnicity), whether included in sub-study, and study site. For weight, we have also adjusted for the source of the measured weight (Clinic versus Self-reported) at each timepoint.
Before natural log transformation, the means (SD) at baseline, 6 M and 12 M in the control arm, respectively were, in mmol/L, 1.08 (0.06), 1.05 (0.07), 1.14 (0.10) and for IPE were 1.01 (0.05), 1.04 (0.06), 1.00 (0.06). The adjusted difference in means (with confidence interval) is presented on the natural log scale, and on taking the anti-log is equivalent to a more interpretable ratio of the geometric means of 1.01 mmoL/l 95% CI (0.90–1.14).
Significantly higher fat-free mass and body water mass were observed in the IPE group compared to the control with adjusted differences in means of 1.07 (0.21–1.93) kg and 0.72 (0.10–1.33) kg, respectively. Additionally, fasting glucose increased in the IPE arm, compared with the inulin control with an adjusted difference in means of 0.11 (0.01–0.21) mmol/L. No changes in other blood biomarkers, waist and hip circumference, blood pressure or BMI were detected (Table 2). No apparent differences in physical activity or lifestyle factors (smoking, drinking or vaping) were detected within or between groups (Supplementary Information, Supplementary Tables S2 and S3).
At 12 months, high compliance with the intervention, determined by a threshold of ≥80% IPE or inulin sachets consumed, was reached by 48% (65/135) and 32% (43/135) of the IPE and inulin control arms, respectively (Table 3). CACE compliance analysis was used to investigate the effect of higher compliance on weight outcomes between groups at a predefined compliance level of ≥50%, and ≥80%, the high compliance level (Supplementary Information; Supplementary Tables S4 and S5). Post-hoc sensitivity analyses identified groups within the study population who exhibited higher levels of compliance, such as older participants, males and those with a lower starting body weight (Supplementary Information; Supplementary Table S6).
Table 3.
Number of participants who reached levels of compliance at 12-month visit.
| Percentage of days compliant over the 12-month follow-up | IPE N = 135 % (N) |
Inulin N = 135 % (N) |
Total N = 270 % (N) |
Difference in proportions (IPE-Inulin) (95% CI) |
|---|---|---|---|---|
| ≥80% | 48 (65) | 32 (43) | 40 (108) | 16.3% (4.8% to 11.5%) |
| ≥50% | 63 (85) | 53 (72) | 58 (157) | 9.6% (−2.1% to 21.3%) |
IPE, inulin-propionate ester. Compliance analyses included those who withdrew from the study entirely and those who did not withdraw but discontinued supplementation. Compliance was taken to be zero after these events.
Analysis of 150 diet diaries from the first 60 participants detected that energy intake reporting was, on average, 40% less than the estimated required energy intake.
Safety data were reported for all participants. There were 340 AEs in 151 (56%) participants, with 160 AEs in 76 (56%) participants in the IPE arm, and 180 AEs in 76 (56%) participants in the inulin control arm. Common, expected and related AEs were gastrointestinal disorders such as bloating, cramping and gas. There were four SAEs, two in the control arm and two in the IPE arm. AE and SAE reporting did not differ substantially between the two arms. There were no unexpected AEs and no SAEs that were related to the IPE or inulin consumption. Incidence of COVID-19 has been documented in infection and infestations in the supplementary information (Supplementary Information; Supplementary Table S8). The Wilson score-based method with no continuity correction was used when there were fewer than five events in either of the arms (25). Table 4, Table 5 summarise AEs and SAEs.
Table 4.
Summary of adverse events by arm.
| IPE (n = 135) % (N) |
Inulin (n = 135) % (N) |
Difference in proportions (95% CI)b (IPE—Inulin) | |
|---|---|---|---|
| Participants reporting ≥1 AE | 56 (76) | 56 (76) | 0.0% (−11.7% to 11.7%) |
| Participants reporting an SAE | 1.5 (2) | 1.5 (2) | 0.0% (−3.9% to 3.9%) |
| Death | 0 (0) | 0 (0) | 0.0% (−2.8% to 2.8%) |
| Adverse events of special interest: | |||
| Participants reporting ≥1 AE in Gastrointestinal disorders SOC | 24 (33) | 33 (45) | −8.9% (−19.4% to 1.9%) |
| Participants reporting at least one AE in infection and manifestations of SOC | 21 (29) | 21 (28) | 0.7% (−9.0% to 10.5%) |
| Participants reporting at least one AE in Food sup taste/texture SOCa | 7 (10) | 4 (6) | 3.0% (−3.0% to 9.1%) |
| No. participants discontinuation from study due to AEa | 3 (4) | 1 (1) | 2.2% (−1.6% to 6.7%) |
AE, adverse event; IPE, inulin-propionate ester; SAE, severe adverse event; SOC, System Organ Class.
3 participants had an AE related to stopping the supplement because they “did not like taking supplements”.
The Wilson score-based method with no continuity correction was used when there were fewer than five events in either of the arms (refer to Newcombe 199824).
Table 5.
Severe adverse events.
| Arm | Duration of SAE (days) | Severity | Months after randomisation | Relationship to study treatment | Outcome | |
|---|---|---|---|---|---|---|
| Stab wound | IPE | 30 | Severe | 10 | Not related | Resolved |
| Hospitalisation | IPE | 61 | Severe | 8 | Not related | Resolved |
| Renal stone removal | Inulin | 1 | Moderate | 4 | Not related | Resolved |
| Meningitis bacterial | Inulin | 17 | Severe | 6 | Not related | Resolved |
IPE, inulin-propionate ester; SAE, severe adverse event.
Discussion
This randomised controlled trial investigated the efficacy of increasing colonic propionate production on the prevention of weight gain in younger adults over one year. This valuable effect was suggested by earlier evidence but had not yet been tested. In this study, IPE had no significant effect, compared with inulin, on the primary outcome of weight gain. Furthermore, neither the IPE nor the control arm participants reached the predicted mean 2 kg gain in body weight. In contrast, an observational study conducted during the same period of the COVID-19 pandemic indicated that the average adult gained 1.57 kg between March and May 2020.25
Previously, a six-month study of IPE intake in a middle-aged cohort (mean age: 54 years) demonstrated a trend towards weight loss, with an adjusted difference (95% CI) in weight of −1.4 kg (−3.07 to 0.27) compared with the inulin control.17 Further, a one-month intervention of IPE with a moderate energy deficit in a younger cohort, reduced body weight (Pre-intervention mean (SD) 77.3 (4.2) kg; post-intervention, 76.6 (4.1) kg, p < 0.05).26 However, it is worth noting that participants for this trial were selected for their phenotypic susceptibility to further weight gain.3
Irrespective of the lack of effect on body weight seen, IPE had a distinct effect on body composition compared with the inulin control. In the current study, fat-free mass increased significantly in the IPE group, this was not accounted for by a change in physical activity measured by IPAQ. There was no significant change in fat mass, contrary to previous studies where lower fat mass26 and lower intra-abdominal fat17 were reported. Nevertheless, an elevated fat-free mass enhances basal metabolic rate and could contribute to longer-term improvements in body composition.27 Although the mechanism for the increased fat-free mass is not clear, this observation has been made in animal studies using a variety of SCFA.28,29 It is proposed that SCFA promote an oxidative skeletal muscle phenotype and increased expression of type I myosin heavy chain proteins.30,31 So far, there are no studies investigating the effect of propionate.
Although fasting glucose was significantly higher after 12 months of IPE intake (0.11 (0.01–0.21) compared with inulin, levels remained within the normal range. The increase of 0.11 mmoL/l is unlikely to be of clinical relevance. Additionally, there was no observed effect on postprandial blood glucose, consistent with findings from previous studies.17,32,33
It is not possible to say with certainty why there are differences between weight maintenance in the current young adult cohort and our previous study.17 One distinction is the age of the participants. Physiological and psychosocial differences driving energy intake may contribute to the differences in the weight gain trajectory between younger and older adults. So far, most studies have recruited middle-aged participants to investigate the effect of increased colonic propionate on metabolism,17,32,34 with one exception.26 The IPE dose of 10 g per day for this study was selected based on previous work suggesting this to be the minimally effective dose in middle-aged adults,17 with no differences seen when the dosage was increased to 20 g per day.32 A cross-sectional study of 153 participants indicated that adults aged 50–65 years have 25% lower SCFA concentrations than adults aged below 50 years.35 The naturally lower concentrations of SCFA seen in older adults may make them more responsive to increased colonic propionate. Therefore, a larger dose may be required to achieve a similar effect in this younger study cohort. In previous work, we have demonstrated that middle-aged men rated high-energy foods as significantly less appealing when consuming IPE.36 These findings in middle-aged, obese adults may not be translatable to younger adults with lower adiposity. Furthermore, younger adults tend to eat outside of the home and snack throughout the day,37 which are both eating behaviours associated with higher energy intake.38 It is also worth noting that the previous study cohort had a higher starting weight and higher plasma lipids and that the results of the previous study were not confounded by COVID-19. The current study highlights the importance of conducting research in an age group where complex drivers of weight gain exist and highlights the need for an array of interventions to combat weight gain.39 These may differ older adults where obesity is more established.
Participant withdrawal rates were lower than predicted and comparable between arms. Compliance analyses indicated that supplement cessation was more common in those consuming inulin than IPE. Therefore, lower compliance did not explain the main trial outcome. However, participants with the poorest compliance with IPE appeared to gain the most weight and those that had high compliance of >80% had greater weight loss.
Notable strengths of this study are the randomised, blinded placebo-controlled design, duration, cohort size and that ≥50% treatment adherence was met by 63% of participants after 12 months. However, this study has some limitations. The dose of IPE may have been inadequate to alter appetite in a group of younger adults as the evidence supporting the use of this dose was based older adults who likely had lower baseline colonic SCFA concentrations. Although dietary data was collected before and after the intervention, it has not been reported in the present paper. After analysing the diet diaries of the first 60 volunteers who completed the study, an average underreporting of 40% of estimated required energy intake was detected. We determined that this level of misreporting made the data too unreliable to be meaningful. It is possible that diets could have changed over the study period, potentially increasing fermentable fibre intake. However, data from the National Diet and Nutrition survey demonstrated a 2-g decrease in dietary fibre intake in 2020.40 Further, from our calculations, the 2.5-fold increase in daily colonic propionate production achieved when IPE releases propionate in the colon, would be difficult to reach by a change in dietary fibre intake.17 Lastly, the control arm was treated with inulin, a fermentable dietary fibre that itself may reduce appetite. This study did not include a negative control in the form of a non-fermentable carbohydrate, like cellulose, as in previous studies. However, as the study aimed to determine the distinct effect of propionate on preventing weight gain, independent of the inulin backbone, inulin was deemed the most appropriate control.
In conclusion, IPE did not differentially affect weight gain, compared to the inulin control, in adults between 20 and 40 years of age, at risk of obesity. This result diverges from previous data which demonstrated that propionate, delivered to the colon, improved weight maintenance in middle-aged overweight adults. The prevention of incremental weight gain in young adults delays the onset of obesity and its associated sequelae. However, the drivers of weight gain and its prevention appear to be more complex during this period than in later life. Further investigation is needed to elucidate the mechanisms behind the observed differential impact of IPE on weight gain prevention between younger and older adults.
Contributors
Professor Gary Frost (ORCID: 0000-0003-0529-6325): Conceptualisation, Funding acquisition, Methodology, Supervision, Resources, Visualisation, Writing—original draft, Writing—review & editing.
Professor Douglas Morrison (ORCID: 0000-0002-4161-5699): Conceptualisation, Funding acquisition, Methodology, Resources, Supervision, Visualisation, Writing—original draft, Writing—review & editing.
Dr Daphne Babalis (ORCID: 0000-0001-9654-4130): Methodology, Project administration, Validation, Writing—original draft.
Dr Christina Prechtl (ORCID: 0000-0003-4122-8899): Methodology, Project administration, Validation, Writing—original draft, Writing—review & editing.
Ms Aisha Anjum (ORCID: 0009-0005-4262-457X): Data curation, Methodology, Validation, Project administration, Writing—original draft.
Professor Mike Lean (ORCID: 0000-0003-2216-0083): Funding acquisition, Supervision.
Professor Waljit Dhillo (ORCID: 0000-0001-5950-4316): Funding acquisition, Methodology, Supervision.
Professor A. Toby Prevost (ORCID: 0000-0003-1723-0796): Conceptualisation, Funding acquisition, Formal analysis, Supervision, Writing—original draft, Writing—review & editing.
Ms Joana C. Vasconcelos (ORCID: 0000-0001-7709-4058): Data curation, Formal analysis, Supervision, Visualisation, Writing—original draft, Writing—review & editing.
Ms Jennifer E. Pugh (ORCID: 0000-0002-5310-2882): Data curation, Investigation, Methodology, Visualisation, Writing—original draft, Writing—review & editing.
Dr Katerina Petropoulou (ORCID: 0000-0003-4680-9246): Data curation, Investigation, Methodology, Writing—review & editing.
Dr Martina Tashkova (ORCID: 0009-0006-3346-7776): Investigation, Methodology.
Dr Sumayya Alshehhi (ORCID: 0009-0000-3933-3054): Investigation, Methodology.
Dr George Thom (ORCID: 0000-0002-8871-9524): Investigation, Methodology.
Ms Louise McCombie (ORCID: 0000-0002-1944-1290): Investigation, Methodology.
Ms Leah Holroyd: (ORCID: 0009-0006-7230-8284) SAG and TSC member, Visualisation, Writing—original draft, Writing—review & editing.
Dr Barzan A. Sadiq (ORCID:0000-0001-8579-1689): SAG member, Writing—original draft, Writing—review & editing.
Professor Tom Preston (ORCID: 0000-0002-9900-5682): Conceptualisation, Funding acquisition, Resources, Methodology.
JCV, AA and ATP accessed and verified the data. The corresponding authors (GF, DM) share responsibility for the decision to submit the manuscript for publication.
Data sharing statement
In this early stage of analysis requests for data access may be made to the corresponding authors for review. Once the data is complete, data will be shared with requestors following the review of scientific proposals.
Declaration of interests
GF, DM, and TP are named inventors of the patent Compounds and their effects on appetite control and insulin sensitivity WO2014020344A1 and are founding directors of a Spinout company aimed at commercialising IPE production. LH and BAS received compensation for their PPI contributions.
Acknowledgements
This project (EME Project: 15/185/16) is funded by the Efficacy and Mechanism Evaluation Programme (EME) Programme, an MRC (Medical Research Council) and NIHR partnership. Infrastructure support was provided by the NIHR Imperial Biomedical Research Centre and the NIHR Imperial Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the funder, the NIHR or the Department of Health and Social Care. All the clinical trials were conducted at the NIHR Imperial Clinical Research Facility and University of Glasgow Clinical Research Facilities; we thank all the NHS staff for their contribution and the trial volunteers who took part in the study. We would also like to thank the Clinical Research Network for the support they provided throughout the study. The production of IPE was funded by internal funding from SUERC.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102844.
Contributor Information
Douglas Morrison, Email: Douglas.morrison@glasgow.ac.uk.
Gary Frost, Email: g.frost@imperial.ac.uk.
Appendix A. Supplementary data
References
- 1.Prevalence of obesity. World obes. Fed. https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity
- 2.Frontier Economics . 2023. Unhealthy numbers: the rising cost of obesity in the UK.https://www.institute.global/insights/public-services/unhealthy-numbers-the-rising-cost-of-obesity-in-the-uk [Google Scholar]
- 3.Lewis C.E., Jacobs D.R., McCreath H., et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 4.Katsoulis M., Lai A.G., Diaz-Ordaz K., et al. Identifying adults at high-risk for change in weight and BMI in England: a longitudinal, large-scale, population-based cohort study using electronic health records. Lancet Diabetes Endocrinol. 2021;9:681–694. doi: 10.1016/S2213-8587(21)00207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill J.O., Wyatt H.R., Peters J.C. The importance of energy balance. Eur Endocrinol. 2013;9:111–115. doi: 10.17925/EE.2013.09.02.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fildes A., Charlton J., Rudisill C., Littlejohns P., Prevost A.T., Gulliford M.C. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health. 2015;105:e54–e59. doi: 10.2105/AJPH.2015.302773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truesdale K.P., Stevens J., Lewis C.E., Schreiner P.J., Loria C.M., Cai J. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. Int J Obes. 2006;30:1397–1407. doi: 10.1038/sj.ijo.0803307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds A.N., Mann J., Cummings J., Winter N., Mete E., Morenga L.T. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 9.Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., Macfarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microb. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne C.S., Chambers E.S., Morrison D.J., Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes. 2015;39:1331–1338. doi: 10.1038/ijo.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Public Health England . GOV.UK; 2020. National diet and nutrition survey.https://www.gov.uk/government/collections/national-diet-and-nutrition-survey [Google Scholar]
- 13.OECD . OECD; 2023. Health at a glance 2023: OECD indicators. [DOI] [Google Scholar]
- 14.Ridaura V.K., Faith J.J., Rey F.E., et al. Cultured gut microbiota from twins discordant for obesity modulate adiposity and metabolic phenotypes in mice. Science. 2013;341 doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boets E., Gomand S.V., Deroover L., et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595:541–555. doi: 10.1113/JP272613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husted A.S., Trauelsen M., Rudenko O., Hjorth S.A., Schwartz T.W. GPCR-mediated signaling of metabolites. Cell Metab. 2017;25:777–796. doi: 10.1016/j.cmet.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Chambers E.S., Viardot A., Psichas A., et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut. 2015;64:1744–1754. doi: 10.1136/gutjnl-2014-307913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pugh J.E., Anjum A., Petropoulou K., et al. 2022. Increase in colonic PRopionate as a method of prEVENTing weight gain in adults aged 20–40 years (iPREVENT): protocol of a multi-centre, double-blind, randomised, parallel-group trial to investigate the efficacy of inulin-propionate ester versus inulin (control) in the prevention of weight gain over 12 months [version 1; peer review: awaiting peer review] published online Oct 10. [DOI] [Google Scholar]
- 19.Polyviou T., MacDougall K., Chambers E.S., et al. Randomised clinical study: inulin short-chain fatty acid esters for targeted delivery of short-chain fatty acids to the human colon. Aliment Pharmacol Ther. 2016;44:662–672. doi: 10.1111/apt.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton P.R., Gurrin L.C., Campbell M.J. Clinical significance not statistical significance: a simple Bayesian alternative to p values. J Epidemiol Community Health. 1998;52:318–323. doi: 10.1136/jech.52.5.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allman-Farinelli M., Partridge S.R., McGeechan K., et al. A mobile health lifestyle program for prevention of weight gain in young adults (TXT2BFiT): nine-month outcomes of a randomized controlled trial. JMIR MHealth UHealth. 2016;4 doi: 10.2196/mhealth.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunn G., Maracy M., Dowrick C., et al. Estimating psychological treatment effects from a randomised controlled trial with both non-compliance and loss to follow-up. Br J Psychiatry. 2003;183:323–331. doi: 10.1192/bjp.183.4.323. [DOI] [PubMed] [Google Scholar]
- 23.White I.R. Uses and limitations of randomization-based efficacy estimators. Stat Methods Med Res. 2005;14:327–347. doi: 10.1191/0962280205sm406oa. [DOI] [PubMed] [Google Scholar]
- 24.Newcombe R.G. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 25.Bakaloudi D.R., Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Chourdakis M. Impact of the first COVID-19 lockdown on body weight: a combined systematic review and a meta-analysis. Clin Nutr. 2022;41:3046–3054. doi: 10.1016/j.clnu.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malkova D., Polyviou T., Rizou E., et al. Moderate intensity exercise training combined with inulin-propionate ester supplementation increases whole body resting fat oxidation in overweight women. Metabolism. 2020;104 doi: 10.1016/j.metabol.2019.154043. [DOI] [PubMed] [Google Scholar]
- 27.Luke A., Schoeller D.A. Basal metabolic rate, fat-free mass, and body cell mass during energy restriction. Metabolism. 1992;41:450–456. doi: 10.1016/0026-0495(92)90083-m. [DOI] [PubMed] [Google Scholar]
- 28.Walsh M.E., Bhattacharya A., Sataranatarajan K., et al. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell. 2015;14:957–970. doi: 10.1111/acel.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo T., Kishi M., Fushimi T., Kaga T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J Agric Food Chem. 2009;57:5982–5986. doi: 10.1021/jf900470c. [DOI] [PubMed] [Google Scholar]
- 30.Gao Z., Yin J., Zhang J., et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L., Fu C., Li F. Acetate affects the process of lipid metabolism in rabbit liver, skeletal muscle and adipose tissue. Animals. 2019;9:799. doi: 10.3390/ani9100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chambers E.S., Byrne C.S., Morrison D.J., et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut. 2019;68:1430–1438. doi: 10.1136/gutjnl-2019-318424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pingitore A., Chambers E.S., Hill T., et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes Metab. 2017;19:257–265. doi: 10.1111/dom.12811. [DOI] [PubMed] [Google Scholar]
- 34.Byrne C.S., Chambers E.S., Preston T., et al. Effects of inulin propionate ester incorporated into palatable food products on appetite and resting energy expenditure: a randomised crossover study. Nutrients. 2019;11:E861. doi: 10.3390/nu11040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salazar N., Arboleya S., Fernández-Navarro T., de los Reyes-Gavilán C.G., Gonzalez S., Gueimonde M. Age-associated changes in gut microbiota and dietary components related with the immune System in adulthood and old age: a cross-sectional study. Nutrients. 2019;11:1765. doi: 10.3390/nu11081765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byrne C.S., Chambers E.S., Alhabeeb H., et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr. 2016;104:5–14. doi: 10.3945/ajcn.115.126706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malam S., Prior G., Phillips R., O'Driscoll C. Food Standards Agency; 2014. The 2014 food and you survey. England bulletin 3. Eating outside the home.https://www.food.gov.uk/sites/default/files/media/document/england-bulletin-3-food-and-you-2014_0.pdf [Google Scholar]
- 38.Lachat C., Nago E., Verstraeten R., Roberfroid D., Van Camp J., Kolsteren P. Eating out of home and its association with dietary intake: a systematic review of the evidence. Obes Rev. 2012;13:329–346. doi: 10.1111/j.1467-789X.2011.00953.x. [DOI] [PubMed] [Google Scholar]
- 39.Wing R.R., Tate D.F., Espeland M.A., et al. Innovative self-regulation strategies to reduce weight gain in young adults: the study of novel approaches to weight gain prevention (SNAP) randomized clinical trial. JAMA Intern Med. 2016;176:755–762. doi: 10.1001/jamainternmed.2016.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Office for Health Improvement and Disparities . 2021. National Diet and Nutrition Survey: diet, nutrition and physical activity in 2020 A follow up study during COVID-19. [published online Sept] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.