Abstract
Even though respiratory dysfunctions are the primary symptom associated with SARS-CoV-2 infection, cerebrovascular events, and neurological symptoms are described in many patients. However, the connection between the neuroimmune profile and the lung's inflammatory condition during COVID-19 and its association with the neurological symptoms reported by COVID-19 patients still needs further exploration. The present study characterizes the SARS-CoV-2 infectivity profile in postmortem nervous and lung tissue samples of patients who died due to severe COVID-19, and the pro-inflammatory factors present in both nervous and lung tissue samples, via a proteomic profiling array. Additionally, Brain-Derived Neurotrophic Factor (BDNF) levels and intracellular pathways related to neuroplasticity/neuroprotection were assessed in the samples. Out of the 16 samples analyzed, all samples but 1 were positive for the viral genome (genes E or N2, but only 3.9% presented E and N2) in the olfactory brain pathway. The E or N2 gene were also detected in all lung samples, with 43.7% of the samples being positive for the E and N2 genes. In the E/N2 positive brain samples, the Spike protein of SARS-CoV-2 co-localized with TUJ-1+ (neuron-specific class III beta-tubulin) and GFAP+ (glial fibrillary acidic protein) astrocytes. IL-6, but not IL-10, expression was markedly higher in most nervous tissue samples compared to the lung specimens. While intracellular adhesion molecule-1 (ICAM-1), interleukin-8 (IL-8), macrophage migration inhibitory factor (MIF), and plasminogen activator inhibitor 1 (PAI-1) were increased in lung samples from SARS-Cov-2 patients, only MIF and IL-18 were detected in nervous tissue samples. Correlation analysis suggested that high levels of IL-6 are followed by increased levels of IL-10 in the brain, but not in lung samples. Our analysis also demonstrated that the presence of comorbidities, such as cardiovascular disease, hypertension, and hypothyroidism, is associated with neuroinflammation, while chronic kidney conditions predict the presence of neurological symptoms, which correlate with lower levels of BDNF in the brain samples. Our results corroborate the hypothesis that a pro-inflammatory state might further impair neural homeostasis and induce brain abnormalities found in COVID-19 patients.
Keywords: SARS-CoV-2, Severe COVID-19, Neuroinflammation, BDNF, Cardiovascular disease, Kidney disease
Highlights
-
•
IL-6 expression is higher in nervous tissue compared to lung specimens of severe COVID-19 patients.
-
•
Cardiac and renal comorbidities contribute to neuroinflammation in severe COVID-19.
-
•
Chronic kidney conditions predict lower BDNF levels in brain samples of severe COVID-19.
1. Introduction
More than two years after the beginning of the COVID-19 pandemic, SARS-CoV-2 infection has caused the death of millions of people worldwide and challenged the health systems and the scientific community to find the best ways to combat the disease (Mohamadian et al., 2021; Singh et al., 2020). Even though SARS-CoV-2 infection is most frequently associated with respiratory dysfunctions, many patients have cerebrovascular events and neurological symptoms (Varatharaj et al., 2020; Lu et al., 2020). Moreover, there are reports of patients without respiratory signs, but with neurological manifestations as the only consequence of SARS-CoV-2 infection (Duong et al., 2020). The neurological symptoms may include dizziness, acute cerebrovascular disorders, headaches, convulsions, encephalopathy, encephalitis, ischemic stroke, changes in consciousness, muscle injury, and anosmia (Mao et al., 2020; Fotuhi et al., 2020), with an increasing number of reports supporting that SARS-CoV-2 is neurotropic.
The novel coronavirus has already been detected in the cerebrospinal fluid of COVID-19 patients and human brain organoids (Huang et al., 2020; Song et al., 2021). Furthermore, human autopsy studies have shown viral RNA transcripts in brain tissues and viral proteins in the endothelial cells within the olfactory bulb of patients who died from COVID-19 complications (Puelles et al., 2020; Solomon et al., 2020; Daly et al., 2020). Studies have tried to elucidate the implications of SARS-CoV-2 neuroinfection, including its long-term consequences (Helms et al., 2020; Al‐Sarraj et al., 2021). RNA of other human coronavirus (HCoVs), such as HCoV-OC43, has been detected in postmortem brain samples from patients with multiple sclerosis (Murray et al., 1992). HCoV-OC43 can quickly propagate between neurons and reach several brain areas, compromising multiple brain functions and, in some cases, producing fatal encephalitis (Arbour et al., 2000). Additionally, HCoV-NL63, whose cell entry receptor is ACE-2, which is the main cell receptor for SARS CoV-2, has been implicated in neurological conditions, including seizures and acute encephalitis (Chiu et al., 2005).
However, there have been controversial results regarding the brain infectivity profile of SARS-CoV-2 and its correlation with poor neurological outcomes. While some studies have reported SARS-CoV-2 genome in olfactory nerve cells, others have failed to find significant virus detection, posing an important question about whether SARS-CoV-2 effectively infects the brain and is unequivocally connected with neurological symptoms in COVID-19 patients (Song et al., 2021; Li et al., 2016; Meinhardt et al., 2021). Therefore, the present study investigated the neuro-immune consequences of COVID-19 in postmortem samples of the brain/olfactory pathway, and their relationship with the in-situ detection of SARS-CoV2.
2. Material and methods
2.1. -Detection of SARS-CoV-2 genome in the samples
SARS-CoV-2 genes E/N2 were detected by RT-qPCR according to the Charité protocol (Meinhardt et al., 2021), using β-actin/RNAse mRNAs as housekeeping genes and total nucleic acids extracted with Trizol® from 250 μL of homogenized cell pellets and supernatants to determine the genome viral load in postmortem tissues and in vitro assays. All real-time PCR assays were performed on a Step-One Plus real-time PCR thermocycler (Applied Biosystems, Foster City, CA, USA), with specific primers and probes (Table 1). Briefly, after reverse transcription with 1 μL of extracted RNA primed with random hexamers using Multiscribe Reverse Transcriptase (Applied Biosystems, Foster City, CA, USA), real-time PCR for SARS-CoV-2 was performed in a final volume of 15 μL, using 3 μL of cDNA, 400 nM forward and reverse primers, 200 nM probe, 0.15 μL of Rox and 7.5 μL of TaqMan master mix (Sigma-Aldrich, St. Louis, MO, EUA), with the following parameters: 95 °C for 3 min (min) and then 45 cycles of 95 °C for 15 s (s), 60 °C for 30 s, with a final soaking at 10 °C.
Table 1.
Primers used for SARS-CoV-2 PCR detection.
| Primer | Sequence | Reference | |
|---|---|---|---|
| N2 | Forward | 5’-TTA CAA ACA TTG GCC GCA AA-3’ | Nalla et al., 2020 |
| Reverse | 5’-GCG CGA CAT TCC GAA GAA-3’ | ||
| Probe | 5’-FAM-ACA ATT TGC CCC CAG CGC TTC AG-BHQ1-3’ | ||
| Forward | 5’-ACAGGTACGTTAATAGTTAATAGCGT-3’ | ||
| E | Reverse | 5’-ATATTGCAGCAGTACGCACACA-3’ | Corman et al., 2020 |
| Probe | 5’-AM-ACACTAGCCATCCTTACTGCGCTTCG-BHQ-1-3’ | ||
| Forward | 5’-AGA TTT GGA CCT GCG AGC G-3’ | ||
| RNAse-P | Reverse | 5’-GAG CGG CTG TCT CCA CAA GT-3’ | Nalla et al., 2020 |
| Probe | 5’-FAM – TTC TGA CCT GAA GGC TCT GCG CG – BHQ-1-3’ | ||
| Forward | 5’ -CCC AGC CAT GTA CGT TGC TA- 3’ | ||
| β-actin | Reverse | 5’-TCA CCG GAG TCC ATC ACG AT-3’ | Proenca-Modena et al., 2012 |
| IL-6 | ProbeForwardReverse | Fam-ACG CCT CTG GCC GTA CCA CTG G-Tamra | |
| 5’ -ATC GGG CTG AAC GGT CAA AG- 3’ | |||
| 5’ -GGC GTC GTG GAT GAC ACA- 3’ | |||
| IL-10 | ForwardReverse | 5’ -TTC CCT GAC CTC CCT CTA ATT -3’ | |
| 5’ -GTC CCC TGG TTT CTC TTC CTA A -3’ |
2.2. Postmortem samples
Postmortem samples were collected from olfactory epithelium/brain (OE/brain samples) by minimally invasive autopsies done on sixteen individuals who died from COVID-19. The study was approved by the National Commission for Research Ethics (CAAE: 32475220.5.0000.5440 and CAAE: 38071420.0.1001.5404). Clinical data and comorbidities of the patients are presented in Table 2. OE/brain Formalin-fixed paraffin-embedded brain tissue samples were obtained and then sectioned into slices of 2–4 μm of thickness. For proteomic assay, proteins were isolated from the organic phase saved from the RNA isolation step of the RT-qPCR analysis. Protein concentration was measured with a protein bicinchoninic acid kit (BCA) (Thermo Fisher Scientific, Inc.).
Table 2.
Clinical data of patients during COVID-19 hospitalization.
| Patient | Comorbidities |
∗Neurological Symptoms |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Time from Covid-19 diagnosis and death (days) | DM | HTN | OB | CP | SMK | ACL | Other diseases/conditions | H | ANM | DIS | |
| 1 | M | 24 | 15 | DM; OB | X | None | X | X | |||||
| 2 | F | 31 | 6 | X | Intestinal and urinary tract obstruction due to tumors | ||||||||
| 3 | F | 69 | 13 | X | X | Chronic Obstructive Lung Disease | |||||||
| 4 | M | 49 | 30 | X | X | X | Lung Aspergillosis Bronchitis, right leg venous insufficiency | ||||||
| 5 | F | 77 | 24 | X | None | X | |||||||
| 6 | M | 74 | 13 | X | X | X | X | Chronic kidney Disease; Previous Cerebrovascular Accident | |||||
| 7 | F | 69 | 12 | X | X | X | X | Hypothyroidism, Dyslipidemia | |||||
| 8 | F | 53 | 26 | X | Tracheal stenosis; Previous Myocardial infarction; Previous Cerebrovascular Accident | ||||||||
| 9 | M | 65 | 10 | X | X | Hypertriglyceridemia; Hypothyroidism | |||||||
| 10 | F | 53 | 19 | X | Hypothyroidism | X | X | ||||||
| 11 | M | 74 | 25 | X | X | X | X | Lung hypertension under investigation; severe sleep apnea; Previous cerebrovascular accident with motor sequelae; Hypothyroidism; Osteoarthritis- knee; Chronic kidney disease | |||||
| 12 | M | 43 | 34 | X | None | X | X | ||||||
| 13 | M | 78 | 38 | X | X | Hypothyroidism | |||||||
| 14 | F | 68 | 11 | X | X | X | X | Chronic kidney Disease; Major Depressive Disorder, dyslipidemia. | |||||
| 15 | F | 83 | 24 | Alzheimer's Disease Hypothyroidism |
|||||||||
| 16 | M | 73 | 17 | Asthma | |||||||||
DM: Diabetes Mellitus; HTN: Hypertension; OB: Obesity; CP: Cardiac Pathologies: SMK: Smoking; AA: Alcoholism; H: Headache; ANM: Anosmia; DIS: Dysgeusia.
2.2.1. Immunofluorescence staining
Sixteen postmortem tissue samples taken from olfactory epithelium/brain were fixed with 4% paraformaldehyde (PFA) for 24 h (h). Tissues were then processed and embedded in paraffin for posterior preparation of 4 μm tissue sections using a microtome and mounted in gelatin-covered glass slides (1 slide per sample). Subsequently, tissue sections were submitted to sequential incubations with organic solvents to remove paraffin and then to an antigen retrieval protocol (30 min in citrate buffer at 70 °C). Slides were then washed 3 times in TBS (Tris Buffered Saline, 50 nM) and incubated for 4 h in a blocking solution [bovine serum albumin (BSA) 1% + 0,25% Triton 100X in TBS). After that, slides were incubated with a combination of primary antibodies for 48 h (human chimeric anti-SARS-CoV-2 Spike S1- GeneScript + mouse monoclonal anti-GFAP- Cell Signaling- 1:500; or human chimeric anti-SARS-CoV-2 Spike S1+ mouse Tuj-1- Millipore, 1:500; or human chimeric anti-SARS-CoV-2 Spike S1- GeneScript + goat polyclonal anti-Iba- Novusbio 1:500). The slides were washed three times in TBS and incubated with secondary antibodies Alexa Fluor, nuclei were stained with Hoestch (Life Technologies), and images were acquired in a confocal microscope (Leica SPE-TS) using Z-stacks, and analysis of colocalization was done using the 3D function of the LAX-Z software.
2.2.2. Quantitative PCR for IL-6 and IL-10 mRNAs
Tissue fragments of the postmortem samples collected through minimally invasive biopsy (intranasal-olfactory pathway/brain and lung) were placed in individual Eppendorf tubes, each containing 500 μL of TRIzol™ (Invitrogen, Thermo Fisher Scientific, USA). The tissues were then subjected to tissue lysis and homogenization using a Tissuelyser. Subsequently, the steps of RNA extraction were carried out with TRIzol™ (TRIzol Plus RNA Purification, Thermo Fisher Scientific, USA), cDNA synthesis (High-Capacity RNA to cDNA™ Kit, Thermo Fisher Scientific, USA), and RT-PCR for the quantification of the expression of the Interleukin-6 (IL-6- primers Table 1), and Interleukin-10 (IL-10- primers Table 1) mRNAs. Real-time PCR assays were performed using specific primers and probes, along with TaqMan Fast Advanced Master Mix (Applied Biosystems™). All assays were conducted following the instructions provided by their respective manufacturers. The obtained values were normalized by the expression of the β-actin gene. The experiment was performed with a total of three biological replicates and results expressed by 2- Δ/ΔCt method.
2.2.3. Proteomic profile of cytokines and chemokines
To measure cytokines and chemokines, purified proteins were obtained from OE/brain and lung tissues. Using a Human Cytokine Array Panel A kit (R&D Systems, Inc.), 36 different cytokines were analyzed according to the manufacturer's instructions. The immune spots were exposed and captured with an Imager AI680 (GE Amersham™), and the data were analyzed with Image Studio Lite 5.2 software (LI-COR, Inc.).
2.2.4. Enzyme-linked immunosorbent assay for the quantification of IL-6, IL-10, ACE-2 and BDNF
IL-6 (cat number- D6050), IL-10 (cat number-DY217b), ACE2 (cat number-DY929) and BDNF (cat number-DY248) expression were measured with commercial ELISA DuoSet kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Briefly, proteins extracted from the supernatant of the TRIZOL phase of brain and lung postmortem samples were analyzed in 96-well microplates (DY990 - R&D Systems, Minneapolis, MN, USA) that were incubated overnight at room temperature with 100 μL of Capture antibodies (IL-6: PART#890045; IL-10: PART#841825; ACE-2: PART#841365; BDNF: PART#840180 - R&D Systems, Minneapolis, MN, USA) diluted in PBS (137 mM NaCl; 2.7 mM KCl; 8.1 mM Na2HPO4; 1.5 mM KH2PO4; pH 7.2–7.4, 0.2 μm filtered). After three washes with Wash Buffer (0.05% Tween® 20 in PBS, pH 7.2–7.4), microplates were blocked with 300 μL/well of Reagent Diluent (1% BSA in PBS, pH 7.2–7.4, 0.2 μm filtered) and incubated at room temperature for 1 h. After another wash step, 100 μL of samples (previously centrifuged and diluted 1:10 in Reagent Diluent) or standards were added to the microplates and incubated for 4 h at room temperature. Microplates were washed once again and 100 μL of detection antibodies (IL-6: PART#890046; IL-10: PART#840196; ACE-2: PART#841366; BDNF: PART#840181 - R&D Systems, Minneapolis, MN, USA) diluted in Reagent Diluent were added and incubated for 2 h at room temperature. After another wash step, 100 μL of Streptavidin conjugated to horseradish-peroxidase (Streptavidin-HRP) diluted in Reagent Diluent were added and incubated at room temperature, protected from light, for 20 min. The microplates were rewashed, and 100 μL/well of Substrate Solution (1:1 mixture of Color Reagent A: H2O2 and Color Reagent B: Tetramethylbenzidine) were added and then incubated for 20 min at room temperature. To stop the reaction, 50 μL of Stop Solution (H2SO4 2N) were added, and absorbance was immediately measured at 450 nm using a BioTek Epoch plate reader (BioTek Instruments, Winooski, VT, USA). Data analysis and calculations were performed with BioTek Gen5 3.05 and Microsoft Excel 2016 software. Results were normalized by total protein of each sample quantified by BCA method (Thermo Fisher Scientific, Inc; Massachusetts- USA).
2.3. Statistical analysis
Student's t-test (using degree of freedom correction, since we do not have the assumption of variance homogeneity) was used to determine differences between groups of patients with different comorbidities or according to the presence or absence of SARS-CoV-2 in the samples. Non-parametric statistical analyses were performed since our data did not follow a normal distribution, and the population variances were not homogeneous. Pearson´sPearson's 2-tailed correlation and Simple Regression Analysis were used to determine the degree of association between cytokine levels, ACE-2, BDNF expression levels, and comorbidities. Data are expressed as mean ± SEM or median and interquartile intervals. All statistical analyses were performed using SPSS version 16.0 (IBM, New York, USA).
3. Results
3.1. SARS-CoV-2 gene products are detectable in postmortem OF/brain tissue samples from COVID-19 patients
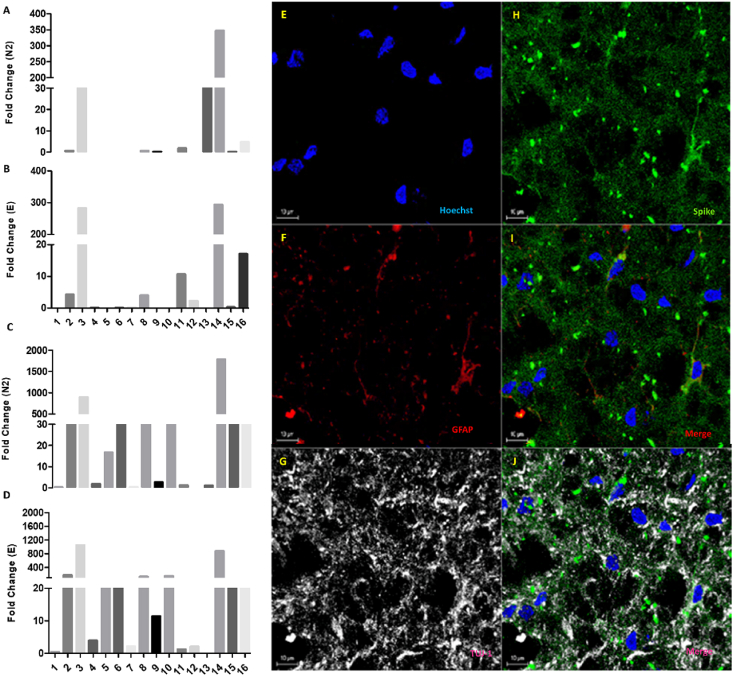
The expression of the SARS-CoV-2 E and N2 genes in nervous tissue as well as lung samples were initially determined. Not all analyzed samples showed a positive relative expression for either E (7 patients) or N2 (1 sample) genes (Fig. 1A–B) (Supplementary Fig. 1). While 3.94% of the neural samples were positive for the E and N2 genes (E and N2 simultaneously - 3 patients), 43.7% of the lung samples were positive for E and N2, indicating differences (in the same patients) between the presence of SARS-CoV-2 genes in the lung and the brain. Noteworthy, even in the OE/brain samples in which the viral genes were detected, the relative gene expression for E and N2 were 2–6 times lower compared to the relative expression found for the same genes in lung samples from the same patients (Fig. 1C–D).
Fig. 1.
SARS-CoV-2 in postmortem samples. SARS-CoV-2 E and N2 genes were detected in some, but not all, postmortem nervous tissue samples from COVID-19 patients. Relative expression of N2 (A- Brain/olfactory epithelium and C- Lung) and E (B- brain/olfactory epithelium and D-lung). Fold change (2ˆΔΔCT) was normalized by the expression of β-actin. E-J:SARS-CoV-2 targets both GFAP+ astrocytes and TUJ1+ neurons in the olfactory nervous tissue. Immunohistochemistry staining of the Spike protein of SARS-CoV-2 (green), GFAP+ cells (red), TUJ1+ cells (grey), and cell nuclei (Hoechst; blue) in postmortem nervous tissue samples collected from the olfactory epithelium of patients who died due to COVID-19. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Altogether, these observations indicate that the nervous tissue is targeted by SARS-CoV-2 in patients with severe cases of COVID-19, although the level of infectivity in this tissue is, as expected, substantially lower than in the respiratory tract.
To characterize the presence of viral proteins in nervous tissue collected from the olfactory epithelium of patients who died due to COVID-19, immunostaining analysis was performed, and confirmed the presence of both TUJ-1+ neurons and GFAP+ astrocytes in the olfactory nervous tissue samples (Fig. 1E–H). The S protein of SARS-CoV-2 was co-localized with both TUJ-1 and GFAP markers, indicating that SARS-CoV-2 infects both cell types in the nervous system (Fig. 1E–J).
3.2. Inflammatory signaling molecules are upregulated in postmortem nervous tissue samples from COVID-19 patients
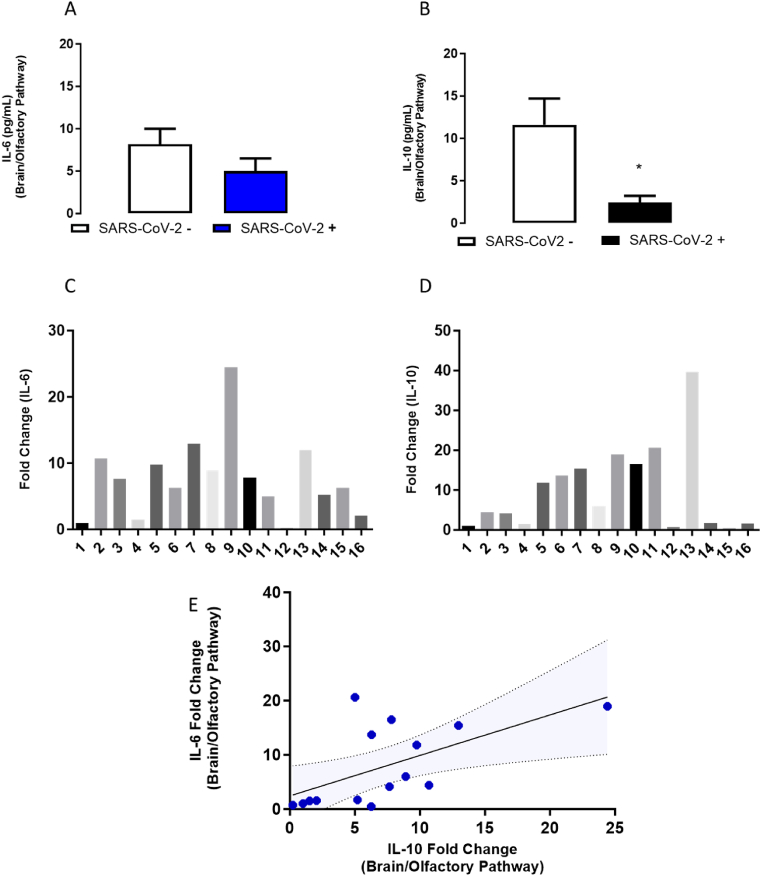
To address whether SARS-CoV-2 neural infection-related inflammatory response led to neuro-inflammation and pathology, we assessed the expression of the pro-inflammatory cytokine IL-6 in all nervous tissue samples. IL-6 levels were not significantly different between the samples positive for E or N2 or E/N2 genes, nor samples negative for E or/and N2 genes (Fig. 2A and C). Interestingly, the magnitude of the change in the mRNA expression of IL-6 was substantially higher in the nervous tissue (t(15.07) = 4.3, p = 0.001, Supplementary Figs. 2A–C) compared to the lung samples. Concerning patient's samples positive detection of SARS-CoV-2 viral genes E, N2 or both in the nervous tissue had decreased levels of the IL-10 anti-inflammatory cytokine (t (Daly et al., 2020)= 2.3, p = 0.04- Fig. 2B and D). Quantification of IL-10 mRNA revealed that the cytokine levels in the nervous tissue exceeded the levels in the lungs for all patients (t(15.08) = 3.3, p = 0.004, Supplementary Figs. 2D–F). In addition to the presence of viral genetic material in situ, the results indicate that COVID-19 strongly modulates neuro-immune responses in the analyzed samples (postmortem brain/olfactory pathway). Simple regression and Pearson's correlation analysis suggested that the levels of IL-6 in samples from OE/brain were positively correlated to the levels of IL-10 (R2 = 0.37; F(1,14) = 7.6, p = 0; 016; r = 0.6; p = 0.02, Fig. 2C). Therefore, the results suggest that SARS-CoV-2 induces a strong immune modulation in the CNS samples analyzed.
Fig. 2.
IL-6 and IL-10 levels detected in postmortem samples of the brain/olfactory pathway from COVID-19 patients. A-B. Expression of IL-10 and IL-6 protein levels (ELISA). C and D-mRNA levels of IL-6 and IL-10 in the brain/olfactory pathway samples positive (N = 8) and negative (n = 8) for SARS-CoV-2 detection from patients who died from COVID-19. C. Sample Regression analysis of IL-6 and IL-10 (mRNA) levels in the brain/olfactory pathway ( ± 95% CI). Bars represent the mean ± SEM. ∗ indicates difference in SARS-CoV2- negative (−) samples.
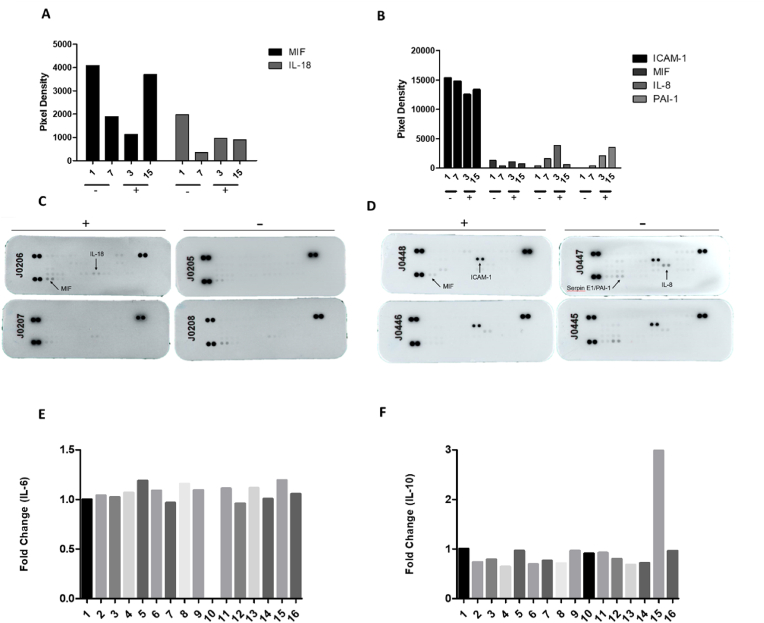
The proteomic array indicated the expression of several inflammatory factors such as the macrophage migration inhibitory factor (MIF) and the pro-inflammatory cytokine IL-18 in OE/brain samples (independently of virus presence). In the lungs, we detected intracellular adhesion molecule-1 (ICAM-1), IL-8, MIF, and plasminogen activator inhibitor 1 (PAI-1) (Fig. 3A–H). We also determined the mRNA levels of IL-6 and IL-10 in lung samples (expressed individually- Fig. 3A–D). Additionally, we detected p-mTOR, p-ERK, and NLRP3 in lung samples, confirming the activation of inflammatory pathways (Supplementary F ig. 3 A-C).
Fig. 3.
Qualitative inflammatory profile in postmortem samples of COVID-19 patients (brain/olfactory pathway and lungs). Relative expression of IL-6 (A-Nervous tissue and B-lungs) and IL-10 (C-Nervous tissue and D-lungs) of 16 patients who died due to severe COVID-19. Fold change (2ˆΔΔCT) normalized by the expression of GAPDH. E-H. Different inflammatory factors are detected in nervous tissue and in lung samples of COVID-19 patients (- Negative for SARS-CoV2, samples 1 and 7; + positive for SARS-CoV-2 samples 3 and 15). Dot blot analysis of brain/olfactory pathway (E, G) and lung (F, H) samples.
Taken together, the results suggest that the presence of SARS-CoV-2 in neural tissue might not be essential to explain the inflammatory environment found in the nervous system of patients with severe cases of COVID-19, with or without neurological complications.
3.3. ACE-2 expression in the brain/olfactory pathway is associated with the presence of neurological symptoms of COVID-19
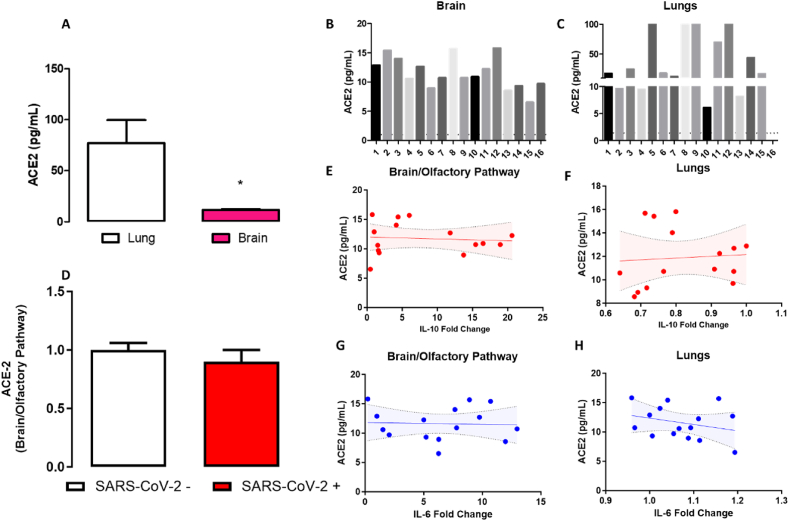
The Angiotensin Converting Enzyme 2 (ACE-2) is the main receptor for SARS-CoV-2 in human cells (Meinhardt et al., 2021). However, the expression of ACE-2 in neural cells seems to be very low in comparison with peripheral organs such as the lungs (Salamanna et al., 2020). Our data indicate that brain/olfactory pathway samples exhibit lower levels of ACE-2 than lung samples (t(15.1) = 2.9, p = 0.011- Fig. 4A). The expression of ACE-2 in brain/olfactory pathway samples was not associated with the presence of SARS-CoV-2 genome, nor correlated with the levels of the IL-6 or IL-10 cytokines in nervous tissue or lung samples.
Fig. 4.
ACE-2 expression in the brain and lungs detected in postmortem samples of COVID-19 patients. A. Expression of ACE-2 levels in brain/olfactory pathway and lungs measured by ELISA. B-C. Individual ACE-2 expression of 16 patients who died from COVID-19 in brain (N = 16) and lungs (N = 16), respectively. D. ACE-2 expression of patients in which SARS-CoV-2 was undetectable (N = 8) and positive for SARS-CoV-2 (N = 8). E-F. Correlation analysis of ACE-2 and IL-10 (mRNA) in the brain/olfactory pathway (E) and lungs (F). G-H. Correlation analysis of ACE-2 and IL-6 (mRNA) in the brain/olfactory pathway (G) and lungs (H). Bars represent mean ± SEM. Correlation graph ( ± 95% CI). ∗ Indicates difference from lung samples (Student´s t-test).
3.4. Comorbidities (cardiovascular disease, obesity, and hypothyroidism) predict worse neuroinflammatory profile
Among all the samples analyzed, only 3 were from patients without comorbidities (Table 2), which is in line with previous observations suggesting that severe cases of COVID-19 are frequently associated with pre-existing chronic conditions (Zhou et al., 2020). The correlation analysis to determine the influence of these comorbidities on the neuroinflammatory profile of the brain/olfactory tissues showed that the presence of those pathologies did not correlate with the variables analyzed, neurological symptoms associated with COVID-19, levels of cytokines, and expression of ACE-2.
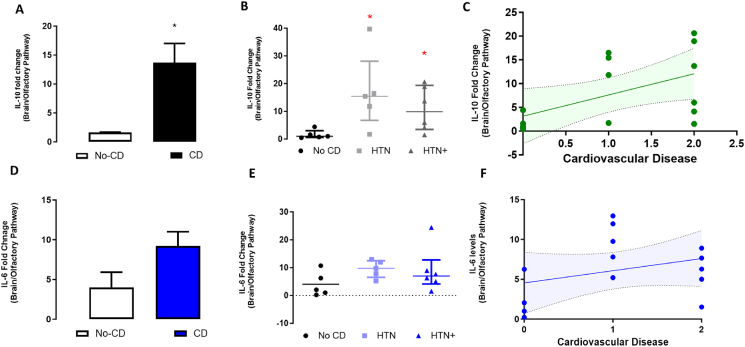
Nonetheless, patients with cardiovascular disease and hypertension had higher levels of IL-10 (Fig. 5A–C), but not IL-6 (Fig. 5D–F), than patients with no cardiovascular pathology (t(10.9) = 3.5, p = 0.005), especially when associated with chronic hypertension (Kruskal Wallis followed by Dunn test, χ2 = 7,8 df = 2, p = 0.03). Simple Regression Analysis also suggested that increased levels of IL-10, but not IL-6 (Fig. 5F), was associated with previous cardiovascular conditions (F (Mohamadian et al., 2021; Al‐Sarraj et al., 2021) = 5,2; p = 0.04)). Additionally, Pearson's correlation analysis also indicated a positive association of IL-10 with the existence and severity of previous cardiovascular conditions (r = o.28, p = 0.02).
Fig. 5.
IL-6 and IL-10 levels related to cardiovascular comorbidities in postmortem samples of COVID-19 patients. A. IL-10 levels in the brain/olfactory pathway of patients with and without cardiovascular disease. B. mRNA levels of IL-10 in the brain/olfactory pathway associated with hypertension. C. Correlation analysis of IL-10 mRNA and cardiovascular disease. D. IL-6 mRNA in the brain/olfactory pathway of patients with and without cardiovascular disease. E. Levels of IL-6 in the brain/olfactory pathway associated with hypertension. F. Correlation analysis of IL-6 levels and cardiovascular disease. CD – cardiovascular disease; HTN – hypertension. HTN + - hypertension + cardiovascular and kidney disease. A and D- N = 5 and 11 respectively; B and D- N = 5, 5, 6 respectively. Bar/Dot plots indicate mean ± SEM. ∗ indicates difference from no CD (A-D- Student´s t-test; B and E−one-way ANOVA followed by Duncan´s posttest.
There was also a positive correlation between hypothyroidism with and higher levels of IL-10 in the nervous tissue sample (correlation-r2 = 0.5542, p = 0.005; Pearson r = 0.75, p = 0.005- Supplementary Fig. 4).
3.5. Hypertension and chronic kidney condition are associated with reduced brain levels of brain-derived neurotrophic factor in COVID-19 patients
The neuroimmune hypothesis of neuropsychiatric disorders suggests that reduced BDNF levels are associate with mental illness, and possibly due to increased neuroimmune modulation inducing a proinflammatory state (Lima et al., 2019), a panorama similar to the one described in COVID-19 patients (Vanderheiden and Klein, 2022), as supported by our own results.
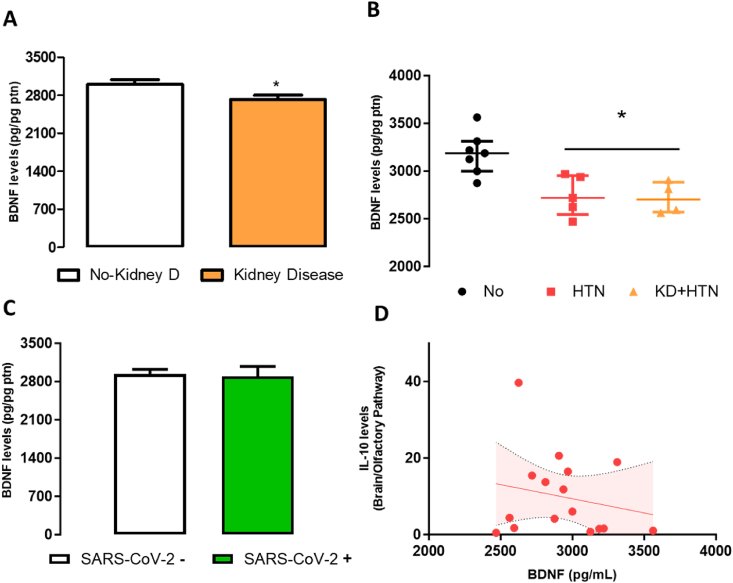
COVID-19 patients with previous chronic kidney disease had lower levels of BDNF when compared with patients without kidney disease (t(Puelles et al., 2020) = 2,30, p = 0.043, Fig. 6A). In addition, the presence of kidney disease correlated negatively with BDNF levels (Spearman-rho R2 = −0.44, p = 0.033), which was not seen with other comorbidities (obesity or lung conditions-data not shown). Curiously, COVID-19 patients with diagnosis of hypertension (without kidney disease or other cardiac pathologies) also exhibited decreased BDNF levels in brain samples (Kruskal Wallis, χ2 = 9.3, df = 2, p = 0.01) (Fig. 6B). Changes in BDNF levels were not associated with the presence of SARS-CoV-2 in brain samples, or with levels of IL-10 (Fig. 6D) or IL-6 (data not shown).
Fig. 6.
BDNF levels correlates with comorbidities in postmortem samples of COVID-19 patients. A. BDNF levels of patients with and without kidney disease. B. BDNF levels of patients with hypertension and kidney disease. C. Expression of BDNF in samples negative for SARS-CoV-2 and positive for SARS-CoV-2 who died from COVID-19 (N = 8/group). D. Correlation analysis between IL-10 and BDNF levels. KD – kidney disease; HTN – hypertension; KD + HTN- kidney disease + hypertension. Bars represent mean ± SEM. ∗ indicates a difference from no KD (Student´s t-test). A and C- N = 7,9 respectively; B- N = 7,5,4 respectively.
4. Discussion
The documented neurological symptoms and brain abnormalities in SARS-CoV-2 infected individuals show that the viral infection may have long-term effects on the nervous system. In this study we analyzed postmortem nervous and lung tissue samples from patients who died from COVID-19 to characterize and compare the infectivity profile of SARS-CoV-2 in both sites, and further investigate the neuro-immune consequences of the infection.
Our results show that the Spike protein of SARS-CoV-2 can be found co-localized with TUJ-1+ neurons and GFAP + astrocytes in the olfactory nervous tissue samples. However, SARS-CoV-2 genome was not detected in all samples, but only 50% of them. A recent systematic review of neuropathological studies of COVID-19 reported a similar rate of detection of SARS-CoV-2 genome in nervous tissue specimens (41.9% within 438 patients from 45 articles) (Cosentino et al., 2021).
Interestingly, the vast majority of these studies used postmortem tissue samples or in vitro models, such as human brain organoids. The research shows that SARS-CoV-2 effectively infects and replicates in many parts of the brain, but to a lesser extent than in other tissues and organs, such as the lungs. In this research, viral infectivity and replication were investigated using RT-qPCR to identify viral RNA (E, N2, and NSP16) and immunofluorescence to target the viral genome and neural markers. Most studies found similar rates of infection to those we have shown in the present study (BECKMAN et al., 2022; SERRANO et al., 2022; HOU et al., 2022)
The relative expression of E and N2 genes was 2–6 times higher in lungs than in nervous tissue. Notably, although there were markedly lower levels of ACE-2 expression in the olfactory nervous tissue when compared to the lungs, our results suggest that the presence of SARS-CoV-2 genome or the levels of the cytokines IL-6 or IL-10 in the brain or lung, were not linked to the expression of ACE-2 in brain/olfactory tissue samples. Thus, these results raise questions about whether the low infectivity rate of the olfactory nervous tissue is sufficient to promote the long-term brain abnormalities that have been reported.
Indeed, aside from olfactory-transmucosal transmission, SARS-CoV-2 could gain access to the CNS through different routes, such as the infection of perivascular compartments by blood-brain barrier crossing, and the vagus/glossopharyngeal-mediated invasion, or by a Trojan horse effect, within migrating leukocytes, further promoting the neuropathological changes widely reported in the literature (Khan et al., 2021; Pontelli et al., 2022; Emmi et al., 2023; Matschke et al., 2020). Considering the Trojan horse effect, it is noteworthy that SARS-CoV-2 was detected in PBMCs from roughly 50% of COVID-19 patients, a rate similar to that found in the nervous tissues (Stein et al., 2022).
Another important feature that could be related to the brain abnormalities found in COVID-19 patients is the SARS-CoV-2-induced pro-inflammatory response favoring neurodegeneration. Interestingly, in the present work, we found that all nervous tissue samples showed expression of both IL-6 and IL-10 pro-inflammatory cytokines, whereas expression for IL-6 was markedly higher in most nervous tissue samples, compared to the lung specimens. Moreover, we have also found that whereas intracellular adhesion molecule-1 (ICAM-1), IL-8, MIF, and plasminogen activator inhibitor 1 (PAI-1) are detected in lung samples, only MIF and IL-18 can be detected in nervous tissue samples, demonstrating distinct inflammatory profiles in the two infection sites.
When analyzing the neuroinflammatory profile of brain/olfactory samples, we found higher levels of IL-10 but not IL-6 in patients with cardiovascular diseases and hypertension, compared to patients without a medical history of cardiovascular pathology. Moreover, higher levels of IL-10 were found in patients with hypothyroidism, whereas IL-6 was found to be increased in patients diagnosed with obesity (Singh and Rai, 2019).
Interestingly, although IL-10 is typically characterized as an anti-inflammatory marker, in the context of inflammatory responses triggered by viral infection, it could be a key to viral persistence and the development of viral reservoirs. Indeed, different studies report that higher levels of IL-10 in tissues infected by different viruses are correlated with an increase in viral persistence (ISLAM et al., 2021; Carlini et al., 2023; HARPER et al., 2022). Thus, these results could indicate a finely tuned balance between pro-inflammatory and anti-inflammatory responses within the CNS, which may influence disease progression and the development of long-term neurological sequelae.
A meta-analysis conducted by Zhou and colleagues (Zhou et al., 2020) has shown that the presence of hypertension was associated with a 3.1-fold increased risk of adverse outcomes in patients with COVID-19, whereas cardiovascular diseases and chronic kidney diseases showed 3.13- and 3.02-times higher risks, respectively.
Besides the differential expression of IL-6 and IL-10, patients with hypertension and chronic kidney disease display lower levels of BDNF in brain/olfactory samples. Although changes in the BDNF levels were not correlated with higher levels of IL-6 and IL-10, reduced expression of neurotrophic factors is a key component in the pathogenesis of different neurological and neuropsychiatric disorders (Schwabenland et al., 2021; Espíndola et al., 2021).
These results suggest that the presence of comorbidities, especially those related to increased expression of pro-inflammatory markers (e.g., chronic hypertension, obesity, and chronic kidney disease), is correlated with worse neuroimmune consequences of SARS-CoV-2 infection in the nervous tissue, and we hypothesize that this can be a predictive risk factor for worse neurological outcomes (Oka et al., 2021).
Hence, although SARS-CoV-2 neuroinvasiveness appears to be lower compared to the respiratory tract, our results corroborate the hypothesis that it is sufficient to induce a pro-inflammatory state that could further impair neural homeostasis and induce brain abnormalities found in COVID-19 patients. Indeed, recent literature shows increased levels of several proinflammatory cytokines in the cerebral spinal fluid of COVID-19 patients, especially IL-6 (Moore and June 2020; Hu et al., 2021). It has been hypothesized that the massive production of pro-inflammatory factors could trigger a cytokine storm, further inducing the activation of microglia and astrocytes, ultimately leading to neurodegeneration (Tay et al., 2020; Azkur et al., 2020; Ramakrishna et al., 2023; Rai et al., 2019).
Our results should be interpreted with caution, given that we cannot exclude the possibility that differences between the infectivity in both sites could be caused by clinical heterogeneity displayed by the patients included in this study. Moreover, an inherent limitation of our study lies in the utilization of post-mortem samples, which inherently precludes the ability to capture real-time dynamics of neural alterations associated with SARS-CoV-2 infection. While our findings shed light on the neuropathological consequences of SARS-CoV-2 infection, they may not fully encapsulate the evolving nature of neural alterations over time. Future studies employing longitudinal assessments are warranted to elucidate the dynamic interplay between viral infection and neural changes with greater temporal resolution. In addition, our study's generalizability may be limited by the relatively small sample size of 16 patients. Furthermore, whereas our findings provide valuable insights into the neural alterations associated with SARS-CoV-2 infection in this cohort, the small sample size inherently restricts the extent to which our results can be extrapolated to broader populations.
It is important to recognize that while strong associations between variables may be identified in our study, determining causality requires rigorous consideration of various factors, including temporal sequence, biological plausibility, multimodal analysis and the presence of confounding variables. We recognize that our results provide a static snapshot of the disease pathology at a single time point, limiting our ability to discern causal pathways or temporal relationships. Therefore, future studies with larger and more diverse cohorts can deepen our knowledge about the relationship between SARS-CoV-2 neuroinfection, neuroimmune consequences, and the neurological outcomes, including the long-lasting ones observed in COVID-19 patients.
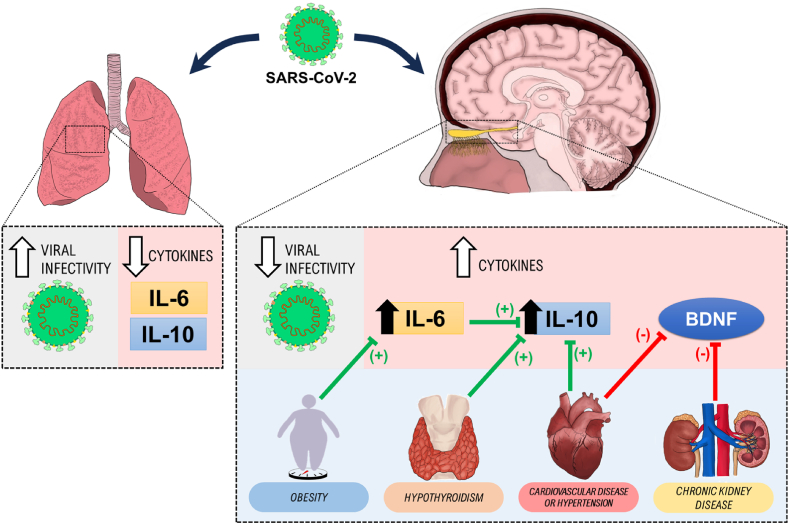
In conclusion, our work demonstrates that SARS-CoV-2 has a relatively low infectivity profile in the olfactory nervous tissue, when compared to the lung infectivity profile, and that both sites have distinct pro-inflammatory states. Moreover, the present work corroborates the hypothesis that neurological and neuropsychiatric outcomes linked with COVID-19 are determined not only by the presence or absence of viral infection in the nervous tissues, but also by distinct neuroimmune consequences and pro-inflammatory profiles that are dependent on patients’ comorbidities (Fig. 7).
Fig. 7.
Schematic representation of the results' summary. Red arrows indicate negative (−) modulation; Green, positive modulation (+). In brief, our findings indicate a higher detection of viral infection in the lung tissue compared to CNS tissues. However, notable variations in the interleukin levels (IL-6 and IL-10), reveal elevated levels in the CNS tissues as opposed to the lungs. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Funding
This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) regular Fellowship (2020/05416–4).
CRediT authorship contribution statement
Rafael R. Ferreira: Writing – original draft, Methodology, Investigation, Data curation. Ronaldo B. Martins: Writing – review & editing, Methodology, Formal analysis, Data curation. Isabela Pires: Writing – original draft, Methodology, Investigation, Formal analysis. Bruno L. Marques: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Karla C.M. Costa: Writing – original draft, Methodology, Investigation. Pedro H.C. Lirio: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Davi S. Scomparin: Methodology, Investigation. Franciele F. Scarante: Writing – review & editing, Methodology, Investigation. Sabrina S. Batah: Visualization, Investigation. Jaime E.C. Hallak: Writing – review & editing, Writing – original draft, Visualization. Jose A. Crippa: Writing – review & editing, Visualization. Livia C.M. Rodrigues: Writing – review & editing, Writing – original draft, Visualization. Rita C. Tostes: Writing – review & editing, Visualization, Resources. Alexandre T. Fabro: Writing – review & editing, Investigation, Conceptualization. Eurico Arruda: Writing – review & editing, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Alline C. Campos: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
We, the authors, attest that there is no conflict of interest associated with this study. The authors have no financial or personal relationships with other individuals or organizations that may influence professional judgment concerning the essential values and the experimental design of our study.
Acknowledgments
We thank our lab colleagues and collaborators for maintaining such an excellent scientific environment that has led to the production of this study, even during one of the most challenging times experienced by humankind: the COVID-19 pandemic. We are also grateful to the Brazilian people who, through the payment of their taxes, indirectly funded this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2024.100855.
Appendix ASupplementary data
The following is/are the supplementary data to this article.
Data availability
Data will be made available on request.
References
- Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000;74(19):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Brüggen M.C., et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKMAN Danielle, et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep. 2022;41(5) doi: 10.1016/j.celrep.2022.111573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini V., Noonan D.M., Abdalalem E., Goletti D., Sansone C., Calabrone L., Albini A. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1161067. PMID: 37359549; PMCID: PMC10287165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S.S., Chan K.H., Chu K.W., Kwan S.W., Guan Y., Poon L.L.M., Peiris J.S.M. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin. Infect. Dis. 2005;40:1721–1729. doi: 10.1086/430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino G., Todisco M., Hota N., Della Porta G., Morbini P., Tassorelli C., et al. Neuropathological findings from COVID‐19 patients with neurological symptoms argue against a direct brain invasion of SARS‐CoV‐2: a critical systematic review. Eur. J. Neurol. 2021;28(11):3856–3865. doi: 10.1111/ene.15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J.L., Simonetti B., Klein K., Chen K.-E., Williamson M.K., Antón-Plágaro C., et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370(6518):861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong L., Xu P., Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav. Immun. 2020;87:33. doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmi A., Rizzo S., Barzon L., Sandre M., Carturan E., Sinigaglia A., et al. Detection of SARS-CoV-2 viral proteins and genomic sequences in human brainstem nuclei. npj Parkinson's Disease. 2023;9(1):25. doi: 10.1038/s41531-023-00467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espíndola O.M., Gomes Y.C., Brandão C.O., Torres R.C., Siqueira M., Soares C.N., et al. Inflammatory cytokine patterns associated with neurological diseases in coronavirus disease 2019. Ann. Neurol. 2021;89(5):1041–1045. doi: 10.1002/ana.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. J. Alzheim. Dis. 2020;76(1):3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARPER Justin, et al. Interleukin-10 contributes to reservoir establishment and persistence in SIV-infected macaques treated with antiretroviral therapy. J. Clin. Investig. 2022;132(8) doi: 10.1172/JCI155251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOU Yuxin, et al. Enhanced replication of SARS-CoV-2 Omicron BA. 2 in human forebrain and midbrain organoids. Signal Transduct. Targeted Ther. 2022;7(1):381. doi: 10.1038/s41392-022-01241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Huang S., Yin L. The cytokine storm and COVID‐19. J. Med. Virol. 2021;93(1):250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.H., Jiang D., Huang J.T. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav. Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISLAM Hashim, et al. Elevated interleukin-10 levels in COVID-19: potentiation of pro-inflammatory responses or impaired anti-inflammatory action? Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.677008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Yoo S.-J., Clijsters M., Backaert W., Vanstapel A., Speleman K., et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184(24):5932–5949. e15. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li H., Fan R., et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59:163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima Giacobbo B., Doorduin J., Klein H.C., Dierckx R.A., Bromberg E., de Vries E.F. Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol. Neurobiol. 2019;56:3295–3312. doi: 10.1007/s12035-018-1283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Li X., Geng D., Mei N., Wu P.-Y., Huang C.-C., et al. Cerebral micro-structural changes in COVID-19 patients–an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J.P., Schröder A.S., Edler C., et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–929. doi: 10.1016/S1474-4422(20)30308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Mohamadian M., Chiti H., Shoghli A., Biglari S., Parsamanesh N., Esmaeilzadeh A. COVID‐19: virology, biology and novel laboratory diagnosis. J. Gene Med. 2021;23(2) doi: 10.1002/jgm.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Murray R.S., Brown B., Brain D., Cabirac G.F. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol.: Official Journal of the American Neurological Association and the Child Neurology Society. 1992;31(5):525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Ueda A., Nakagawa T., Kikuchi Y., Inoue D., Marumo S., et al. SARS-CoV-2-related progressive brain white matter lesion associated with an increased cerebrospinal fluid level of IL-6. Intern. Med. 2021;60(19):3167–3170. doi: 10.2169/internalmedicine.8123-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontelli C., Castro A., Martins B., et al. SARS-CoV-2 productively infects primary human immune system cells in vitro and in COVID-19 patients. J. Mol. Cell Biol. 2022;14(4) doi: 10.1093/jmcb/mjac021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai S.N., Dilnashin H., Birla H., et al. The role of PI3K/akt and ERK in neurodegenerative disorders. Neurotox. Res. 2019;35(3):775–795. doi: 10.1007/s12640-019-0003-y. [DOI] [PubMed] [Google Scholar]
- Ramakrishna K., Nalla L.V., Naresh D., et al. WNT-Β catenin signaling as a potential therapeutic target for neurodegenerative diseases: current status and future perspective. Diseases. 2023;11(3):89. doi: 10.3390/diseases11030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanna F., Maglio M., Landini M.P., Fini M. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front. Med. 2020;7 doi: 10.3389/fmed.2020.594495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabenland M., Salié H., Tanevski J., Killmer S., Lago M.S., Schlaak A.E., et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity. 2021;54(7):1594–1610. e11. doi: 10.1016/j.immuni.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERRANO Geidy E., et al. SARS-CoV-2 brain regional detection, histopathology, gene expression, and immunomodulatory changes in decedents with COVID-19. J. Neuropathol. Exp. Neurol. 2022;81(9):666–695. doi: 10.1093/jnen/nlac056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Rai S.N. Factors affecting obesity and its treatment. Obesity Medicine. 2019;16 [Google Scholar]
- Singh N., Rai S.N., Singh V., Singh M.P. Molecular characterization, pathogen-host interaction pathway and in silico approaches for vaccine design against COVID-19. J. Chem. Neuroanat. 2020;110 doi: 10.1016/j.jchemneu.2020.101874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S., Mukerji S.S., Keller K., Ali A.S., et al. Neuropathological features of covid-19. N. Engl. J. Med. 2020;383(10):989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., Lu-Culligan A., Prado A.V., Skriabine S., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218(3) doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S.R., Ramelli S.C., Grazioli A., Chung J.-Y., Singh M., Yinda C.K., et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022:1–6. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheiden A., Klein R.S. Neuroinflammation and COVID-19. Curr. Opin. Neurobiol. 2022 doi: 10.1016/j.conb.2022.102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A., Thomas N., Ellul M.A., Davies N.W., Pollak T.A., Tenorio E.L., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatr. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yang Q., Chi J., Dong B., Lv W., Shen L., et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;99:47–56. doi: 10.1016/j.ijid.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.