Abstract
Objectives
Comprehensive preoperative management involves the identification and optimization of medical comorbidities while avoiding excessive healthcare utilization. While diabetes and heart disease are major causes of morbidity that can worsen surgical outcomes, further study is needed to evaluate how well current perioperative strategies mitigate their risks. This study employs an exact matching protocol to isolate the effects of both diabetes and cardiovascular disease on spine surgery outcomes.
Methods
4680 consecutive patients undergoing single-level, posterior-only lumbar fusion were retrospectively enrolled. Univariate logistic regression was performed on comorbidity subgroups, then coarsened exact matching (CEM) was employed for patients with diabetes or cardiovascular disease. Patients were matched 1:1 on ten patient and procedural characteristics known to affect neurosurgical outcomes. Separate pairs of exact-matched cohorts were generated to isolate both cardiovascular disease (matched n = 192), and diabetes (matched n = 380). Primary outcomes were surgical complications; length of stay; discharge disposition (home vs. non-home); and 30- and 90-day Emergency Department (ED) visits, readmissions, reoperations, and mortality.
Results
Cardiovascular disease and diabetes subgroups were not associated with short term outcomes after matching to control for confounders. Compared to univariate statistics, this method demonstrates that confounding control variables may drive outcomes more than these comorbidities themselves.
Conclusion
Between otherwise exactly matched patients undergoing lumbar fusion, diabetes and cardiovascular disease posed no greater risk of short-term adverse outcomes. This suggests proper selection criteria for surgical candidates and effective current perioperative strategies to mitigate these common comorbidities. Further studies are warranted to assess and optimize the cost-effectiveness of preoperative management for patients with common comorbidities.
Keywords: Lumbar fusion, Diabetes mellitus, Heart disease, Outcomes
1. Introduction
Lumbar fusion is a common procedure performed for a variety of spinal pathologies.1 Despite low baseline complication and mortality rates, there remains an ongoing need to improve the value of lumbar fusion surgery performed in an aging population in a costly healthcare landscape.1, 2, 3, 4, 5, 6 Key strategies gaining recent attention for value-based surgical care include perioperative planning and risk-factor optimization. To facilitate this process, many patient-specific risk factors for markers of poor surgical outcomes are being studied to guide the selection of surgical techniques and focus targets for perioperative management.7, 8, 9, 10
Medical comorbidity is an important factor considered in perioperative management and has governed recent interest in literature. Elevated Charlson Comorbidity Index (CCI), a composite risk score of comorbidities predictive of long-term survival, has been shown to be associated with adverse short-term outcomes (such as readmission and reoperation) from lumbar surgery in univariate and multivariable analyses.7,11, 12, 13, 14 In addition to composite measures like the CCI or global assessments of disease severity like the ASA classification, some individual comorbidities have also been studied with multivariable modeling approaches. These studies have variably shown that malignancy, rheumatoid disease, diabetes, and/or asthma have predictive value with respect to patient outcomes following spinal surgery.15,16
Diabetes and cardiovascular disease are two of the most common conditions in the developed western world, exhibiting huge costs to society.17, 18, 19 To mitigate the deleterious effects of these comorbidities on surgical outcomes, many surgeons require extensive preoperative workups, including cardiac function testing via echocardiography or cardiac stress testing. In addition, some surgeons partner with PCPs or endocrinologists to improve patients’ glycemic control before surgery. However, in lumbar fusion surgery specifically, the direct effect of these comorbidities on outcomes, controlling for other related medical and demographic factors, requires more intentional study. This analysis retrospectively evaluates matched patients with and without these disease factors to provide a more accurate understanding of their effect on lumbar fusion surgery outcomes. We employ coarsened exact matching (CEM), which matches patients 1:1 when they equate along each covariate, compared to methods like propensity score matching, which combines all covariates into a single composite score. This provides a novel method to investigate associations in a clinically interpretable way: how does the variable of interest associate with the measured outcome between people who exactly match along every other covariate? These results will aid in evaluating current perioperative management strategies and guide future studies on cost-effectiveness in perioperative care.
2. Methods
2.1. Patient selection
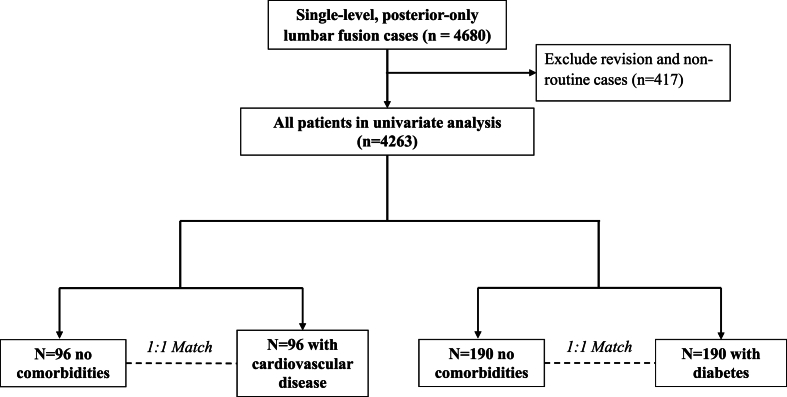
A total of 4680 consecutive cases of adult patients undergoing single-level posterior-only lumbar fusion at a single academic medical center were prospectively enrolled and retrospectively studied. Limiting cases to those that were non-emergent, inpatient admissions, using general anesthesia, and had clean wounds, there were 4263 cases analyzed further (Fig. 1).
Fig. 1.
Patient selection and study design.
2.2. Data extraction
Patient characteristics and surgical outcome data were extracted from the electronic medical record (EMR) using EpiLog, a non-proprietary system integrated with the EMR to streamline data collection and quality improvement initiatives. Patient variables included median household income (MHI) cross-referenced to zip-code (adjusted to 2016 US dollars), body mass index (BMI), age, sex, race, American Society of Anesthesiologists (ASA) score, smoking status, prior surgical history, insurance type (public vs private), and all factors of the CCI. Outcomes measured included length of stay, discharge home vs non-home, and 30- and 90- day readmissions, emergency department (ED) evaluation, reoperation, and all-cause mortality.
2.3. Comorbidities and statistical analysis
Comorbidity groups were derived from the EMR via ICD codes. Diabetes is coded either complicated (DMC, n = 5) or uncomplicated (DMU, n = 234). Cardiovascular disease groups included congestive heart failure (CHF, n = 12), peripheral vascular disease (PVD, n = 62), prior myocardial infarction (MI, n = 11), and prior stroke (CVA, n = 52).
Patients with the disease categories were compared to those with no comorbidities (n = 2329), as measured by the CCI, excluding the age component of the CCI score. First, univariate logistic regression was performed to determine how the individual disease categories correlated to the short-term surgical outcomes. Next, coarsened exact matching (CEM) was employed separately for diabetes (DMU and DMC, matched n = 380) and for cardiovascular disease (CHF/PVD/MI/CVA, matched n = 192). 10 patient level variables were coded into categorical levels and patients with the disease group were exactly matched 1:1 to those without any medical comorbidities along all 10 covariates. Covariates (and binning categories) included: gender (male/female), ASA grade (exact), age (binned by decade), smoking history (prior/never), insurance type (public/private), prior surgery (binary), prior surgery in 30 days (binary), length of surgery (below/above median), BMI (cachectic, normal [18.5–30], obese), and MHI (below/above median). McNemar's test was compared categorical outcomes differences between matched patients with the observed comorbidity and without comorbidity. Nonparametric testing was used for continuous outcomes.
3. Results
3.1. Patient demographics
Before matching, patients with heart disease tended to be older, have higher ASA scores, be publicly insured, and have more prior surgeries than patients without comorbidity. Patients with diabetes tended to be older, have higher BMI and ASA, be publicly insured, and be non-white compared to patients without comorbidity. After matching, there were no differences between matched groups. Key information on patient demographics is presented in Table 1and Table 2.
Table 1.
Demographics in the cohort of cardiovascular disease (CVD) analysis.
| Before Matching |
After Matching |
|||||
|---|---|---|---|---|---|---|
| No Comorbidities (n = 2329) | CVD (n = 137) | p-value | No Comorbidities (n = 96) | CVD (n = 96) | p-value | |
| Gender (n, %) | ||||||
| Male | 1050 (45.08 %) | 63 (45.99 %) | 0.8367 | 43 (44.79 %) | 43 (44.79 %) | 1 |
| Female | 1279 (54.92 %) | 74 (54.01 %) | 53 (55.21 %) | 53 (55.21 %) | ||
| Age (mean, [range]) | ||||||
| 58.75 [15,90] | 67.38 [34, 85] | <0.0001 | 67.94 [35, 90] | 67.29 [34,84] | 0.8294 | |
| Race (n, %) | ||||||
| White | 1959 (84.11 %) | 113 (82.48 %) | 0.067 | 85 (88.54 %) | 82 (85.42 %) | 0.1563 |
| Non-White | 370 (15.9 %) | 24 (17.52 %) | 11 (11.5 %) | 14 (14.6 %) | ||
| BMI (mean, [range]) | ||||||
| 29.26 [15,54.4] | 28.86 [13.64, 44.76] | 0.7319 | 27.99 [18.6, 44.61] | 28.96 [19.75, 44.76] | 0.21 | |
| Tobacco Use (n, %) | ||||||
| 333 (14.30 %) | 18 (13.14 %) | 0.9312 | 5 (5.21 %) | 5 (5.21 %) | 1 | |
| Insurance Type (n, %) | ||||||
| Private | 1329 (57.1 %) | 44 (32.1 %) | <0.0001 | 29 (30.2 %) | 29 (30.2 %) | 1 |
| Public | 1000 (42.9 %) | 93 (67.9 %) | 67 (69.8 %) | 67 (69.8 %) | ||
| Prior Surgery (mean, [range]) | ||||||
| 0.52 [0,11] | 0.93 [0,8] | <0.0001 | 0.73 [0,7] | 0.51 [0,4] | 0.20 | |
| Prior Surgery 30D (mean, [range]) | ||||||
| 0.027909 [0,3] | 0.05 [0,2] | 0.1082 | 0.00 [0,0] | 0.00 [0,0] | 1 | |
| ASA Score (mean, [range]) | ||||||
| 2.24 [1,4] | 2.58 [2,4] | <0.0001 | 2.55 [2,3] | 2.55 [2,3] | 1 | |
Table 2.
Demographics of the Cohort in Diabetes (DM) analysis.
| Before Matching |
After Matching |
|||||
|---|---|---|---|---|---|---|
| No Comorbidities (n = 2329) | DM (n = 239) | p-value | No Comorbidities (n = 190) | DM (n = 190) | p-value | |
| Gender (n, %) | ||||||
| Male | 1050 (45.08 %) | 113 (47.28 %) | 0.5394 | 88 (46.32 %) | 88 (46.32 %) | 1 |
| Female | 1279 (54.92 %) | 126 (50.55 %) | 102 (53.68 %) | 102 (53.68 %) | ||
| Age (mean, range) | ||||||
| 58.75 [15,90] | 64.05 [33,86] | <0.0001 | 63.71 [29,85] | 63.98 [33,86] | 0.8514 | |
| Race (n, %) | ||||||
| White | 1959 (84.11 %) | 184 (76.99 %) | 0.0189 | 172 (90.53 %) | 148 (77.89 %) | 0.1024 |
| Non-White | 370 (15.9 %) | 24 (23.01 %) | 18 (9.47 %) | 42 (22.11 %) | ||
| BMI (mean, range) | ||||||
| 29.26 [15,54.40] | 33.02 [18.14, 56.58] | <0.0001 | 31.53 [20.2, 54.5] | 32.79 [19.8, 56.58] | 0.09 | |
| Tobacco Use (n, %) | ||||||
| 333 (14.30 %) | 21 (8.97 %) | 0.0617 | 10 (5.26 %) | 10 (5.26 %) | 1 | |
| Insurance Type (n, %) | ||||||
| Private | 1329 (57.1 %) | 109 (45.6 %) | 0.0002 | 85 (44.7 %) | 85 (44.7 %) | 1 |
| Public | 1000 (42.9 %) | 130 (54.4 %) | 105 (55.3 %) | 105 (55.3 %) | ||
| Prior Surgery (mean, range) | ||||||
| 0.52 [0,11] | 0.70 [0,12] | 0.0887 | 0.44 [0,7] | 0.55 [0,12] | 0.86 | |
| Prior Surgery 30D (mean, range) | ||||||
| 0.03 [0,3] | 0.02 [0,2] | 0.574 | 0.01 [0,2] | 0.01 [0,1] | 1 | |
| ASA Score (mean, range) | ||||||
| 2.24 [1,4] | 2.54 [2,3] | <0.0001 | 2.49 [2,3] | 2.49 [2,3] | 1 | |
3.2. Univariate analysis
DMU was associated with less home discharge (OR 0.53, p = 0.0002) (see Table 2). DMC was associated with increased surgical complications (OR = 13.4, p = 0.013) and death at 30 days (OR = 60, p = 0.016). CHF was associated with death at 30 and 90 days (OR = 26.6, p = 0.040; OR = 86.72, p < 0.001). MI was associated with death at 30 and 90 days (both OR = 28.9, p = 0.036). PVD was associated with surgical complication (OR = 5.44, p < 0.001), non-home discharge (OR = 0.40, p = 0.002), and death at 90 days (OR = 16.2, p = 0.005). CVA was associated with readmission at 90 days (OR = 2.3, p = 0.024).
3.3. Matched analysis
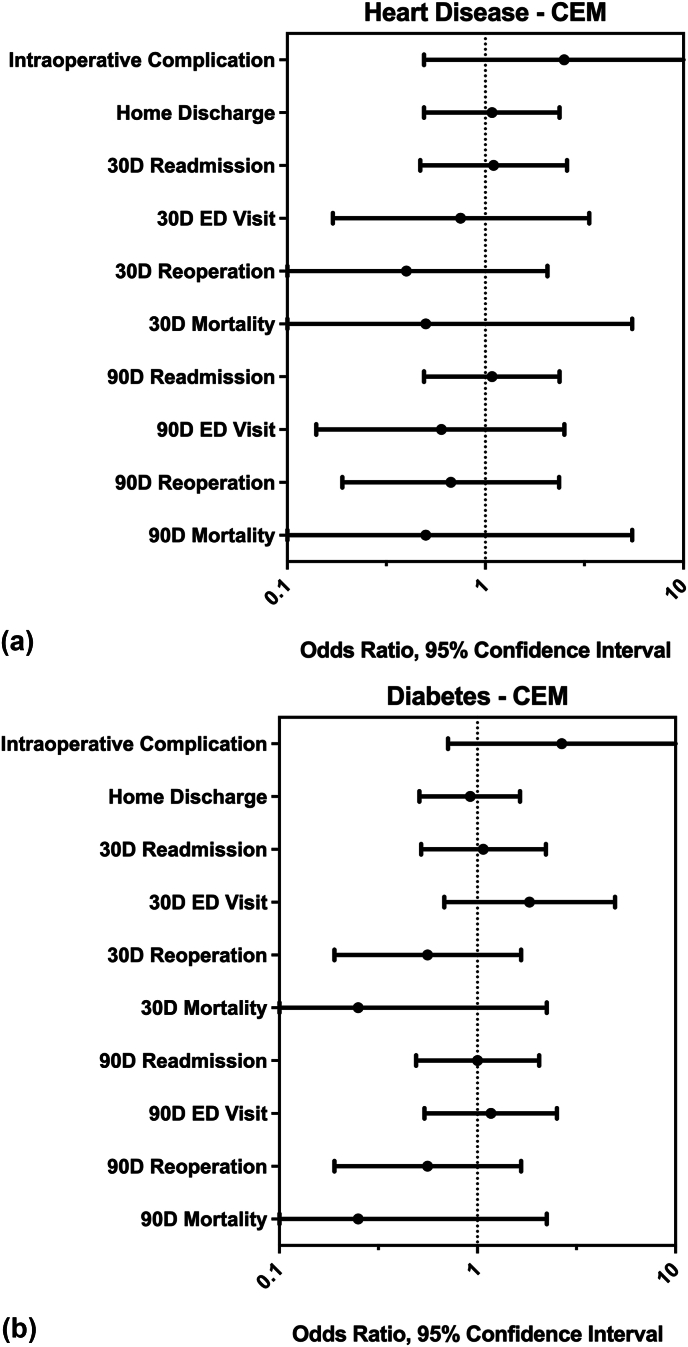
After CEM, neither diabetes nor cardiovascular disease groups were found to be significantly associated with any of the outcome categories (Fig. 2, Table 3). While not statistically significant, for both disease groups, the mean odds ratios of reoperation and mortality at both 30 and 90 days fell below one, indicating lower odds of these events in the group with the index comorbidity. Length of stay in patients with cardiovascular disease (mean = 104.8 h) was not significantly different from matched peers without comorbidity (mean = 98.8 h, p = 0.57). Length of stay in patients with diabetes (mean = 88.2 h) was not significantly different from matched peers without comorbidities (mean = 92.4 h, p = 0.25). Fig. 2.
Fig. 2.
Results of coarsened exact matching. Odds ratios and 95 % confidence intervals are shown for analyzed outcomes, separated by heart disease (A) and diabetes (B). No significant differences in the measured outcomes were found between the matched cohorts.
Table 3.
Results of coarsened exact matching. Odds ratios and 95 % confidence intervals shown for analyzed outcomes, separated by diabetes and heart disease. No significant differences were found between the matched cohorts.
| Diabetes |
Heart Disease |
|||
|---|---|---|---|---|
| OR (95 % CI) | P-value | OR (95 % CI) | P-value | |
| Intraoperative Complication | 2.67 (0.71–10.05) | 0.132 | 2.50 (0.49–12.89) | 0.257 |
| Home Discharge | 0.92 (0.51–1.64) | 0.768 | 1.08 (0.49–2.37) | 0.841 |
| 30D Readmission | 1.07 (0.52–2.22) | 0.853 | 1.10 (0.47–2.59) | 0.827 |
| 30D ED Visit | 1.83 (0.68–4.96) | 0.225 | 0.75 (0.17–3.35) | 0.706 |
| 30D Reoperation | 0.56 (0.19–1.66) | 0.285 | 0.40 (0.08–2.06) | 0.257 |
| 30D Mortality | 0.25 (0.03–2.24) | 0.180 | 0.50 (0.05–5.51) | 0.564 |
| 90D Readmission | 1.00 (0.49–2.05) | 1.000 | 1.08 (0.49–2.37) | 0.842 |
| 90D ED Visit | 1.17 (0.54–2.52) | 0.695 | 0.60 (0.14–2.51) | 0.480 |
| 90D Reoperation | 0.56 (0.19–1.66) | 0.285 | 0.67 (0.19–2.36) | 0.527 |
| 90D Mortality | 0.25 (0.03–2.24) | 0.180 | 0.50 (0.05–5.51) | 0.564 |
4. Discussion
This study examines the impact of diabetes and heart disease on short-term outcomes of lumbar fusion surgery in a single-center cohort study. Controlling for important patient-level demographic and medical factors with CEM, we observed no differences in short-term surgical outcomes for patients with these conditions. The findings of this matching protocol suggest associations between these conditions and medical outcomes may be driven by confounding variables such as age, ASA score, or insurance status. Taken together, our findings may suggest that despite the known risks of diabetes and heart disease to surgical outcomes, current perioperative medical practice at this single institution is well-equipped to care for these specific medical needs in the immediate term, and more work needs to be done to study the best markers for optimizing patients before surgery.
Diabetes and heart disease are major causes of morbidity and mortality in Western societies and have been linked to various adverse surgical outcomes in other surgical domains.20,21,22, 23, 24 Diabetes causes chronic micro and macrovascular complications, and cardiovascular disease impairs circulation, causes chronic inflammation, and a dysregulated sympathetic state.17,18,25 These factors, combined with the controlled insult of surgical trauma, likely worsen surgical results. Despite these proven biological relationships, selecting appropriate patients and perioperative strategies aimed at medically optimizing patients with these comorbidities are routinely implemented in clinical practice and may mitigate the adverse effects of these conditions.26, 27, 28,29,30
For patients with cardiovascular disease, diabetes, or their risk factors, collaboration with and referral to the primary team managing their comorbidity (e.g. primary care, cardiology, endocrinology) may involve risk stratification tools, screening tests, and potential interventions to optimize the comorbidity.31,32 This preoperative decision-making may drive the non-differences found in our matching protocol - appropriate surgical decision making and medical management might mitigate the effects of these conditions for given ages and functional statuses. As a result, the relationship of these conditions to poor outcomes following surgery observed in our univariate analysis may be better explained by the other related factors controlled by our matching protocols. It may also be that the short-term nature (30 and 90 days) of our results are driven mainly by pathology and surgical intervention, while longer-term study would be needed to assess differences directly attributable to diabetes and heart disease after accounting for other variables.
We interestingly observed a trend towards lower reoperation and short-term mortality rates in the matched patients with the studied conditions compared to those without the conditions. The trend in reoperation may reflect surgeons’ decisions to reoperate considering the comorbidity profile; that is, surgeons in this cohort may prefer not to reoperate on patients with these conditions. The trend towards less operative mortality is similarly interesting and may reflect the abundance of care and preoperative medical management taken before operating on patients with these conditions.
As diabetes and heart disease only continue to increase in prevalence in our aging population, the added value of medical optimization in relation to their added costs of holistic surgical care must be further evaluated, and cost-effective measures to optimize care and mitigate the underlying drivers of these conditions must be implemented.
5. Limitations
The findings of this study come with notable limitations. As a retrospective analysis, this study matched and compared within a cohort of patients selected for and undergoing surgery. We demonstrate that, for our sample with a selection bias of those chosen for surgery, outcomes were not observably different with or without the disease states. In addition, this study is performed at a single medical center in a very specific type of surgery. This study is intentionally limited such that a highly homogeneous cohort of patients could be compared in direct matching. With relatively low baseline rates of adverse outcomes for lumbar fusion, the effects of comorbidities on outcomes may be below our power for detection. Larger studies with multicenter or national registries have shown associations between these medical conditions and patient outcomes. However, the findings from our matched cohort and further study of specific practices that medically optimize comorbidities in clinical practice may provide important lessons for healthcare systems aimed at increasing value and lowering costs.
One further limitation of this study is the short-term outcome time frame. While this study evaluates two common chronic diseases, we intentionally chose to study these outcomes as they are routinely used in healthcare systems research and surgical reimbursement models, with demonstrated and understandable importance to payers, providers, and patients.
6. Conclusion
This study examines the impact of diabetes and heart disease on short-term outcomes of lumbar fusion surgery in a single-center cohort study. Controlling for important patient-level demographic and medical factors with CEM, we observed no differences in surgical outcomes for patients with these conditions. Though we did observe associations in univariate statistics, the findings of our matching protocol suggest that those associations are better explained by the variables that were controlled for, such as age, ASA score, or insurance status. Taken together, our findings may suggest that despite the known risks of diabetes and heart disease to surgical outcomes, current perioperative medical practice at this single institution are well-equipped to care for these medical needs. Further study must examine the cost-effectiveness of these strategies and optimize patient selection criteria.
Sources of funding
NRM received support from the Bernadette and Kevin McKenna Family Research Fund.
Ethics committee approval
This study was approved by the IRB at the Hospital of the University of Pennsylvania. The IRB number for this study is: 832794. All ethical guidelines and rules were followed to protect patient privacy.
CRediT authorship contribution statement
Ryan S. Gallagher: Writing – original draft, Project administration, Conceptualization. Connor A. Wathen: Writing – review & editing, Project administration, Conceptualization. Ritesh Karsalia: Writing – review & editing, Validation. Austin J. Borja: Writing – review & editing, Validation, Conceptualization. Tara Collier: Formal analysis, Data curation. Jianbo Na: Formal analysis, Data curation. Scott McClintock: Formal analysis, Data curation. Paul J. Marcotte: Writing – review & editing, Supervision, Data curation. James M. Schuster: Writing – review & editing, Supervision, Data curation. William C. Welch: Writing – review & editing, Supervision, Data curation. Neil R. Malhotra: Writing – review & editing, Supervision, Project administration, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The EpiLog Project and The Bernadette and Kevin McKenna Family Research Fund.
Abbreviations:
- BMI
body mass index
- CCI
Charlson Comorbidity Index
- CEM
coarsened exact matching
- CHF
congestive heart failure
- CVA
stroke; cardiovascular accident
- DMC
complicated diabetes
- DMU
uncomplicated diabetes
- ED
emergency department
- EMR
electronic medical record
- MHI
median household income
- MI
myocardial infarction
- PVD
peripheral vascular disease
References
- 1.Martin B.I., Mirza S.K., Spina N., Spiker W.R., Lawrence B., Brodke D.S. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2019;44(5):369–376. doi: 10.1097/brs.0000000000002822. [DOI] [PubMed] [Google Scholar]
- 2.Beckerman D., Esparza M., Lee S.I., et al. Cost analysis of single-level lumbar fusions. Global Spine J. 2020;10(1):39–46. doi: 10.1177/2192568219853251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tosteson A.N.A., Skinner J.S., Tosteson T.D., et al. The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years. Spine. 2008;33(19):2108–2115. doi: 10.1097/brs.0b013e318182e390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glassman S.D., Polly D.W., Dimar J.R., Carreon L.Y. The cost effectiveness of single-level instrumented posterolateral lumbar fusion at 5 Years after surgery. Spine. 2012;37(9):769–774. doi: 10.1097/brs.0b013e3181e03099. [DOI] [PubMed] [Google Scholar]
- 5.Reisener M.J., Pumberger M., Shue J., Girardi F.P., Hughes A.P. Trends in lumbar spinal fusion-a literature review. J Spine Surg. 2020;6(4):752–761. doi: 10.21037/jss-20-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi K., Ando K., Nishida Y., Ishiguro N., Imagama S. Epidemiological trends in spine surgery over 10 years in a multicenter database. Eur Spine J. 2018;27(8):1698–1703. doi: 10.1007/s00586-018-5513-4. [DOI] [PubMed] [Google Scholar]
- 7.Lubelski D., Feghali J., Nowacki A.S., et al. Patient-specific prediction model for clinical and quality-of-life outcomes after lumbar spine surgery. J Neurosurg Spine. 2021;34(4):580–588. doi: 10.3171/2020.8.spine20577. [DOI] [PubMed] [Google Scholar]
- 8.Glauser G., O'Connor A., Brintzenhoff J., Roth S.C., Malhotra N.R., Cabey W.V. A scoping review of the literature on the relationship between social and structural determinants of health and neurosurgical outcomes. World Neurosurg. 2021;158:24–33. doi: 10.1016/j.wneu.2021.10.109. [DOI] [PubMed] [Google Scholar]
- 9.Elmallah R.D., Cherian J.J., Robinson K., Harwin S.F., Mont M.A. The effect of comorbidities on outcomes following total knee arthroplasty. J Knee Surg. 2015;28(5):411–416. doi: 10.1055/s-0035-1549023. [DOI] [PubMed] [Google Scholar]
- 10.Pugely A.J., Martin C.T., Gao Y., Mendoza-Lattes S. Causes and risk factors for 30-day unplanned readmissions after lumbar spine surgery. Spine. 2014;39(9):761–768. doi: 10.1097/brs.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 11.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. https://www.ncbi.nlm.nih.gov/pubmed/3558716 [DOI] [PubMed] [Google Scholar]
- 13.Borja A.J., Connolly J., Kvint S., et al. Charlson Comorbidity Index score predicts adverse post-operative outcomes after far lateral lumbar discectomy. Clin Neurol Neurosurg. 2021;206 doi: 10.1016/j.clineuro.2021.106697. [DOI] [PubMed] [Google Scholar]
- 14.Winter E., Detchou D.K., Glauser G., et al. Predicting patient outcomes after far lateral lumbar discectomy. Clin Neurol Neurosurg. 2021;203 doi: 10.1016/j.clineuro.2021.106583. [DOI] [PubMed] [Google Scholar]
- 15.Akins P.T., Harris J., Alvarez J.L., et al. Risk factors associated with 30-day readmissions after instrumented spine surgery in 14,939 patients. Spine. 2015;40(13):1022–1032. doi: 10.1097/brs.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 16.Khor S., Lavallee D., Cizik A.M., et al. Development and validation of a prediction model for pain and functional outcomes after lumbar spine surgery. Jama Surg. 2018;153(7):634. doi: 10.1001/jamasurg.2018.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S., Khunti K., Davies M.J. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/s0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 18.Libby P., Buring J.E., Badimon L., et al. Atherosclerosis. Nat Rev Dis Prim. 2019;5(1):56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 19.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics—2022 update: a report from the American heart association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/cir.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 20.Arrighi-Allisan A.E., Neifert S.N., Gal J.S., et al. Diabetes is predictive of postoperative outcomes and readmission following posterior lumbar fusion. Global Spine J. 2022;12(2):229–236. doi: 10.1177/2192568220948480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne J.A., Cook C., Pietrobon R., Bethel M.A., Richardson W.J. Diabetes and early postoperative outcomes following lumbar fusion. Spine. 2007;32(20):2214–2219. doi: 10.1097/brs.0b013e31814b1bc0. [DOI] [PubMed] [Google Scholar]
- 22.He X., Fei Q., Sun T. Metabolic syndrome increases risk for perioperative outcomes following posterior lumbar interbody fusion. Medicine. 2020;99(38) doi: 10.1097/md.0000000000021786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye I.D., Wagner S.C., Butler J.S., Sebastian A., Morrissey P.B., Kepler C. Risk factors for adverse cardiac events after lumbar spine fusion. Internet J Spine Surg. 2018;12(5):638–643. doi: 10.14444/5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad W., Fernandez L., Bell J., et al. Assessment of postoperative outcomes of spine fusion patients with history of cardiac disease. J Am Acad Orthop Sur. 2022;30(8):e683–e689. doi: 10.5435/jaaos-d-21-00850. [DOI] [PubMed] [Google Scholar]
- 25.Bloom M.W., Greenberg B., Jaarsma T., et al. Heart failure with reduced ejection fraction. Nat Rev Dis Prim. 2017;3(1) doi: 10.1038/nrdp.2017.58. [DOI] [PubMed] [Google Scholar]
- 26.Wijeysundera D.N., Pearse R.M., Shulman M.A., et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet. 2018;391(10140):2631–2640. doi: 10.1016/s0140-6736(18)31131-0. [DOI] [PubMed] [Google Scholar]
- 27.Pannell L.M.K., Reyes E.M., Underwood S.R. Cardiac risk assessment before non-cardiac surgery. European Hear J - Cardiovasc Imaging. 2013;14(4):316–322. doi: 10.1093/ehjci/jes288. [DOI] [PubMed] [Google Scholar]
- 28.Kindler M., Wanner P.M., Filipovic M. Cardiac risk in non-cardiac surgery: a review. Trends Anaesth Critical Care. 2018;21:6–12. doi: 10.1016/j.tacc.2018.04.013. [DOI] [Google Scholar]
- 29.Smilowitz N.R., Berger J.S. Perioperative cardiovascular risk assessment and management for noncardiac surgery. JAMA. 2020;324(3):279–290. doi: 10.1001/jama.2020.7840. [DOI] [PubMed] [Google Scholar]
- 30.Palermo N.E., Garg R. Perioperative management of diabetes mellitus: novel approaches. Curr Diabetes Rep. 2019;19(4):14. doi: 10.1007/s11892-019-1132-7. [DOI] [PubMed] [Google Scholar]
- 31.Fleisher L.A., Fleischmann K.E., Auerbach A.D., et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2014;130(24):e278–e333. doi: 10.1161/cir.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 32.Roth S.G., Chanbour H., Gupta R., et al. Optimal hemoglobin A1C target in diabetics undergoing elective cervical spine surgery. Spine J. 2022;22(7):1149–1159. doi: 10.1016/j.spinee.2022.02.014. [DOI] [PubMed] [Google Scholar]