Abstract
Reovirus type 1 Lang (T1L) infects the mouse intestinal mucosa by adhering specifically to epithelial M cells and exploiting M-cell transport to enter the Peyer's patches. Oral inoculation of adult mice has been shown to elicit cellular and humoral immune responses that clear the infection within 10 days. This study was designed to determine whether adult mice that have cleared a primary infection are protected against viral entry upon oral rechallenge and, if so, whether antireovirus secretory immunoglobulin A (S-IgA) is a necessary component of protection. Adult BALB/c mice that were orally inoculated on day 0 with reovirus T1L produced antiviral S-IgA in feces and IgG in serum directed primarily against the reovirus ς1 attachment protein. Eight hours after oral reovirus challenge on day 21, the Peyer's patches of previously exposed mice contained no detectable virus whereas Peyer's patches of naive controls contained up to 2,300 PFU of reovirus/mg of tissue. Orally inoculated IgA knockout (IgA−/−) mice cleared the initial infection as effectively as wild-type mice and produced higher levels of reovirus-specific serum IgG and secretory IgM than C57BL/6 wild-type mice. When IgA−/− mice were rechallenged on day 21, however, their Peyer's patches became infected. These results indicate that intestinal S-IgA is an essential component of immune protection against reovirus entry into Peyer's patch mucosa.
Secretory immunoglobulin A (S-IgA) is the predominant immunoglobulin on the intestinal mucosal surface and is considered to be a first line of immune defense, protecting the mucosa against adherence and invasion by enteric pathogens (32). There is evidence that S-IgA prevents contact of pathogens with mucosal surfaces by facilitating entrapment of pathogens in mucus followed by peristaltic or ciliary clearance (24, 48). In addition, IgA may directly block or sterically hinder the microbial attachment proteins that mediate epithelial attachment or may even intercept incoming pathogens within epithelial cell vesicular compartments (8, 9, 24, 28). The importance of S-IgA in protection against mucosal viral infections has been supported by studies in which protection was associated with the presence of specific IgA in secretions (for a review, see reference 34). On the other hand, there is evidence that S-IgA is not essential and that IgG alone can prevent mucosal infection (11, 39, 40, 56). The development of a transgenic mouse in which the IgA switch and constant regions are deleted has provided a valuable model in which T-cell function and production of other immunoglobulin isotypes are normal or elevated but IgA is absent from serum and secretions (22). Immunization-challenge experiments using this IgA knockout (IgA−/−) model have indicated that IgA is not necessary for protection against influenza virus infection of respiratory epithelium (29), herpes simplex virus infection of the vaginal epithelium (44), Helicobacter pylori colonization of the gastric mucosa (6), or rotavirus infection of the intestinal epithelium (41).
The relative importance of S-IgA in protection against mucosal entry of other pathogens cannot be predicted from the above studies, however, because each microorganism has a preferred site of invasion and a distinct strategy for subverting epithelial barrier function and establishing mucosal infection. A striking example is the mouse pathogen reovirus that exploits the transepithelial transport activity of M cells to enter the Peyer's patch mucosa and initiate infection (63). After oral ingestion of reovirus type 1 Lang (T1L), the outer capsid of native virions is processed by proteases in the lumen of the intestine (5, 7), resulting in intermediate subviral particles (ISVPs) that adhere selectively to M-cell surfaces (2). Adherent viruses are transcytosed in vesicles to the intraepithelial M-cell pocket and the subepithelial tissue, and over the next 2 days, reovirus replicates in cells of the Peyer's patch mucosa (17, 42). In neonates, the infection then spreads systemically, but in adult mice the infection is usually limited to the mucosa, although viral antigens and/or antigen-sensitized cells later appear in the mesenteric lymph nodes and spleen (17). Infection of adult mice by reovirus T1L results in host immune responses that include specific serum IgG, S-IgA, and cytotoxic T lymphocytes (CTLs) (26, 27, 46, 58), and the infection is cleared within about 10 days (27). There is evidence that both CTLs and serum antibodies contribute to clearance of an established infection (4, 54). However, it is not known whether mice that have cleared an initial infection are protected against reinfection of Peyer's patches upon oral rechallenge and, if so, whether IgA is essential for protection.
In suckling mice, serum IgG alone was unable to prevent entry or early replication of reovirus in Peyer's patches. Reovirus-specific, neutralizing IgG monoclonal antibodies (MAbs) passively transferred by intravenous injection failed to inhibit uptake and local replication of orally administered reovirus T1L in Peyer's patches, although they did prevent systemic spread (52, 53). In suckling mice orally challenged with reovirus type 3 Dearing (T3D), reovirus replication in the intestinal mucosa was prevented in pups that were suckled on orally immunized (but not subcutaneously immunized) dams (14). Rodent milk contains high levels of IgG that is transferred from the intestine into the neonatal circulation by receptor-mediated transcytosis (45), but in this case, protection was attributed to the reovirus-specific S-IgA antibodies in milk that were present only in the orally immunized dams. In the intestinal lumens of normal adult mice, there is abundant IgA but little IgG (21). Although this suggests that S-IgA would be required to prevent M-cell adherence and entry of reovirus in adults, the relative importance of S-IgA in protection of the intestinal mucosa against reovirus reinfection has not been directly tested. A complicating factor is that IgA as well as IgA-antigen complexes (but not IgG or IgM) selectively adheres to apical surfaces of M cells in adult mice (35, 59). Thus, the presence of specific IgA in the intestinal lumen during oral reovirus challenge could result in two very different outcomes: IgA-coated viral particles could be entrapped in mucus and cleared, or IgA could facilitate M-cell-mediated viral uptake and infection.
In this study, we sought to assess the role of IgA antibodies in protection against entry of reovirus (T1L) into Peyer's patches of adult mice. In an active immunization-rechallenge protocol, adult mice that had cleared an initial infection and produced both intestinal IgA and serum IgG antibodies directed against reovirus T1L outer capsid proteins were protected against reinfection of Peyer's patches upon oral rechallenge. When IgA knockout mice were subjected to the same protocol, they cleared the initial infection but their Peyer's patches became infected upon oral rechallenge, despite the presence of antiviral IgG in serum and elevated antiviral IgM in secretions. The results of this study indicate that S-IgA is a crucial component of mucosal protection against reovirus and that antireovirus IgA protects by preventing adherence of virus to M cells of the Peyer's patch epithelium.
MATERIALS AND METHODS
Virus growth and purification.
Mouse L929 fibroblast cells (L cells) were grown in suspension culture in Joklik minimal essential medium (Irvine Scientific, Santa Ana, Calif.) containing 5% fetal calf serum (HyClone Laboratories, Logan, Utah), 2 mM glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (all from Gibco BRL, Grand Island, N.Y.). Purified T1L virions were prepared using second-passage L cells infected with plaque-purified reovirus as previously described (16). Virus was released from infected cells by freezing-thawing and sonication, recovered from lysates through two Freon 113 (trichlorotrifluoroethane) extractions, and then purified by cesium chloride gradient centrifugation. The virus band was removed, dialyzed extensively against dialysis buffer (150 mM NaCl, 15 mM MgCl2, 10 mM Tris, pH 7.4) at 4°C, and stored at 4°C in dialysis buffer. The concentration of viral particles was calculated from protein concentration (16), and concentration of infectious virus was determined by plaque assay (53). The purity of viral preparations was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis on 10% polyacrylamide reducing gels (7).
Mice.
Adult female BALB/c and C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, Mass.). IgA−/− (C57BL/6 × 129/Sv) mice were originally generated and described by Harriman and collaborators (22) and were generously provided to us by John Nedrud, Case Western Reserve University. Animals were maintained in the animal resource facility at the Children's Hospital, and all animal procedures were conducted in strict compliance with the Guidelines for Animal Experimentation established by Harvard Medical School, the Children's Hospital, and the National Institutes of Health.
Quantitation of viral entry into Peyer's patch tissue by plaque assay.
Mice were anesthetized by intraperitoneal administration of Avertin, 250 mg/kg of body weight (2,2,2-tribromoethanol; Aldrich, Milwaukee, Wis.), and sacrificed by cervical dislocation. Small intestines were removed and placed in incomplete Dulbecco's minimal essential medium (Gibco BRL) on ice. Peyer's patches were excised and collected in preweighed microcentrifuge tubes containing 1 ml of gelatin saline, pH 7 (per liter, 8 g of NaCl, 0.03 g of CaCl2, 0.17 g of MgCl2-6H2O, 1.2 g of H3BO3, 0.05 g of Na2B4O7-10H2O, 3.0 g of gelatin), with 2% Fungibact (Irvine Scientific). Tissue weight was determined, and Peyer's patches were disrupted by freezing-thawing twice, followed by probe sonication. Tissue plaque assays were done as previously described (56), and concentration of infectious virus in Peyer's patch tissue was expressed as PFU per milligram of tissue.
Challenge-rechallenge assay.
For initial inoculations, mice were given 2 × 107 PFU of reovirus in 500 μl of phosphate-buffered saline (PBS) intragastrically using a feeding needle. Naive mice were not intubated. On day 21, feces and serum were collected from all mice and subsets of animals from the reovirus-exposed and naive groups were orally challenged. Appropriate challenge doses, defined as doses that resulted in measurable infections in the Peyer's patches of all unprotected animals, were determined by pilot studies as 2 × 107 PFU for BALB/c mice and 5 × 108 PFU for C57BL/6 and IgA−/− (C57BL/6 × 129/Sv) mice. All groups of mice were sacrificed 8 h after rechallenge, and reovirus PFU per milligram of Peyer's patch tissue was determined as described above.
Evaluation of reovirus-specific antibodies in secretions and serum.
Feces were collected from all mice on day 21 and placed in preweighed microcentrifuge tubes containing 1 ml of PBS containing 0.5% (wt/vol) nonfat dry milk and protease inhibitors (aprotinin, 1 μg/ml [Sigma]; leupeptin, 5 μg/ml [Sigma]; aminoethylbenzenesulfonyl fluoride, 48 μg/ml [Calbiochem, La Jolla, Calif.]; and bestatin, 1 μg/ml [Sigma]). Fecal pellets were disrupted by vortexing, and supernatants were obtained by centrifugation at maximum speed in an Eppendorf microcentrifuge for 20 min at 4°C. Aliquots of supernatants were stored at −20°C. For enzyme-linked immunosorbent assays (ELISAs), 96-well flat-bottomed plates (Nunc MaxiSorp, Roskilde, Denmark) were coated overnight with 2 × 1011 viral particles/ml in PBS at 4°C in a humidified chamber. Plates were washed in PBS containing 0.05% Tween (PBS-Tween), and nonspecific protein binding sites were blocked by addition of blocking buffer (PBS-Tween with 1% fetal calf serum). Serial twofold dilutions of serum and fecal supernatants in blocking buffer were applied in duplicate. Known concentrations of MAbs RB8 (anti-μ1c IgA) and 5C6 (anti-ς1 IgG) were used as standards for reovirus-specific IgA and IgG, respectively. A reovirus-specific IgM standard was not available, and so a preparation of pooled serum containing reovirus-specific IgM was assigned an arbitrary unit value and used as standard; concentrations in samples were expressed as ELISA units per milliliter. After washing in PBS-Tween, secondary biotinylated goat anti-mouse IgA, IgG, or IgM (Southern Biotechnology Associates, Birmingham, Ala.) was added at a 1:3,000 dilution in blocking buffer. Bound antibody was detected with a 1:5,000 dilution of streptavidin-horseradish peroxidase (Pierce, Rockford, Ill.) and the TMB one-component peroxidase-substrate detection system (Kirkegaard and Perry Laboratories, Gaithersburg, Md.). Plates were read at 650 nm in a SpectraMax 250 plate reader using the Softmax ELISA analysis program.
Evaluation of total IgA, IgG, and IgM levels in serum and feces.
Fecal supernatants were prepared as described above. Ninety-six-well flat-bottomed plates (Nunc MaxiSorp) were coated with goat anti-mouse IgA (Southern Biotechnology Associates), goat anti-mouse IgG (Cappel, Durham, N.C.), or goat anti-mouse IgM (Cappel). Plates were washed in PBS-Tween and blocked as described above. Serial twofold dilutions of fecal supernatants or serum were applied in duplicate to the plates, along with standards. Standards were purified mouse monoclonal IgA (Southern Biotechnology Associates), purified mouse serum IgG (Sigma), and purified mouse monoclonal IgM (TEPC 183; Sigma). After washing with PBS-Tween, secondary antibodies were applied and detected as described above.
Western blot analysis.
Gradient-purified reovirus was boiled in sample buffer (0.5 M Tris-Cl, 2% β2-mercaptoethanol, 0.1% bromophenol blue, 20% glycerol, 4% sodium dodecyl sulfate). Viral proteins were separated by electrophoresis on 10% polyacrylamide gels and transferred to nitrocellulose (Bio-Rad). Strips were blocked in PBS–5% fetal calf serum–0.1% Tween, incubated with individual fecal and serum samples, and then washed in PBS–0.05% Tween. Biotinylated goat anti-mouse IgA, IgG, or IgM (1:3,000; Southern Biotechnology Associates) was added, followed by incubation with streptavidin-horseradish peroxidase (1:500; Pierce). After washing, blots were developed with the Opti-4CN kit (Bio-Rad).
MAbs and passive immunization protocol.
The reovirus-specific IgA and IgG MAbs used in this study were as follows. MAb 4A3 (IgG2b) is specific for reovirus outer capsid protein μ1c, 10G10 (IgG2a) is specific for the outer capsid protein ς3, and 5C6 (IgG2a) is specific for the viral attachment protein ς1 (55). Two IgA MAbs were previously obtained by fusion of Peyer's patch cells after mucosal immunization of BALB/c mice: IgA RB3 is specific for the ς3 protein and IgA RB8 is specific for μ1c. Both were produced by cloned hybridoma cells as a mixture of monomers, dimers, and higher polymers (59). Hybridoma cells were grown in a Tecnomouse hollow-fiber apparatus (Integra Biosciences, Lowell, Mass.). Total IgA and IgG concentrations in Tecnomouse culture supernatants were measured by ELISA as previously described (21). BALB/c mice were used to assess the effects of orally administered IgA and IgG MAbs on viral entry. Unanesthetized mice were inoculated intragastrically with 500 μl of PBS containing 50 μg of reovirus-specific IgA or IgG MAb and 2 × 107 PFU of reovirus T1L. Peyer's patch tissue was removed 8 h after oral inoculation, and virus was quantitated by plaque assay.
Statistics.
The Statview 5.0.1 computer program (Abacus Concepts, Berkeley, Calif.) was used for all calculations and statistical analyses. Results were logarithmically transformed to obtain geometric means. Between-group comparisons were performed by unpaired two-tailed t test at the 99% confidence level. Results of all statistical analyses were considered significant only if P values were 0.01 or less.
RESULTS
Reovirus-specific antibodies in secretions and serum of orally inoculated adult BALB/c mice.
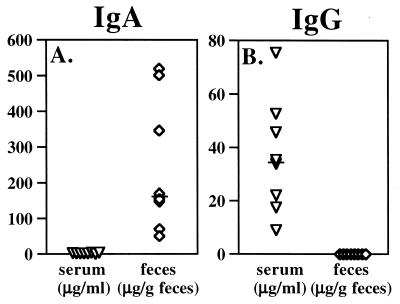
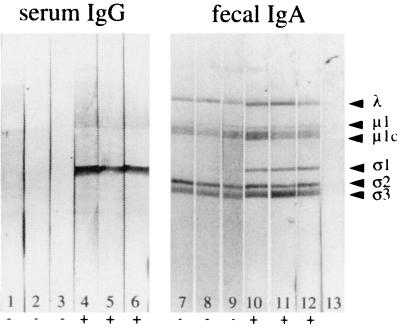
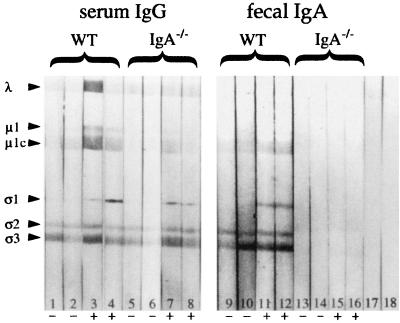
In pilot experiments, 2 × 107 PFU of T1L was identified as an oral challenge dose that consistently resulted in the presence of virus in Peyer's patches of adult BALB/c mice, as detected by viral plaque assays of Peyer's patch tissue at 8 h after feeding. Peyer's patch PFU measured at this time interval represents virus that has entered via M cells and is undergoing early local replication (17, 23). Two groups (eight mice per group) were orally inoculated on day 0 with reovirus, while two groups of control naive mice received no virus. On day 21, about 10 days after the viral infection had been cleared, samples of serum and feces were collected from all 32 mice. A group of eight reovirus-exposed mice and a group of eight naive controls were then orally challenged with reovirus, and 8 h later, Peyer's patches were collected and viral entry was evaluated by plaque assay. Reovirus-specific IgA, IgG, and IgM antibodies present in serum and feces at the time of challenge were measured by ELISA. In all mice exposed to virus on day 0, reovirus-specific IgA antibodies were present in feces at day 21 (Fig. 1). Reovirus-specific IgG was undetectable in fecal supernatants of these mice, but a specific serum IgG response was present (Fig. 1). To determine the antigen specificity of these humoral responses, fecal supernatants and serum samples were applied to Western blots of reovirus proteins (Fig. 2). IgA immunoglobulins in fecal samples from naive control animals bound nonspecifically to multiple reovirus protein bands including ς3 and μ1c (Fig. 2, lanes 7 to 9), so that the extent to which fecal IgA in reovirus-exposed mice specifically recognized these two outer capsid proteins could not be determined. Fecal IgA from naive mice did not bind to the ς1 band, however, and in fecal samples from mice previously exposed to virus, IgA antibodies specific for ς1 were consistently present (Fig. 2, lanes 10 to 12). Western blots also revealed that the serum IgG response to reovirus was focused primarily on the ς1 protein (Fig. 2, lanes 4 to 6). Thus, all mice that were inoculated with reovirus on day 0 had anti-ς1 IgA antibodies in intestinal secretions and anti-ς1 IgG antibodies in serum at the time of oral challenge on day 21.
FIG. 1.
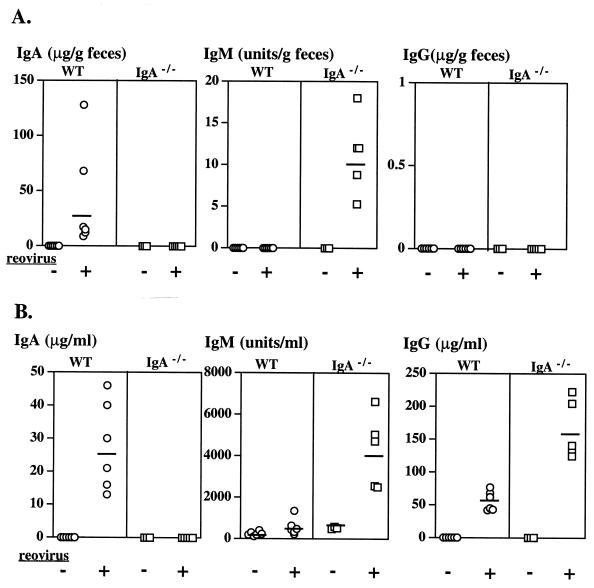
Reovirus-specific antibodies in serum and intestinal secretions of eight adult BALB/c mice, 21 days after oral inoculation with reovirus T1L. Fecal antibodies were almost exclusively of the IgA isotype (A), while the serum response was dominated by IgG (B). Each symbol represents an individual mouse, and bars indicate medians.
FIG. 2.
Western blots of reovirus proteins showing anti-ς1 serum IgG and fecal IgA antibodies in BALB/c mice, 21 days after oral reovirus inoculation. Serum and fecal supernatants from six naive control mice and eight reovirus-inoculated mice were tested; three representative samples from each group are shown. Lanes 1 to 3, sera from naive control mice showed no antireovirus immunoreactivity. Lanes 4 to 6, sera from reovirus-exposed mice contained IgG antibodies directed primarily against the ς1 protein. Lanes 7 to 9, IgA in fecal extracts from naive control mice bound nonspecifically to reovirus proteins including ς3 and μ1c, but not to ς1. Lanes 10 to 12, fecal extracts from reovirus-exposed mice showed the presence of anti-ς1 IgA antibodies. IgA antibodies specific for other viral proteins could not be visualized because of nonspecific binding. Lane 13, control strip exposed to secondary antibody alone.
Previous mucosal infection and clearance of reovirus protects adult mice against subsequent mucosal challenge.
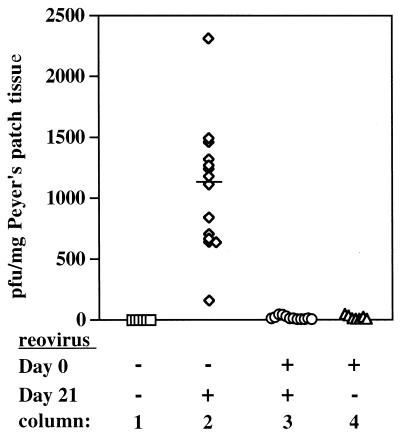
A second set of BALB/c mice were then orally inoculated with reovirus (or not inoculated) on day 0 and rechallenged (or not) on day 21. Peyer's patches were collected 8 h after challenge for plaque assay. Seven mice inoculated on day 0 that were not rechallenged had cleared the infection from the Peyer's patch mucosa by day 21 (Fig. 3, column 4). All 14 of the naive mice that were orally challenged with reovirus on day 21 had infectious virus in their Peyer's patches 8 h later (Fig. 3, column 2). However, 12 mice that had previously cleared a reovirus infection showed no evidence of reinfection (Fig. 3, column 3). Whether virus was prevented from entering the Peyer's patch by secreted IgA antibodies or neutralized within the patch by serum IgG antibodies or virus-specific CTLs could not be determined in these normal mice. To address this issue, we repeated the above experiment using IgA−/− mice.
FIG. 3.
Reovirus in Peyer's patches of naive and reovirus-immunized BALB/c mice, 8 h after mice were rechallenged (or not) on day 21. Column 1, naive mice had no infectious virus in their Peyer's patches (n = 6). Column 2, naive mice orally challenged with reovirus (2 × 107 PFU) had infectious virus in Peyer's patches 8 h after challenge (n = 14). The bar represents the median PFU per milligram of tissue. Column 3, mice orally inoculated with reovirus on day 0 were completely protected against oral reovirus rechallenge on day 21 (n = 12). Column 4, mice orally inoculated with reovirus on day 0 had cleared virus from their Peyer's patches by day 21 (n = 7). Each symbol represents an individual mouse.
Preexposure to reovirus did not prevent mucosal infection upon oral challenge with reovirus in IgA−/− mice.
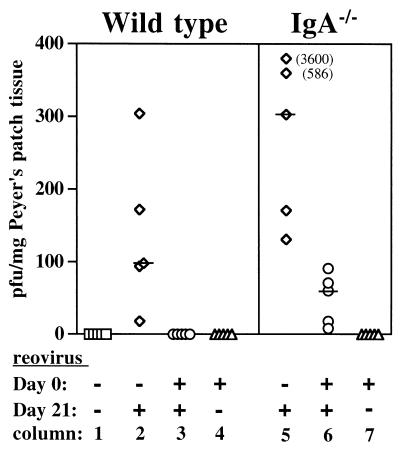
To determine if reovirus-immunized IgA−/− mice would be protected against viral entry upon oral rechallenge, groups (five mice per group) of IgA−/− mice and C57BL/6 controls were inoculated (or not) on day 0 and challenged (or not) 21 days later. Although C57BL/6 mice were not an optimal match, they were preferable to BALB/c mice as controls for the C57BL/6 × 129/Sv IgA−/− mice. Feces and serum samples were collected on the day of challenge for subsequent ELISA analysis as described below. Determination of PFU in Peyer's patch tissue taken on day 21 from immunized mice that were not rechallenged showed that virus had been cleared from the intestines of both C57BL/6 controls and IgA−/− mice by this time (Fig. 4, columns 4 and 7). Comparison of Peyer's patch tissues from naive IgA−/− and naive wild-type mice that were challenged with reovirus on day 21 showed that both were infected at 8 h after challenge, and the difference between these two groups was not significant (Fig. 4, columns 2 and 5). On average, however, the mucosa of IgA−/− mice contained higher amounts of virus, suggesting that IgA in secretions of wild-type mice may have provided some nonspecific protection. Peyer's patch tissues from previously exposed, wild-type C57BL/6 mice were completely virus free 8 h after rechallenge (Fig. 4, column 3), confirming the results in BALB/c mice. In contrast, Peyer's patch tissues from previously exposed IgA−/− mice consistently contained infectious virus (Fig. 4, column 6), although in significantly lower quantities than IgA−/− mice that had not been previously exposed (Fig. 4, column 5).
FIG. 4.
Viral entry into Peyer's patches of naive and reovirus-exposed C57BL/6 (wild-type) and IgA−/− mice, 8 h after oral rechallenge with 5 × 108 PFU of reovirus at day 21. Column 1, Peyer's patches of naive wild-type mice contained no virus. Column 2, naive wild-type mice orally challenged with reovirus had infectious virus in Peyer's patches 8 h after challenge. Column 3, previously inoculated wild-type mice were completely protected against oral reovirus rechallenge on day 21. Column 4, wild-type mice orally inoculated with reovirus on day 0 had cleared virus from their Peyer's patches by day 21. Column 5, naive IgA−/− mice orally challenged with reovirus had infectious virus in Peyer's patches 8 h after challenge. The median PFU per milligram of tissue was higher than in comparable wild-type mice shown in column 2, but the difference was not significant (P = 0.086). Column 6, previously inoculated IgA−/− mice were not protected against oral reovirus rechallenge on day 21, although levels of infectious virus in Peyer's patches were significantly lower than those in naive IgA−/− challenged with reovirus (P = 0.01). Column 7, IgA−/− mice orally inoculated with reovirus on day 0 had cleared virus from their Peyer's patches by day 21. Each symbol represents an individual mouse, and bars indicate the medians for each group.
Total and reovirus-specific antibodies in secretions and serum of orally inoculated IgA knockout mice.
Humoral immune responses to reovirus in IgA−/− and control mice were analyzed in serum and fecal samples collected 21 days after oral inoculation in two separate sets of mice. One set, consisting of four groups (wild type and IgA−/−, inoculated and naive), was used to measure levels of total immunoglobulins and reovirus-specific antibodies; these mice were not rechallenged. Reovirus-specific antibodies were measured in the second set described above (five mice per group), which were inoculated (or not) on day 0 and rechallenged (or not) on day 21 as shown in Fig. 4. Levels of reovirus-specific antibodies in the two sets of mice were comparable. ELISA analysis confirmed that there was no detectable IgA in intestinal secretions or serum of IgA−/− mice as previously documented (22), but there were high levels of IgA (mean, 940 μg/ml) in feces of C57BL/6 controls. We also confirmed that mean total IgM levels in fecal samples were low but were significantly higher in IgA−/− mice (3.1 μg/g of feces) than in wild-type mice (1.0 μg/g of feces) (P ≤ 0.0001). Total IgG levels in feces of IgA−/− and wild-type mice were low and comparable, ranging from 1 to 8.5 μg of IgG/g of feces. In the serum of IgA−/− mice, total IgM levels were significantly higher (mean, 1,040 μg/ml) than in C57BL/6 controls (mean, 363 μg/ml) (P = 0.0027). Total IgG levels in IgA−/− mice (mean, 12.04 mg/ml) were also significantly higher than in wild-type controls (mean, 2.28 mg/ml) (P = 0.0079), confirming the original description of these mice (22).
Reovirus-specific antibodies in fecal and serum samples are shown in Fig. 5. Antireovirus IgA antibodies were present in feces of wild-type C57BL/6 mice (although at lower levels than in BALB/c mice) but were not present in the IgA−/− mice as expected (Fig. 5A). However, reovirus-specific IgM was detected in the feces of IgA−/− mice, whereas it was undetectable in wild-type mice. In neither group were reovirus-specific IgG antibodies detected in fecal supernatants. As expected, reovirus-specific IgA was present in sera of wild-type mice but was undetectable in IgA−/− mice (Fig. 5B). Levels of reovirus-specific serum IgM mirrored total IgM levels: IgA−/− mice (but not wild-type controls) produced detectable levels of specific IgM. Reovirus-specific serum IgG levels were also higher in IgA−/− mice (mean, 161 μg/ml) than in wild-type mice (mean, 54 μg/ml), and this difference was significant. Taken together, the viral plaque assays and ELISA data showed that protection of previously exposed C57BL/6 mice against Peyer's patch infection was associated with high levels of specific and total IgA in secretions, and the absence of IgA in secretions of IgA−/− mice resulted in an inability to prevent viral entry and replication. Although elevated IgM antibodies in secretions and high levels of IgG and IgM antibodies in serum of the IgA−/− mice appeared to have provided partial protection against reovirus challenge, the Peyer's patches nevertheless became infected.
FIG. 5.
Reovirus-specific IgA, IgM, and IgG levels in feces and serum of wild-type (WT) and IgA−/− mice, either naive (−) or reovirus exposed (+), on day 21 after oral inoculation. Each symbol represents an individual mouse, and bars indicate geometric means for each group. IgM levels are expressed as arbitrary ELISA units based on a standard pooled serum preparation from reovirus-immunized mice. IgA and IgG levels are measured as micrograms per milliliter of serum or micrograms per gram of feces, determined using antireovirus monoclonal IgA (monomer-dimer mixture) or IgG as standard. (A) Reovirus-specific IgA, IgM, and IgG in feces. No specific IgA was detected in IgA−/− mice, as expected. Specific IgM was detected, but levels were very low (mean, 10.4 U/g) compared to serum IgM levels (mean, 3,984 U/ml, shown in panel B). No reovirus-specific IgG was detected in feces of WT or IgA−/− mice. (B) Reovirus-specific IgA, IgM, and IgG in serum. Specific serum IgA levels in reovirus-inoculated C57BL/6 mice were higher (mean, 25 μg/ml) than in comparable BALB/c mice shown in Fig. 1. IgA−/− mice had significantly higher levels of specific serum IgM and IgG than did WT mice (for both, P ≤ 0.001).
Western blot analysis of fecal supernatants from reovirus-inoculated mice confirmed that IgA−/− mice had no detectable reovirus-specific IgA antibodies in secretions (Fig. 6, lanes 13 to 16) while wild-type C57BL/6 mice had IgA antibodies specific for ς1 (Fig. 6, lanes 11 and 12). Responses to μ1c and ς3 could not be assessed due to nonspecific binding of fecal IgA to these proteins. Sera of both wild-type and IgA−/− mice contained specific IgG antibodies directed against ς1 (Fig. 6, lanes 3 and 4 and lanes 7 and 8), and some IgG reactivity against μ1c and other viral proteins was inconsistently observed. No specific IgM in sera or secretions of control or knockout mice was detected on Western blots (data not shown).
FIG. 6.
Western blot analysis of serum and fecal supernatants from C57BL/6 (wild-type [WT]) and IgA−/− mice collected 21 days after oral reovirus inoculation. Serum and fecal supernatants of two representative mice from each group are shown. Lanes 1 and 2, sera from naive control mice showed no anti-ς1 immunoreactivity although nonspecific binding of IgG to other reovirus proteins was seen. Lanes 3 and 4, anti-ς1 IgG antibodies were consistently present in the sera of reovirus-exposed WT mice. IgG binding to other proteins was variable. Lanes 5 and 6, sera from naive IgA−/− mice showed some nonspecific IgG binding to some reovirus proteins but not to ς1. Lanes 7 and 8, sera from IgA−/− mice orally inoculated with reovirus contained anti-ς1 IgG antibodies. Lanes 9 and 10, fecal supernatants of naive control WT mice did not contain detectable anti-ς1 IgA antibodies, although nonspecific IgA binding to other reovirus proteins was observed. Lanes 11 and 12, anti-ς1 IgA antibodies were detected in fecal supernatants of reovirus-exposed WT mice. Lanes 13 to 16, no reovirus-specific or nonspecific IgA was detected in fecal supernatants of naive or reovirus-exposed IgA−/− mice. Lanes 17 and 18, control strips exposed to secondary goat anti-mouse IgG and to IgA, respectively.
Effects of antireovirus MAbs on virus entry into Peyer's patch mucosa.
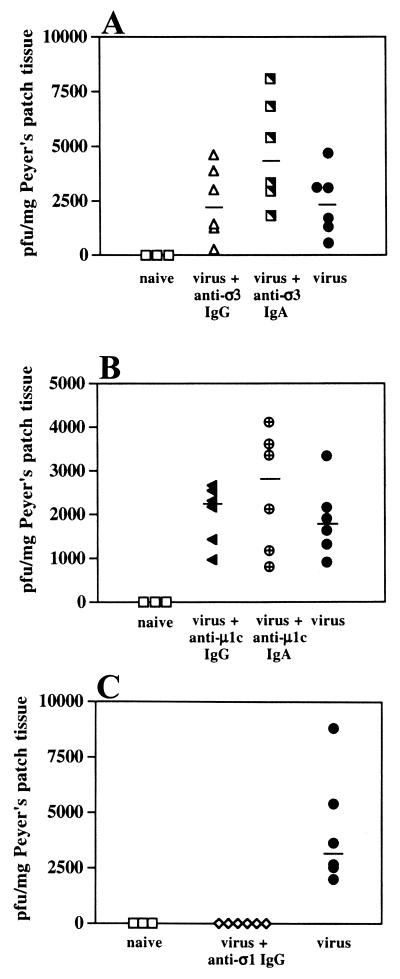
In the immunized BALB/c and C57BL/6 wild-type mice described above, protection against rechallenge was consistently associated with the presence of fecal IgA antibodies directed against the ς1 attachment protein. Whether antibodies against μ1c and ς3 proteins were also present could not be determined because of nonspecific IgA binding on Western blots, but we considered their presence likely because IgA lymphoblasts recovered from mouse Peyer's patches of reovirus-exposed mice were previously shown to be specific for these antigens (59). The outer capsid protein responsible for reovirus adherence to M cells has not been identified, but previous studies using other enteric pathogens have shown that IgAs against abundant microbial surface antigens can protect the mucosa by immune exclusion, even if the antigen is not directly involved in host cell adherence (9, 33, 61). Monoclonal IgA antibodies specific for the abundant outer capsid proteins μ1c and ς3 (but not the viral attachment protein ς1) were available. We therefore sought to determine whether specific luminal IgA MAbs directed against the viral outer capsid may protect against infection of mouse Peyer's patches by preventing M-cell contact or may allow viral entry by mediating adherence to M cells. Groups of mice were orally challenged with reovirus mixed with antireovirus IgA MAbs (at a ratio of about 6 × 104 IgA dimers or 12 × 104 IgA monomers per viral particle), and viral entry in Peyer's patch tissue was measured 8 h later. Other groups received IgG MAbs against μ1c and ς3 for comparison, and control mice received virus with no antibody. Neither the IgG nor the IgA MAbs directed against ς3 prevented reovirus entry (Fig. 7A). This is consistent with the fact that this protein is removed by proteases in the intestinal lumen (7). The μ1c protein is not removed, however, and thus, anti-μ1c IgA MAbs would be expected to remain associated with viral particles. However, neither the IgA nor the IgG MAbs directed against μ1c reduced viral entry or early replication (Fig. 7B). Thus, the IgA MAbs apparently failed to prevent viral contact with the mucosa by immune exclusion and failed to prevent M-cell-mediated reovirus uptake.
FIG. 7.
Effects of antireovirus MAbs on viral entry into Peyer's patches in BALB/c mice. Aliquots of reovirus (2 × 107 PFU) were mixed with 50 μg of MAb and inoculated intragastrically into groups of six mice. Naive mice (n = 3) were not inoculated, and control mice (n = 6) received virus only. After 8 h, Peyer's patches were removed and viral entry was assessed by plaque assay. Each symbol represents an individual mouse, and bars indicate median PFU. (A) Neither anti-ς3 IgG nor anti-ς3 IgA MAb affected viral entry. Although viral titers in the patches were somewhat higher in the presence of the anti-ς3 IgA MAb than with the anti-ς3 IgG MAb in the experiment shown, this difference was not significant and was not observed when the experiment was repeated (data not shown). (B) Neither anti-μ1c IgG nor anti-μ1c IgA MAb affected viral entry. (C) Anti-ς1 IgG MAb 5C6 prevented viral infection of Peyer's patches in all mice tested (n = 6).
In the absence of an IgA MAb specific for the viral attachment protein ς1, we tested an available anti-reovirus T1L IgG MAb, 5C6 (55), which recognizes the head region of the ς1 attachment protein (10). MAb 5C6 was neutralizing in cell culture assays and protected neonatal mice against intracranial injection of reovirus T1L (53). When mixed with virus (at a ratio of about 1.2 × 105 molecules of IgG per viral particle) and fed to mice, this MAb consistently blocked viral infection of Peyer's patch mucosa (Fig. 7C). Although significant amounts of IgG are not normally present in mouse intestinal secretions, this result served to validate the passive feeding protocol by showing that MAbs remained associated with virus in the intestinal lumen. More importantly, it indicated that specific blocking of the viral attachment protein ς1 prevented M-cell-mediated entry whereas blocking of other outer capsid proteins did not.
DISCUSSION
The results of this study show that antireovirus IgA secreted into the intestines of immunized mice plays a crucial role in protection against mucosal reinfection. This is consistent with the well-documented role of S-IgA in preventing pathogen entry into mucosal tissues (24, 32, 48). From studies of a variety of viral pathogens including reovirus and rotavirus, it is generally agreed that cell-mediated immunity plays a major role in clearing viral infections but that antibodies are essential for prevention of infection (18, 19, 26, 27, 30, 31). The relative importance of S-IgA and IgG antibodies in mucosal protection is still controversial, however. Studies in which antirotavirus IgG was induced in serum by immunization or passively delivered onto mucosal surfaces indicated that IgG alone can be sufficient for protection (13, 39, 40), and this has been supported by recent studies using IgA knockout mice (41). In addition, wild-type and IgA−/− mice immunized with influenza virus subunit vaccines along with adjuvants (either cholera holotoxin and B subunit or interleukin-12) showed equivalent levels of protection against pulmonary influenza virus challenge, and protection was attributed to the presence of specific serum IgG in the IgA−/− mice that presumably entered the lungs and airways (3, 29, 57).
It is not surprising that IgG can be sufficient to protect the respiratory tract where significant amounts of IgG, transudated from serum or produced locally, are normally present in secretions (34). More surprising is the report that immunized IgA−/− mice were protected against oral rotavirus challenge (41), since IgG levels in small intestinal secretions of mice are normally very low (21). Indeed, IgG levels in intestinal secretions of the normal mice that were rotavirus immunized and protected were low as expected, but the immunized IgA−/− mice (which were also protected) had elevated antiviral IgG levels in intestinal secretions (41). This suggests that, in the absence of IgA, rotavirus infection of villus epithelial cells caused a defect in epithelial barrier function, perhaps similar to that observed in rotavirus-infected epithelial monolayers (38), which persisted even after the initial infection had been cleared. In addition, serum IgG that diffuses freely from fenestrated villus capillaries and percolates between villus epithelial cells (1) might have restricted rotavirus spread within the epithelium. In contrast, the reovirus-immunized IgA knockout mice in our study did not have elevated antiviral IgG in intestinal secretions and were not protected against reinfection despite high levels of antireovirus IgG in serum.
This apparent discrepancy may reflect the fact that reovirus, unlike rotavirus, initiates infection only in the Peyer's patches (17, 37). Although reovirus-specific serum IgG antibodies against the viral attachment protein could theoretically prevent initial target cell infection and cell-to-cell spread of reovirus in the Peyer's patch mucosa (60), there is evidence that serum IgG diffuses poorly into Peyer's patch mucosa where capillaries are nonfenestrated (1). Indeed, neutralizing antireovirus IgG administered systemically failed to prevent viral entry or early replication in Peyer's patches of suckling mice (51, 52). Similarly, serum IgG failed to prevent the early stage of Peyer's patch infection in the IgA−/− adult mice in this study. The presence of antireovirus IgM was not sufficient for protection against reinfection in our experiments, although it has been suggested that secretory IgM might compensate for IgA in individuals with IgA deficiency (15). It is important to note that the levels of virus in the Peyer's patches of our immunized IgA−/− mice were lower than in unimmunized IgA−/− mice after oral reovirus challenge, suggesting that antiviral serum IgG (and possibly secretory IgM) did provide some protection against entry or early viral replication. However, in the absence of IgA it was not sufficient to completely prevent mucosal infection.
When naive mice that had no reovirus-specific antibodies were orally challenged with reovirus, the IgA−/− mice on average had higher viral titers in their Peyer's patches than did wild-type mice. This is not likely to be due to impaired cell-mediated immunity. Although a recent report suggested that the absence of IgA results in defective T-cell help (3), others had shown that T-cell functions are normal in the IgA−/− mice (22, 29). It is possible that C57BL/6 mice have more innate protective factors in the intestine than do C57BL/6 × 129/Sv mice. However, we consider it likely that the presence of S-IgA in the wild-type mice provided some degree of nonspecific mucosal protection against reovirus. Nonspecific IgA protection has been observed in studies of urinary tract infections with Escherichia coli, where protection proved to be due to interaction of bacterial lectin-like attachment proteins with IgA oligosaccharides (50, 62). Fecal IgA interacted nonspecifically with some reovirus proteins on Western blots, but whether such interactions occurred with intact viral particles in vivo is not known. Alternatively, the endogenous IgA that accumulates on M-cell surfaces of normal mice may have provided nonspecific protection by sterically hindering access of viral particles to the relevant M-cell surface binding sites. In any case, only the mice that had specific antireovirus IgA in intestinal secretions were completely protected against infection of Peyer's patch mucosa.
In mice previously exposed to reovirus, the Peyer's patch tissue presumably contained virus-specific CTLs. Both CD4+ and CD8+ T cells have been shown to be involved in protection against systemic reovirus disease (55), and enteric reovirus infection induced reovirus-specific CTLs in Peyer's patch tissue after intestinal priming (26). However, cell-mediated immunity would come into play only after the virus has entered the Peyer's patch and succeeded in infecting target cells. Indeed, there is evidence that CTLs alone are unable to prevent early proliferation in the mucosa. In studies using SCID mice, adoptive transfer of reovirus-immune, B-cell-depleted spleen cells prior to oral challenge did not prevent initial entry or replication of virus in intestinal tissue (4). Reovirus-specific CTLs would thus not be expected to prevent the initial infection of Peyer's patch target cells that was measured in our immunized IgA−/− mice at 8 h after challenge, although they could eventually have cleared infected cells and limited the infection.
In the process of establishing a mucosal infection in the Peyer's patches of naive mice, reovirus survives passage through the protease-rich environment of the intestinal lumen and then exploits specific binding sites exposed on M-cell surfaces. Enzymes in the intestinal lumen cleave off the reovirus ς3 outer capsid protein, which exposes the putative fusion protein μ1c and allows the viral attachment protein ς1 to extend from the viral surface (16, 20, 36). On the epithelial surface, the virus adheres to specific oligosaccharides on apical membranes of M cells containing α(2-3)-linked sialic acid (K. J. Silvey et al., submitted for publication), which results in rapid vesicular transport across the epithelial barrier. In the small intestinal secretions of normal adult mice, the only immune effector available is S-IgA. S-IgA diffuses freely through mucus gels (12), but when complexed with antigen, its avidity for mucins increases (49), and this appears to be the basis for its ability to intercept pathogens and prevent mucosal contact by immune exclusion. Secretion of monoclonal IgA antibodies directed against microbial surface antigens that were nonneutralizing in cell culture systems has been shown to protect the small intestines of mice against bacterial and viral pathogens (9, 33, 47, 61). On this basis, we assume that immune exclusion played a role in the protection against reovirus challenge observed in our immunized wild-type mice. Studies using IgA or IgM MAbs against rotavirus (9), Sendai virus (28), and human immunodeficiency virus (8) have suggested an additional mechanism of protection in which secretory antibodies being exported by receptor-mediated transepithelial transport intercept incoming viruses within intracellular compartments of epithelial cells. It should be noted that anti-ς1 IgA could not protect against reovirus by this mechanism because reovirus enters via M cells in the follicle-associated epithelium where IgA export does not occur (43).
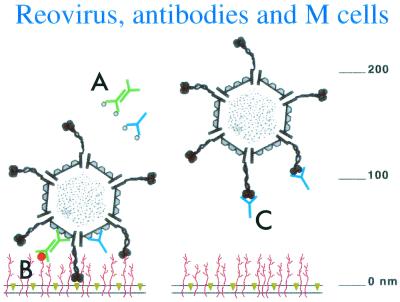
The passive protection results in which IgAs against virus major outer capsid proteins did not prevent uptake and infection appear inconsistent with the protection associated with IgA secretion in immunized normal mice. However, it is important to note that our passive feeding protocol involved loading large amounts of virus and antibodies into the intestinal lumen. This may have resulted in considerable contact of virus-antibody complexes with the epithelium and M cells. Previous studies demonstrated that IgA adheres selectively to the apical membranes of M cells in mice and that adherent IgA-coated particles are transported across the epithelial barrier (59). M-cell adherence of IgA-coated reovirus could explain the fact that neither of the nonneutralizing IgA MAbs, which were directed against the outer capsid proteins ς3 and μ1c, prevented viral entry. If the ς3 protein along with associated IgA antibodies was cleaved off in the lumen by intestinal proteases, the virus could have adhered to M cells via the ς1 adhesin. If the anti-ς3 IgA inhibited cleavage of ς3, it is possible that the IgA itself could have mediated M-cell adherence. We have assumed, but not proven, that the anti-μ1c antibodies could remain associated with the virus in the intestine because μ1c cleavage products are not lost from the viral surface and our anti-μ1c IgA recognizes ISVPs (59). Anti-μ1c IgA on the viral surface may not have prevented interaction of the ς1 attachment protein with M cells, because the length of dimeric IgA is not thought to exceed 30 to 35 nm, whereas the extended ς1 is at least 40 nm long (16, 25) (Fig. 8). The fact that actively immunized, normal mice that secreted IgA against the viral attachment protein ς1 were protected, together with the observation that the anti-ς1 IgG MAb 5C6 was the only antibody that completely prevented Peyer's patch infection, suggested that IgA antibodies directed against ς1 might have played a particularly important role in protection of the immunized mice. Whether anti-ς1 IgA antibodies can protect against viral entry by preventing M-cell adherence in spite of the potential IgA–M-cell interaction is unknown. This issue must be resolved in future studies when appropriate IgA MAbs directed against the ς1 viral attachment protein become available.
FIG. 8.
Cartoon summarizing possible interactions of MAbs with reovirus in the passive protection experiments. The M-cell plasma membrane with integral glycolipids (gold) and glycoproteins (red), along with reovirus ISVPs (adapted from reference 37), is drawn approximately to scale (note nanometer scale at right). (A) Neither IgG (blue) nor dimeric IgA (green) directed against the ς3 outer capsid protein prevented M-cell attachment and Peyer's patch infection, presumably because ς3 is cleaved off by digestive proteases. (B) Anti-μ1c IgG (blue) or dimeric IgA (green) may have failed to prevent reovirus attachment via the extended ς1 attachment protein. Thus, reovirus coated with anti-μ1c IgA could have adhered to M cells via either ς1 or IgA. (The red dot represents a putative IgA receptor on the M cell.) (C) Anti-ς1 IgG may have prevented entry and infection by blocking the interaction of the ς1 viral attachment protein with M-cell receptors.
ACKNOWLEDGMENTS
This work was supported by NIH Research Grant HD17557 and by NIH Center Grant DK34854 to the Harvard Digestive Diseases Center. Katherine Silvey was partially supported by an NIH Training Grant to the Committee on Immunology, Harvard Medical School.
We thank John Nedrud, Department of Pathology, Case Western Reserve University, for providing IgA knockout mice for these studies. We are indebted to William Lucas and David M. Knipe, Department of Microbiology and Molecular Genetics, Harvard Medical School, for assistance and expertise.
Footnotes
This study is dedicated to the memory of our friend and colleague Bernard N. Fields.
REFERENCES
- 1.Allan C H, Trier J S. Structure and permeability differ in subepithelial villus and Peyer's patch follicle capillaries. Gastroenterology. 1991;100:1172–1179. [PubMed] [Google Scholar]
- 2.Amerongen H M, Wilson G A R, Fields B N, Neutra M R. Proteolytic processing of reovirus is required for adherence to intestinal M cells. J Virol. 1994;68:8428–8432. doi: 10.1128/jvi.68.12.8428-8432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arulanandam B P, Raeder R H, Nedrud J G, Bucher D J, Le J, Metzger D W. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J Immunol. 2001;166:226–231. doi: 10.4049/jimmunol.166.1.226. [DOI] [PubMed] [Google Scholar]
- 4.Barkon M L, Haller B L, Virgin H W., IV Circulating immunoglobulin G can play a critical role in clearance of intestinal reovirus infection. J Virol. 1996;70:1109–1116. doi: 10.1128/jvi.70.2.1109-1116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bass D M, Bodkin D, Dambrauskas R, Trier J S, Fields B N, Wolf J L. Intraluminal proteolytic activation plays an important role in replication of type 1 reovirus in the intestines of neonatal mice. J Virol. 1990;64:1830–1833. doi: 10.1128/jvi.64.4.1830-1833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard T G, Czinn S J, Redline R W, Sigmund N, Harriman G, Nedrud J G. Antibody-independent protective mucosal immunity to gastric helicobacter infection in mice. Cell Immunol. 1999;191:74–80. doi: 10.1006/cimm.1998.1421. [DOI] [PubMed] [Google Scholar]
- 7.Bodkin D K, Nibert M L, Fields B N. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol. 1989;63:4676–4681. doi: 10.1128/jvi.63.11.4676-4681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bomsel M, Heyman M, Hocini H, Lagaye S, Beiec L, Dupont C, Desgranges C. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–287. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- 9.Burns J W, Siadat-Pajouh M, Krishnaney A A, Greenberg H B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 10.Chappell J D, Duong J L, Wright B W, Dermody T S. Identification of carbohydrate-binding domains in the attachment proteins of type 1 and type 3 reoviruses. J Virol. 2000;74:8472–8479. doi: 10.1128/jvi.74.18.8472-8479.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements M L, Betts R F, Tierney E L, Murphy B R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cone R A. Mucus. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. San Diego, Calif: Academic Press; 1999. pp. 43–64. [Google Scholar]
- 13.Conner M E, Crawford S E, Barone C, Estes M K. Rotavirus vaccine administered parenterally induces protective immunity. J Virol. 1993;67:6633–6641. doi: 10.1128/jvi.67.11.6633-6641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuff C F, Lavi E, Cebra C K, Cebra J J, Rubin D H. Passive immunity to fatal reovirus serotype 3-induced meningoencephalitis mediated by both secretory and transplacental factors in neonatal mice. J Virol. 1990;64:1256–1263. doi: 10.1128/jvi.64.3.1256-1263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham-Rundles C. Immunodeficiency and mucosal immunity. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. San Diego, Calif: Academic Press; 1999. pp. 939–948. [Google Scholar]
- 16.Dryden K A, Wang G, Yeager M, Nibert M L, Coombs K M, Furlong D B, Fields B N, Baker T S. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J Cell Biol. 1993;122:1023–1041. doi: 10.1083/jcb.122.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan J Y, Boyce C S, Cuff C F. T-helper 1 and T-helper 2 cytokine responses in gut-associated lymphoid tissue following enteric reovirus infection. Cell Immunol. 1998;188:55–63. doi: 10.1006/cimm.1998.1350. [DOI] [PubMed] [Google Scholar]
- 18.Franco M A, Greenberg H B. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800–7806. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franco M A, Tin C, Greenberg H B. CD8+ T cells can mediate almost complete short-term and partial long-term immunity to rotavirus in mice. J Virol. 1997;71:4165–4170. doi: 10.1128/jvi.71.5.4165-4170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlong D B, Nibert M L, Fields B N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988;62:246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harriman G R, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, Zhang Y, Mbawuike I N. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol. 1999;162:2521–2529. [PubMed] [Google Scholar]
- 23.Kauffman R S, Wolf J L, Finberg R, Trier J S, Fields B N. The ς1 protein determines the extent of spread of reovirus from the gastrointestinal tract of mice. Virology. 1983;124:403–410. doi: 10.1016/0042-6822(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 24.Lamm M E. Interactions of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 25.Lee P W K, Gilmore R. Reovirus cell attachment protein s1: structure-function relationships and biogenesis. In: Tyler K L, Oldstone M B A, editors. Reoviruses I: structure, proteins, and genetics. Berlin, Germany: Springer-Verlag; 1998. pp. 137–153. [DOI] [PubMed] [Google Scholar]
- 26.London S D, Rubin D H, Cebra J J. Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer's patches. J Exp Med. 1987;165:830–847. doi: 10.1084/jem.165.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Major A S, Cuff C F. Effects of the route of infection on immunoglobulin G subclasses and specificity of the reovirus-specific humoral immune response. J Virol. 1996;70:5968–5974. doi: 10.1128/jvi.70.9.5968-5974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazanec M B, Kaetzel C S, Lamm M E, Fletcher D, Nedrud J G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mbawuike I N, Pacheco S, Acuna C L, Switzer K C, Zhang Y, Harriman G R. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J Immunol. 1999;162:2530–2537. [PubMed] [Google Scholar]
- 30.McNeal M M, Barone K S, Rae M N, Ward R L. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virology. 1995;214:387–397. doi: 10.1006/viro.1995.0048. [DOI] [PubMed] [Google Scholar]
- 31.McNeal M M, Rae M N, Ward R L. Evidence that resolution of rotavirus infection in mice is due to both CD4 and CD8 cell-dependent activities. J Virol. 1997;71:8735–8742. doi: 10.1128/jvi.71.11.8735-8742.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mestecky J, Moro I, Underdown B J. Mucosal immunoglobulins. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. San Diego, Calif: Academic Press; 1999. pp. 133–152. [Google Scholar]
- 33.Michetti P, Mahan M J, Slauch J M, Mekalanos J J, Neutra M R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy B R. Mucosal immunity to viruses. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. San Diego, Calif: Academic Press; 1999. pp. 695–707. [Google Scholar]
- 35.Neutra M R, Mantis N J, Frey A, Giannasca P J. The composition and function of M cell apical membranes: implications for microbial pathogenesis. Semin Immunol. 1999;11:171–181. doi: 10.1006/smim.1999.0173. [DOI] [PubMed] [Google Scholar]
- 36.Nibert M L. Structure of mammalian orthoreovirus particles. In: Tyler K L, Oldstone M B A, editors. Reoviruses I: structure, proteins, and genetics. Berlin, Germany: Springer-Verlag; 1998. pp. 1–30. [DOI] [PubMed] [Google Scholar]
- 37.Nibert M L, Furlong D B, Fields B N. Mechanisms of viral pathogenesis. Distinct forms of reoviruses and their roles during replication in cells and host. J Clin Investig. 1991;88:727–734. doi: 10.1172/JCI115369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obert G, Peiffer I, Servin A L. Rotavirus-induced structural and functional alterations in tight junctions of polarized intestinal Caco-2 cell monolayers. J Virol. 2000;74:4645–4651. doi: 10.1128/jvi.74.10.4645-4651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Offit P A, Clark H F. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J Virol. 1985;54:58–64. doi: 10.1128/jvi.54.1.58-64.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Offit P A, Shaw R D, Greenberg H B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986;58:700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Neal C, Harriman G R, Conner M E. Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A. J Virol. 2000;74:4102–4109. doi: 10.1128/jvi.74.9.4102-4109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Organ E L, Rubin D H. Pathogenesis of reovirus gastrointestinal and hepatobiliary disease. In: Tyler K L, Oldstone M B A, editors. Reoviruses II: cytopathogenicity and pathogenesis. Berlin, Germany: Springer-Verlag; 1998. pp. 67–83. [DOI] [PubMed] [Google Scholar]
- 43.Pappo J, Owen R L. Absence of secretory component expression by epithelial cells overlying rabbit gut-associated lymphoid tissue. Gastroenterology. 1988;95:1173–1177. doi: 10.1016/0016-5085(88)90347-2. [DOI] [PubMed] [Google Scholar]
- 44.Parr M B, Harriman G R, Parr E L. Immunity to vaginal HSV-2 infection in immunoglobulin A knockout mice. Immunology. 1998;95:208–213. doi: 10.1046/j.1365-2567.1998.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodewald R. Distribution of immunoglobulin G receptors in the small intestine of the young rat. J Cell Biol. 1980;85:18–32. doi: 10.1083/jcb.85.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubin D H, Anderson A O, Lucis D. Potentiation of the secretory IgA response by oral and enteric administration of CP 20,961. Ann N Y Acad Sci. 1983;409:866–870. [Google Scholar]
- 47.Ruggeri F M, Johansen K, Basile G, Kraehenbuhl J P, Svensson L. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J Virol. 1998;72:2708–2714. doi: 10.1128/jvi.72.4.2708-2714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell M W, Kilian M, Lamm M E. Biological activities of IgA. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. San Diego, Calif: Academic Press; 1999. pp. 225–240. [Google Scholar]
- 49.Saltzman W M, Radomsky M L, Whaley K J, Cone R A. Antibody diffusion in human cervical mucus. Biophys J. 1994;66:508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svanborg-Eden C, Svennerholm A M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary epithelial cells. Infect Immun. 1978;22:790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tyler K L, Virgin IV H W, Bassel-Duby R, Fields B N. Antibody inhibits defined stages in the pathogenesis of reovirus serotype 3 infection of the central nervous system. J Exp Med. 1989;170:887–900. doi: 10.1084/jem.170.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tyler K L, Mann M A, Fields B N, Virgin H W., IV Protective anti-reovirus monoclonal antibodies and their effects on viral pathogenesis. J Virol. 1993;67:3446–3453. doi: 10.1128/jvi.67.6.3446-3453.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tyler K L, Bronson R T, Byers K B, Fields B N. Molecular basis of viral neurotropism: experimental reovirus infection. Neurology. 1985;35:88–92. doi: 10.1212/wnl.35.1.88. [DOI] [PubMed] [Google Scholar]
- 54.Virgin H W, IV, Tyler K L. Role of immune cells in protection against and control of reovirus infection in neonatal mice. J Virol. 1991;65:5157–5164. doi: 10.1128/jvi.65.10.5157-5164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Virgin H W, IV, Mann M A, Fields B N, Tyler K L. Monoclonal antibodies to reovirus reveal structure/function relationships between capsid proteins and genetics of susceptibility to antibody action. J Virol. 1991;65:6772–6781. doi: 10.1128/jvi.65.12.6772-6781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Virgin H W, IV, Bassel-Duby R, Fields B N, Tyler K L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing) J Virol. 1988;62:4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner D K, Clements M L, Reimer C B, Snyder M, Nelson D L, Murphy B R. Analysis of immunoglobulin G antibody responses after administration of live and inactivated influenza A vaccine indicates that nasal wash immunoglobulin G is a transudate from serum. J Clin Microbiol. 1987;25:559–562. doi: 10.1128/jcm.25.3.559-562.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinstein P D, Cebra J J. The preference for switching to IgA expression by Peyer's patch germinal center B cells is likely due to the intrinsic influence of their microenvironment. J Immunol. 1991;147:4126–4135. [PubMed] [Google Scholar]
- 59.Weltzin R, Lucia-Jandris P, Michetti P, Fields B N, Kraehenbuhl J P, Neutra M R. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989;108:1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitton J L, Oldstone M B A. Immune responses to viruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 345–374. [Google Scholar]
- 61.Winner L S, III, Mack J, Weltzin R A, Mekalanos J J, Kraehenbuhl J P, Neutra M R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991;59:977–998. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wold A, Mestecky J, Tomana M, Kobata A, Ohbayashi H, Endo T, Svanborg-Eden C. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990;58:3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolf J L, Rubin D H, Finberg R, Kauffman R S, Sharpe A H, Trier J S, Fields B N. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981;212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]