Abstract
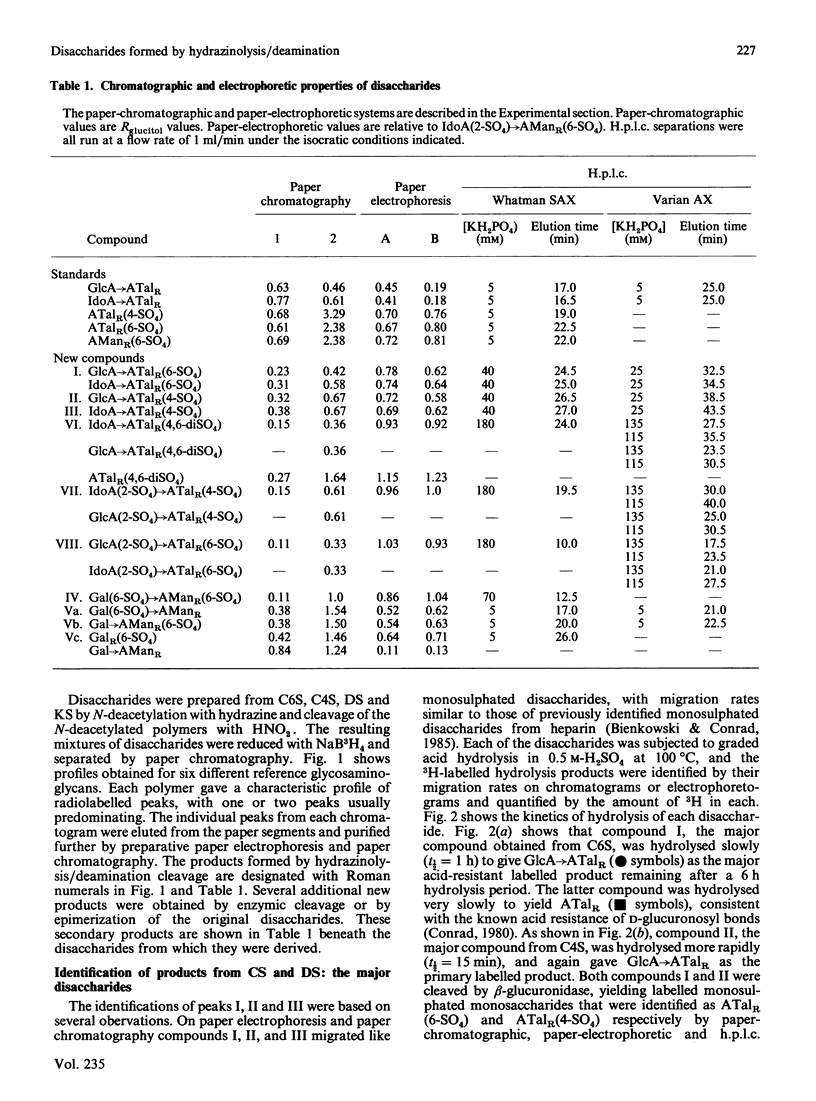
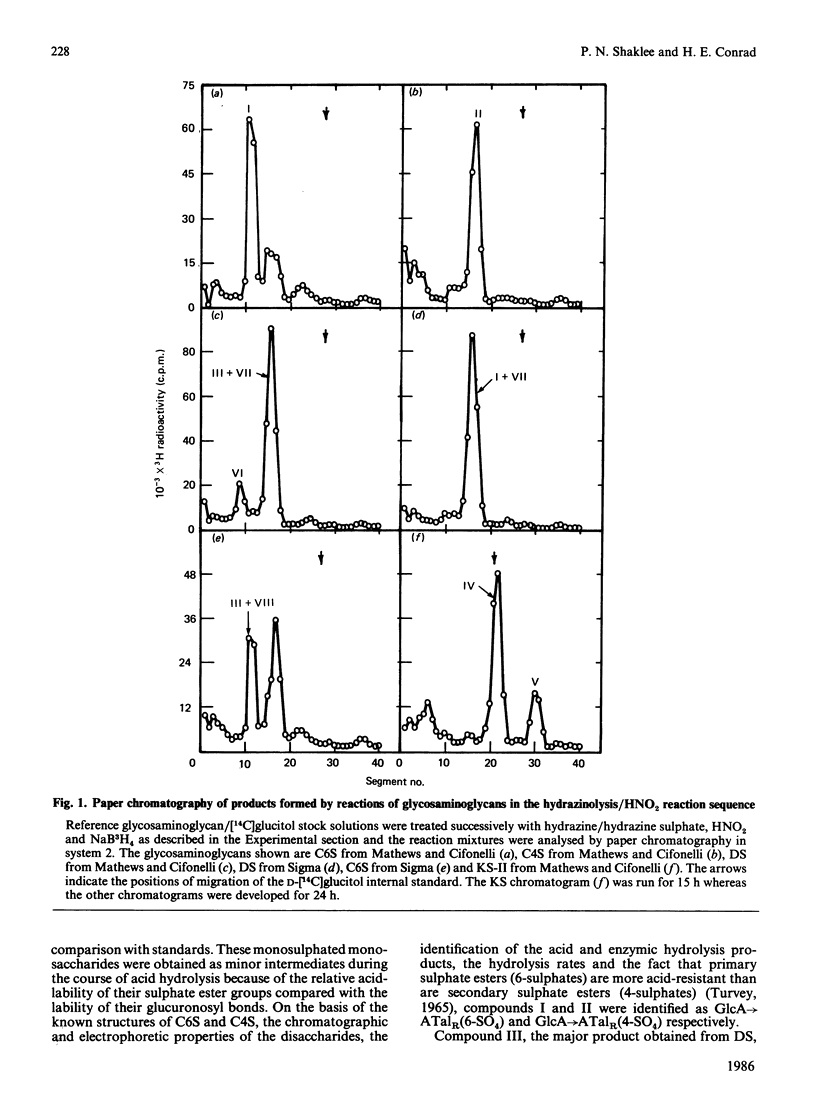
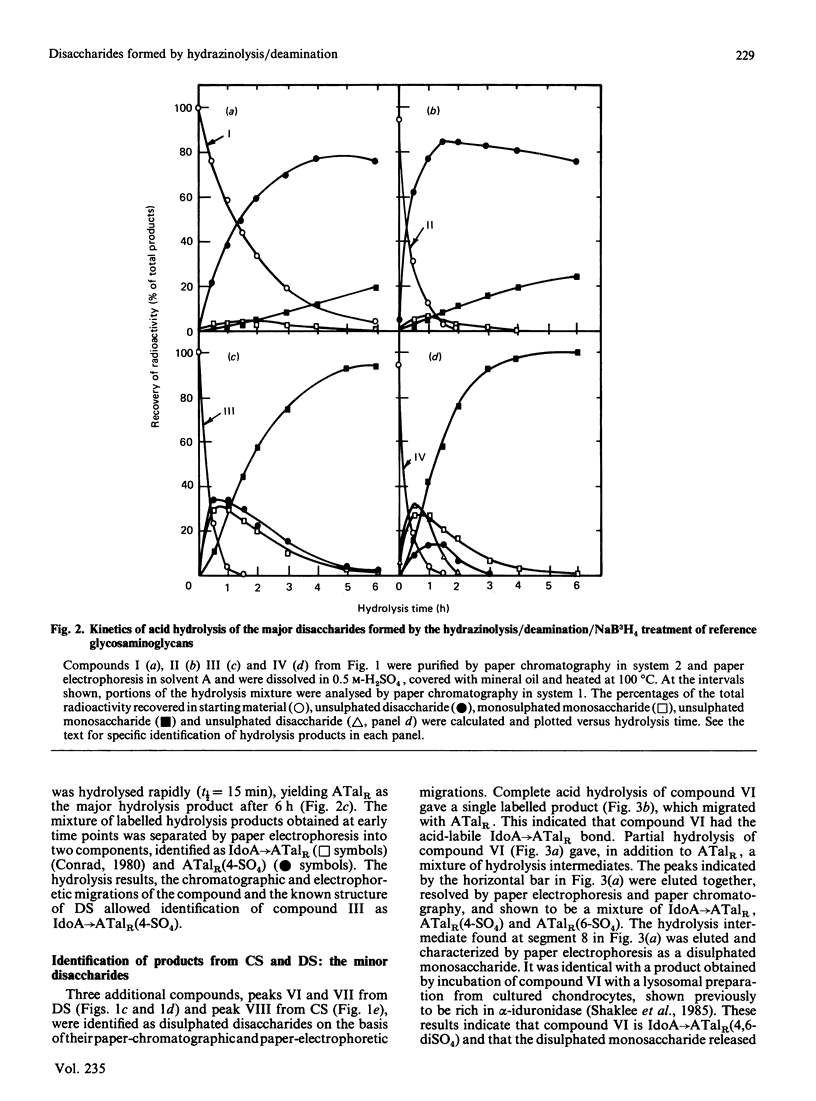
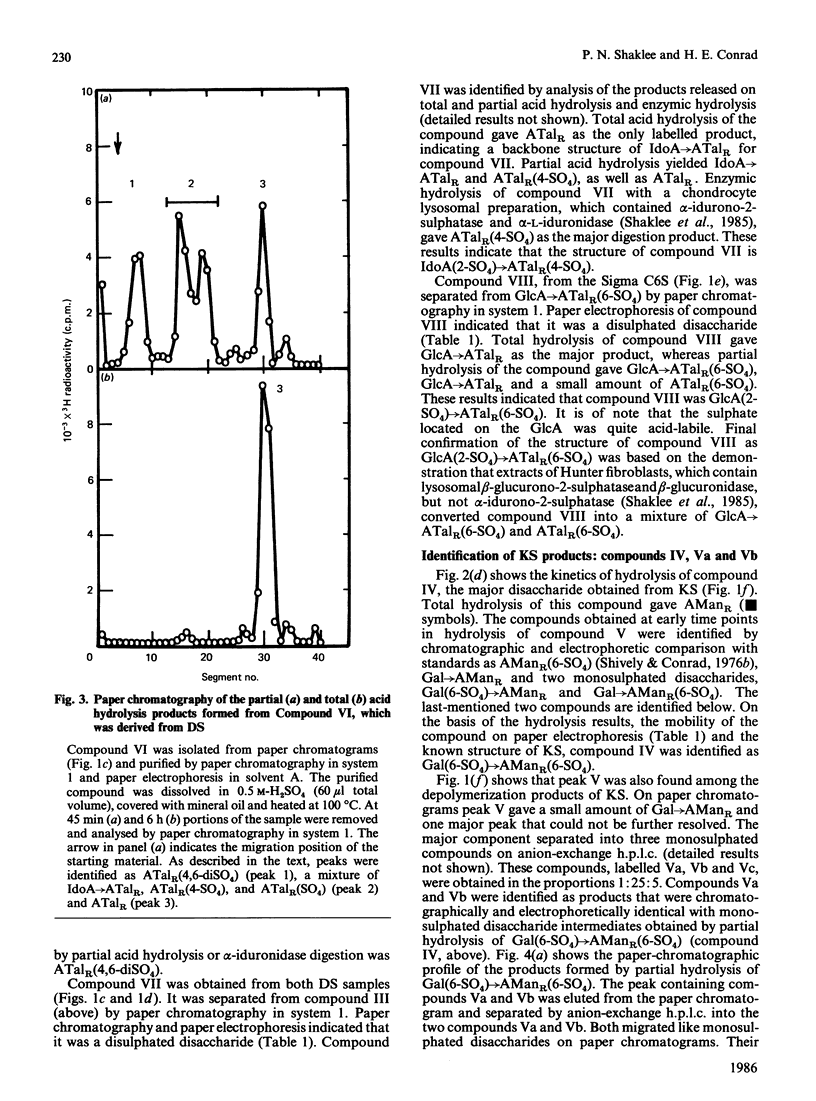
Chondroitin 4-sulphate, chondroitin 6-sulphate, dermatan sulphate and keratan sulphate were N-deacetylated by treatment with hydrazine and then cleaved with HNO2 at pH 4.0, and the resulting products were reduced with NaB3H4. This reaction sequence cleaved the glycosaminoglycans at their N-acetyl-D-glucosamine or N-acetyl-D-galactosamine residues, which were converted into 3H-labelled 2,5-anhydro-D-mannitol (AManR) or 2,5-anhydro-D-talitol (ATalR) residues respectively. The end-labelled disaccharides, composed of D-glucuronic acid (GlcA), L-iduronic acid (IdoA) or D-galactose (Gal) and one of the anhydrohexitols, were identified as follows: both chondroitin 4-sulphate and chondroitin 6-sulphate gave GlcA----ATalR(4-SO4), GlcA----ATalR(6-SO4), IdoA----ATalR (4-SO4) and GlcA(2-SO4)----ATalR(6-SO4); dermatan sulphate gave IdoA----ATalR(4-SO4), GlcA----ATalR(4-SO4), GlcA----ATalR(6-SO4)----IdoA(2-SO4)ATalR(4-SO4) and IdoA----ATalR (4,6-diSO4); keratan sulphate gave Gal(6-SO4)----AManR(6-SO4), Gal----AManR(6-SO4), Gal(6-SO4)----AManR and Gal----AManR. Several additional disaccharides were generated by treatment of the uronic acid-containing disaccharides with hydrazine to epimerize their uronic acid residues at C-5. A number of these disaccharides were found to be substrates for lysosomal sulphatases and glycuronidases. Methods were developed for the separation of all of the disaccharide products by h.p.l.c. The rate of N-deacetylation of chondroitin 4-sulphate by hydrazinolysis was significantly lower than the rate of N-deacetylation of chondroitin 6-sulphate or chondroitin. Dermatan sulphate was N-deacetylated at an intermediate rate. The relative amounts of disaccharides obtained from chondroitin 4-sulphate, chondroitin 6-sulphate and dermatan sulphate under optimum hydrazinolysis/deamination conditions were comparable with the amounts of the corresponding products released from the polymers by chondroitinase treatment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowski M. J., Conrad H. E. Structural characterization of the oligosaccharides formed by depolymerization of heparin with nitrous acid. J Biol Chem. 1985 Jan 10;260(1):356–365. [PubMed] [Google Scholar]
- Blake D. A., Conrad H. E. Hybrid glycosaminoglycans synthesized by monolayers of chick embryo arterial fibroblasts. Biochemistry. 1979 Nov 27;18(24):5475–5482. doi: 10.1021/bi00591a033. [DOI] [PubMed] [Google Scholar]
- Conrad H. E. The acid lability of the glycosidic bonds of L-iduronic acid residues in glycosaminoglycans. Biochem J. 1980 Nov 1;191(2):355–363. doi: 10.1042/bj1910355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney S. R., Conrad H. E., Glaser J. H. A high-performance liquid chromatography approach for isolation and sequencing of chondroitin sulfate oligosaccharides. Anal Biochem. 1980 Oct;108(1):25–34. doi: 10.1016/0003-2697(80)90689-2. [DOI] [PubMed] [Google Scholar]
- Delaney S. R., Leger M., Conrad H. E. Quantitation of the sulfated disaccharides of heparin by high performance liquid chromatography. Anal Biochem. 1980 Jul 15;106(1):253–261. doi: 10.1016/0003-2697(80)90145-1. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Matsumura G. Endo-beta-galactosidase of Escherichia freundii. Purification and endoglycosidic action on keratan sulfates, oligosaccharides, and blood group active glycoprotein. J Biol Chem. 1976 Oct 25;251(20):6218–6225. [PubMed] [Google Scholar]
- Glaser J. H., Conrad H. E. Chick embryo liver beta-glucuronidase. Comparison of activity on natural and artificial substrates. J Biol Chem. 1979 Jul 25;254(14):6588–6597. [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Selective depolymerisation of keratan sulfate: production of radiolabelled substrates for 6-O-sulfogalactose sulfatase and beta-D-galactosidase. Carbohydr Res. 1983 Jun 16;117:263–274. doi: 10.1016/0008-6215(83)88092-6. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Muller V. J. Selective depolymerisation of dermatan sulfate: production of radiolabelled substrates for alpha-L-iduronidase, sulfoiduronate sulfatase, and beta-D-glucuronidase. Carbohydr Res. 1983 Oct 28;122(2):227–239. doi: 10.1016/0008-6215(83)88334-7. [DOI] [PubMed] [Google Scholar]
- Jacobsson I., Hök M., Pettersson I., Lindahl U., Larm O., Wirén E., von Figura K. Identification of N-sulphated disaccharide units in heparin-like polysaccharides. Biochem J. 1979 Apr 1;179(1):77–87. doi: 10.1042/bj1790077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson W., Gundlach M. W., Schmid T. M., Conrad H. E. Selective hydrolysis of chondroitin sulfates by hyaluronidase. Biochemistry. 1984 Jan 17;23(2):368–375. doi: 10.1021/bi00297a028. [DOI] [PubMed] [Google Scholar]
- Nakagawa H., Yamada T., Chien J. L., Gardas A., Kitamikado M., Li S. C., Li Y. T. Isolation and characterization of an endo-beta-galactosidase from a new strain of Escherichia freundii. J Biol Chem. 1980 Jun 25;255(12):5955–5959. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Shaklee P. N., Conrad H. E. Hydrazinolysis of heparin and other glycosaminoglycans. Biochem J. 1984 Jan 1;217(1):187–197. doi: 10.1042/bj2170187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaklee P. N., Glaser J. H., Conrad H. E. A sulfatase specific for glucuronic acid 2-sulfate residues in glycosaminoglycans. J Biol Chem. 1985 Aug 5;260(16):9146–9149. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Saito H., Yamagata T., Anno K., Seno N., Kawai Y., Furuhashi T. Formation of three types of disulfated disaccharides from chondroitin sulfates by chondroitinase digestion. J Biol Chem. 1968 Apr 10;243(7):1543–1550. [PubMed] [Google Scholar]
- Turvey J. R. Sulfates of the simple sugars. Adv Carbohydr Chem Biochem. 1965;20:183–218. doi: 10.1016/s0096-5332(08)60299-4. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


