Abstract
The use of insects as a food source is not a new idea, but it has gained momentum in recent years due to the need for sustainable protein source in livestock feedstuffs and for more environmentally friendly organic waste treatment. In the case of black soldier fly larvae, Hermetia illucens, research has focused on their ability to convert organic waste into usable nutrients and their potential as a protein source for animal and human consumption. In this study, black soldier fly larvae were reared on raw food waste (FW) mixed with garlic peel waste (G) and hydronic growth media waste (H) and the proximate composition and bioactive potential of black soldier fly larvae extract (SFL) were compared. Analysis showed that protein content of SFL fed with G was 4.21% higher and lipid content was 9.93% lower than FW. Similar results were obtained for SFL fed with H. Antioxidant activity of SFL-G was higher than that of SFL-FW and SFL-H. SFL-G treatment exhibited enhanced anti-inflammatory and anti-adipogenesis activities as well compared to SFL-FW. Current results suggested that feeding black soldier fly larvae with food waste added with garlic peel and hydroponic growth media waste resulted in increased nutritional value, polyphenol content and bioactivity for SFLs. In this context, garlic peel waste-added food waste was suggested a promising substrate for black soldier fly larvae to obtain high-quality protein source with enhanced antioxidant, anti-inflammatory and anti-adipogenic potential.
Keywords: Adipogenesis, Antioxidant, Anti-inflammatory, Black soldier fly, Hermetia illucens, Garlic peel
INTRODUCTION
The food demand is steadily increasing parallel to increasing population and improved living standards along with quantity of food waste which causes a worldwide problem [1]. The demand for food is estimated to increase by more than 60% in 30 years, globally [2,3]. Coupled with changes in diets towards more animal-based products such as fish, milk and egg, future holds a great deal of need for livestock, poultry, and fishing [4]. Therefore, protein shortage is a global concern as sustainable animal husbandry depends on procurement of protein raw materials to be used in animal feed [5]. Up to date, the main ingredient for animal feed which is rich in protein is soybean meal. However, soybean cultivation comes with several problems such as diminishing land availability, deforestation, and threats to biodiversity [6,7]. Moreover, both soybean cultivation and other conventional sources of protein are being economically unfavorable as the cost of animal feeds allocate approximately 70% of total husbandry costs [8]. This led researchers to focus their efforts to alternative protein sources which are comparably less damaging to environment and provides quality protein.
In this context, using insects has gained momentum in recent years due to the increasing need for sustainable protein sources [9]. Although the use of insects as a food source is not a new idea and insects have been part of a diet in some cultures worldwide for centuries, approval of the use of insect protein in animal feed by governments and relevant agencies has opened the door for more widespread utilization of insects in both research and industrial fields [10,11]. Studies showed that insects are indeed promising protein sources with high-quality protein content among other essential nutrients such as vitamins and minerals [12–14]. Recent studies showed that although chicken feed prepared with insect meals resulted in altered products, it showed potential to replace chicken feed prepared with soybean meal [15]. Similarly, Toral et al. showed that insect-based protein rich ruminant feeds were comparable to traditional soybean meal-based feed and among four tested insects Tenebrio molitor feed exhibited very favorable ruminal intestinal digestibility and degradation [16]. However, the accumulation of toxins, heavy metals and other harmful substances by the insects are the main factors that limit wide use of insect protein as animal feed and led to several regulations and tests to eliminate risk of insect-triggered toxicity [17].
The use of insects in recent years is not limited to as a protein source. Insects are part of bioconversion processes of organic food waste. Sustainable management of food industry waste is one of the most alarming challenges of the current decade [18]. Instead of aiming at the elimination of food waste, biological waste treatment enables beneficial food waste management. Biological processes such as composting, and biogas production are very popular solutions to increasing food waste problems as they are both economically and environmentally beneficial [19,20]. Using living organisms such as insects is another attractive food waste management technique. Insects reared on food waste simultaneously treat the food waste to produce digestates and provide biomass growth rich in protein and fat [21,22]. The former is widely utilized in agriculture as fertilizer, biogas production and composting. On the other hand, insect biomass can be utilized as protein source for animal or human consumption as previously mentioned.
The larvae of black soldier fly, Hermetia illucens, have been the subject of research in recent years due to its potential role in food waste treatment and as protein source [23]. The use of black soldier fly larvae in organic waste treatment and as a protein source is gaining interest due to several factors. Firstly, black soldier fly larvae extracts (SFL) offer sustainable alternative to traditional protein sources, such as soybean meal, which is often produced using environmentally harmful methods [6,7,15]. Also, using organic waste as feed for black soldier fly larvae can help reduce the amount of waste going to landfills, which can reduce greenhouse gas emissions and other environmental impacts [5]. In addition, the use of black soldier fly larvae in organic waste treatment can provide a valuable source of nutrients for agriculture. The larvae produce a nutrient-rich compost that can improve soil health and reduce the need for chemical fertilizers [24]. Also, this compost can be used in biogas production with higher yields compared to raw plant food waste [25]. It was reported that black soldier fly larvae were efficient at converting food waste into organic digestate; significantly decreased volume and weight against enhanced nutritional value [26,27]. Other studies also suggested that SFL can be used as protein source for agricultural feedstuffs and given its nutritional value it might replace traditional protein sources such as soybean meal [28]. The studies also hinted that with proper legislation, SFL sourced nutrients are also suitable for human consumption [28–30]. However, use of black soldier fly larvae for treatment of organic waste is a safer and widely adapted method to utilize these insects compared to use of black soldier fly protein as animal feed due to possibility of toxicity. Studies showed that black soldier fly larvae body composition heavily affected by the breeding environment especially by the presence of heavy metals [31]. Microbial toxins and pesticides might not alter the body composition in a negative way [32] but it has been shown that cadmium and lead could be accumulated in black soldier fly body at high concentrations [33] which raises a safety concern for animal feeds containing insect protein. Thus, in its current state, monitoring, and regular testing of insect sources for heavy metals are necessary. Although this might hinder its wide use as animal feed in several countries, future trends are expected to provide safer solutions to utilize this vast protein source.
Garlic peel is an agricultural waste often discarded or incinerated despite being a good source of bioactive substances and nutrients [34]. In this context, hydroponic growth systems, which were developed to provide environmentally friendly sustainable agriculture, produce significant amount waste, especially in terms of used growth medium. Reuse of this waste was suggested by several studies reporting its nutrient-rich composition [35]. In the current study, the proximate composition of SFL obtained from larvae reared on different organic waste substrates were compared. Black soldier fly larvae were reared on raw food waste containing garlic peel or hydroponic growth media waste. In addition, the effect of different organic waste on the bioactive properties of SFL were investigated in terms of antioxidant, anti-inflammatory and anti-adipogenic activities.
MATERIALS AND METHODS
Preparation of different organic wastes
Food waste to be fed to black soldier fly larvae was obtained from Geoje Food Waste Intermediate Treatment Industry. Food waste was ground and heated up to 110°C for 30 min prior to feeding. Garlic peel waste was purchased from Namhae Garlic Processing Plant. Hydroponic growth media waste was used coconut fiber growth media and was kindly given by Sacheon-gun tomato farms. Three different organic waste substrate was prepared as follows: Group 1 contained 100% raw food waste (FW), Group 2 contained 80% (w/w) raw food waste and 20% garlic peel waste (G) and Group 3 contained 80% raw food waste and 20% hydroponic growth media waste (H).
Soldier fly larvae feeding and harvest
Black soldier fly larvae (5 days old-post hatching; 300 g) were obtained from Daum agricultural and bred for 10 days in 25°C containers (200 l capacity with width, height, and depth of 550 cm × 1,100 cm × 330 cm) with 60% humidity and fed only once at the beginning of rearing (42 kg total feed). At the end of the day 10, larvae from different feeding groups were collected, separated from waste, and washed. Next, larvae were dried for 24 h at 65°C and subsequently ground to obtain SFL. This extract was kept at −20°C until use. For the assays SFL was dissolved in 10% dimethyl sulfoxide (DMSO) unless otherwise noted.
Proximate composition
SFL from different feeding groups was subjected to proximate composition analysis using the nitrogen to protein conversion factors following the methods reported by Janssen et al. [36]. The total moisture, protein and fat content was measured. Also, total polyphenol content of SFLs were measured by Folin–Denis’ reagent (47742, Merck, Darmstadt, Germany). Briefly, 100 μL SFL samples with different concentrations were added to 500 μL 1N Folin–Denis’ reagent (Merck), and kept at room temperature for 3 min, and then 7.5% Na2CO3 (400 μL) was slowly added to the solution. Samples were kept at room temperature for another 90 min. Subsequently tubes were centrifuged at 4°C. Supernatants were used to detect optical density at 760 nm using a microplate reader (Multiskan GO, Tecan, Grodig, Austria). Total polyphenol content was measured using a standard curve which was established using gallic acid as a standard, and the results were presented in milligram gallic acid equivalent per 100 g of extract (mg/100 g).
Antioxidant activity of black soldier fly larvae extracts
Antioxidant properties of SFLs from different feeding groups were examined by cell-free scavenging assays using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and nitric oxide (NO) as substrates.
The DPPH (Sigma-Aldrich, St. Louis, MO, USA) stock solution (150 μM) was prepared by dissolving DPPH in 100% EtOH. In a 96-well plate well, 100 μL SFL sample was mixed with 100 μL DPPH solution and the plate was kept for 30 min in the dark at a room temperature. Finally, DPPH scavenging was measured by optical density of the wells at 520 nm, calculated with a microplate reader (Multiskan GO, Tecan). The control group contained the same volume of ethanol and DPPH solution without any sample. Group with only ethanol was used as blank. Relative percentage-based scavenging of the DPPH free radical was quantified compared to the control.
NO free radical scavenging activity was measured as previously reported with slight modifications. Briefly, 500 μL of 10 mM sodium nitroprusside solution was added to 500 μL of the SFL sample dissolved in 20 mM phosphate buffer (pH 7.4). Reaction was progressed at 25°C for 150 min, and the tubes were centrifuged (12,000×g, 10 min). Production of NO was measured by using the supernatants and performing Griess reaction. One milliliter of Griess solution was added to the supernatant and the mixture was kept at 25°C for 10 min. The absorbance value of mixture was measured at 542 nm using a multiplate reader (Multiskan GO, Tecan). Ascorbic acid was used as a positive control group. NO scavenging effect was calculated as a relative percentage compared to untreated control.
Cytotoxicity of black soldier fly larvae extracts
Prior to conduct in vitro assays using cell lines, any cytotoxic presence of SFL was measured by MTT assay. Briefly, RAW264.7 mouse macrophages and 3T3-L1 mouse pre-adipocytes were transferred into 96-well plates with a density of 3 × 103 cells per well and kept for 24 h in incubators. Both cell lines were fed with Dulbecco’s Modified Eagle Medium (DMEM; Gibco BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum (FBS; Gibco BRL), 100 U/mL penicillin (Gibco-BRL), and 100 mg/ml streptomycin (Gibco-BRL). After 24 h, cells were added with varying concentrations of SFLs for the next 48 h. Next, culture medium was swapped with 100 μl of MTT reagent (0.05%, m/v) and the plates were kept in incubators for 4 h, after which the reaction was stopped by adding 100% DMSO to each well. Absorbance values were then measured at 540 nm (Multiskan GO, Tecan). Cell viability was quantified as the absorbance value of each well and given as relative percentage of the untreated control.
Anti-inflammatory activity of black soldier fly larvae extracts
Anti-inflammatory effect of SFLs was screened in RAW264.7 mouse macrophages. Inflammatory response in RAW264.7 cells were induced by lipopolysaccharide (LPS) stimulation and NO levels were measured as an indicator of inflammation. The RAW264.7 cells were seeded into wells (1.0×104 cell/well) of a 96 well plate and fed with DMEM medium containing 10% FBS for 24 h at 37°C incubators with a 5% CO2 atmosphere. After 24 h, medium was replaced with fresh one containing LPS (final conc. 1.0 μg/mL) and plates were incubated for another 1 h. Subsequently, the SFL samples were added to the wells and treatment lasted for 48 h. After 48 h, culture medium was harvested from wells and centrifuged. The supernatants were collected and mixed with Griess reagent (Sigma-Aldrich) at 1:1 ratio. The mixture was left at room temperature for 15 min. and the absorbance value of mixture was measured at 540 nm using a multiplate reader. Anti-inflammatory effect was measured via the NO production levels which were given as a relative percentage of untreated control.
Anti-adipogenesis activity of black soldier fly larvae extract s
Anti-adipogenic activity of SFL samples was evaluated in 3T3-L1 mouse pre-adipocyte cell line. Cells were induced to differentiate into mature adipocytes and antiadipogenic properties of SFLs were recorded via their ability to decrease intracellular lipid accumulation. Briefly, 3T3-L1 cells were seeded in 6-well plates (1 × 104 cells/well) and fed with DMEM containing 10% FBS. After cells reached confluency, medium was changed with differentiation medium (DMEM containing insulin [5 μg/mL], methylisobutylxanthine [0.5 mM], and dexamethasone [0.25 μM]) along with or without SFL samples (day 0). After two days of incubation, differentiation medium was swapped with feeding medium (DMEM containing insulin [5 μg/mL]). Feeding medium was changed with fresh one every 2 days until intracellular lipid droplets were visible (day 8). The intracellular lipid droplets were then stained with Oil Red O. Briefly, differentiated cells at day 8 in 6-well plates were washed with phosphate-buffered saline and fixed on wells by adding 1 mL formaldehyde (3.7%, v/v in distilled water) and incubating for 1 h. Fixed cells were then washed again and incubated with filtered Oil red O staining solution (0.5% Oil Red O stain, w/v in a mixture of 60% isopropanol and 40% distilled water) for 1 h at room temperature. Subsequently stain was removed from wells, and red lipid droplets were observed under a light microscope (Nikon, Tokyo, Japan). The level of accumulated lipid droplets was measured by quantification of the retained stain in the wells. Quantification was carried out by eluting the stain from the cells with addition of 100% isopropanol. The amount of stain was then calculated by the measuring absorbance at 500 nm using a microplate reader (Multiskan GO, Tecan). Lipid accumulation was given as a relative percentage of lipid levels in untreated fully differentiated control group.
Statistical analysis
The data were presented as mean ± SD (n = 3) where applicable. Significant differences between the means of the different treatment groups were expressed at the p<0.05 level calculated by one-way analysis of variance (ANOVA) coupled with Duncan’s multiple range post-hoc test (SAS v9.1, SAS Institute, Cary, NC, USA).
RESULTS AND DISCUSSION
In this study, black soldier fly larvae were reared on different organic waste substrates for comparison. Three different feeding group was established by mixing raw food waste with garlic peel waste from garlic processing industry and coconut fiber growth media waste from hydroponic farming. By doing this, it was aimed to treat wide range of organic waste while improving the black soldier fly larvae biomass in terms of nutritional value and bioactive potential.
Proximate composition of black soldier fly larvae extracts
The SFL samples obtained from black soldier fly larvae reared on three different feeding groups were first compared by their proximate composition. Analysis showed that SFL from raw food waste feeding group (SFL-FW) recorded 4.73 ± 0.32% moisture, 35.29 ± 0.21% crude protein and 38.77 ± 2.86% fat content (Table 1). The SFL from raw food waste-garlic peel waste feeding group (SFL-G) recorded a higher crude protein content with 39.08 ± 0.36% along with lower values of fat and moisture, 28.80 ± 2.94% and 3.13±0.12% respectively. A similar trend was observed from the analysis of SFL from raw food waste-hydroponic growth media waste feeding group (SFL-H) with 3.70 ± 0.08% moisture, 36.17±0.21% crude protein and 28.11 ± 2.82% fat content.
Table 1. Proximate composition of black soldier fly larvae extracts.
| Sample | Moisture (%) | Crude protein (%) | Fat (%) |
|---|---|---|---|
| SFL-FW | 4.73 ± 0.32a1) | 35.29 ± 0.21c | 38.77 ± 2.86a |
| SFL-G | 3.13 ± 0.12c | 39.08 ± 0.36a | 28.80 ± 2.94b |
| SFL-H | 3.70 ± 0.08b | 36.17 ± 0.21b | 28.11 ± 2.82b |
Date are means ± SD.
Data with different superscript letters are significanly different, whereas same superscript letters mean no significant difference as revealed by Duncan’s multiple range post-hoc test (p < 0.05) compared within same test group (moisture, crude protein and fat).
Results showed that by altering the waste composition, protein yield of SFL biomass was increased while fat content was lowered. Studies showed that most of SFL that were aimed to be used in feed industry in Korea are subjected to defatting process in order to drop the fat levels to the required amounts due to high fat content of SFLs [37,38]. Current results indicated that by adding garlic peel waste to the feeding substrate fat content of SFL was decreased by 9.97% without any further procedure. It also yielded 3.79% more protein. The black soldier fly larvae contain high amounts of fat which hinders the direct production of animal feed [17]. Therefore, the larvae are subjected to defatting process to remove the excess fat prior to use as a feedstock. Thus, higher protein yield with decreased fat composition of SFL-G suggests enriching larvae feed with garlic waste is a promising approach to obtain more favorable biomass.
Comparison of bioactivities of soldier fly larvae extracts
Yielding high-quality protein as an alternative to traditional sources is not the only benefit of SFL utilization. Studies showed that SFL exhibited various bioactivities which may prove beneficial to animal for which SFL used as feedstuff. Reports already documented that SFL has antimicrobial properties against several bacterial strains, including Escherichia coli, Staphylococcus aureus, and Salmonella enterica [39,40]. Also, SFL has been found to possess antioxidant and anti-inflammatory properties which were attributed to the presence bioactive compounds such as phenols and flavonoids [41,42]. In this context, total polyphenol content analysis was conducted for both feeds and SFLs. Analysis of feeds revealed that garlic peel added waste (G) had higher polyphenol content than that of food waste and hydroponic growth waste added waste (H) (Table 2). Analysis of SFLs revealed that all extracts had significantly higher levels of polyphenols (p < 0.05) compared to feed materials (Table 3). SFL-FW contained 1,513.23 ± 28.18 mg polyphenol per 100 g of dry matter. This content was significantly increased to 1,992.40 ± 20.81 for SFL-G. However, SFL-H recorded a lower polyphenol content with 1422.60±7.86 mg polyphenol per 100 g of dry matter. Compared with the polyphenol contents of FW, G and H, which were ,1022.16±29.75, 1,166.27 ± 50.63 and 823.14 ±1.70 respectively, SFL-H recorded the highest increase by 72.82% followed by 70.83% for SFL-G and 48.04% for SFL-FW. Results indicated that enriched food wastes resulted in elevated levels of polyphenol transformation by black soldier flies. Increasing evidence suggests that polyphenol rich ingredients translate into beneficial effects in overall health, mainly due to their antioxidant potential [43]. In this context, garlic peel waste enriched food waste resulting in increased polyphenol content in SFLs was speculated to be a way to produce more bioactive ingredients.
Table 2. Total polyphenol contents of feed.
| Sample | Total polyphenol contents (mg GAE/100 g dry matter) |
|---|---|
| FW | 1,022.16 ± 29.75b |
| G | 1,166.27 ± 50.63a |
| H | 823.14 ± 1.70c |
Values with different letters are significantly different (p < 0.05).
GAE, gallic acid equivalent; FW, raw food waste; G, garlic peel waste added food waste; H, hydroponic growth media waste added food waste.
Table 3. Total polyphenol contents of black soldier fly larvae extract (SFL) obtained from larvae fed with feeds.
| Sample | Total polyphenol contents (mg GAE/100 g dry matter) |
|---|---|
| SFL-FW | 1,513.23±28.18b |
| SFL-G | 1,992.40±20.81a |
| SFL-H | 1,422.60±7.86c |
Values with different letters are significantly different (p < 0.05).
GAE, gallic acid equivalent; FW, raw food waste; G, garlic peel waste added food waste; H, hydroponic growth media waste added food waste.
Antioxidant activity
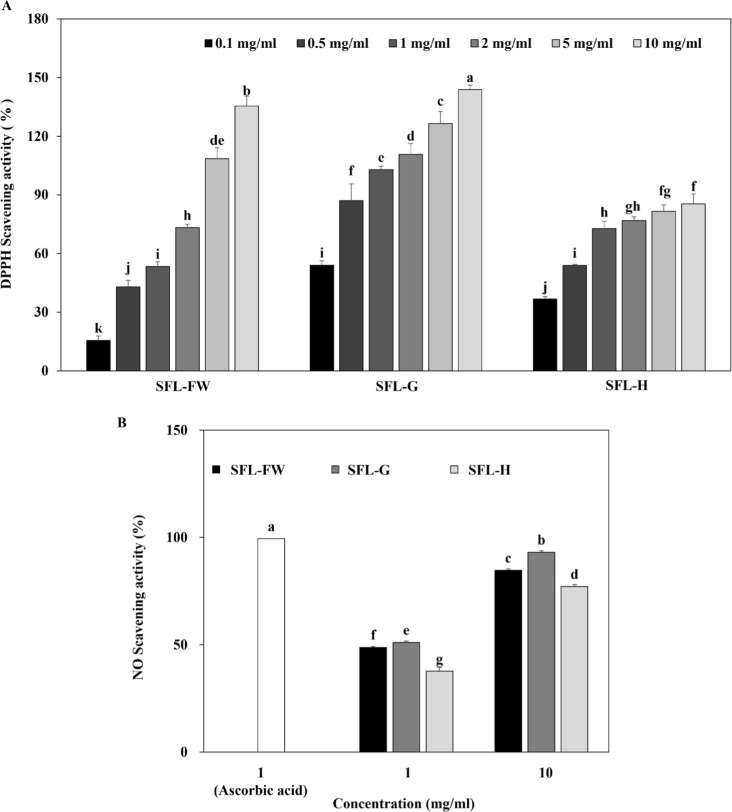
First, antioxidant potential of three different SFLs were evaluated by their DPPH and NO scavenging activities. Results showed that all three SFLs exerted significant DPPH scavenging activity in a dose-dependent manner (Fig. 1A). Both, SFL-FW and SFL-G showed dose-dependent increase in scavenging activity until the highest concentration of 10 mg/mL while SFL-H scavenging activity was not increased by increasing doses after 2 mg/mL proportional to other samples. At 1 mg/ml concentration, SFL-FW exhibited 53.34% DPPH scavenging while SFL-G ang SFL-H scavenging activity was recorded as 102.94% and 72.79% respectively. The IC50 values for this antioxidant activity were calculated to be 1.11 mg/mL, 0.09 mg/ml and 0.42 mg/mL for SFL-FW, SFL-G and SFL-H. Similar results were observed in NO scavenging activity of SFLs. At 10 mg/mL concentration, SFL-FW NO scavenging activity was 84.68% against 93.12% of SFL-G and 77.10% SFL-H (Fig. 1B). Positive control ascorbic acid exerted 99.44% scavenging effect at 1 mg/mL concentration. Parallel to polyphenol content results, addition of garlic peel waste notably increased antioxidant potential of SFL. Although it scavenged NO higher than FW group, H was showed not to affect antioxidant potential of SFL significantly which was also apparent as decreased polyphenol content. Nevertheless, garlic peel waste was suggested to beneficial addition to black soldier fly larvae feeding substrate to enhance its antioxidant potential. Studies mainly reported the antioxidant properties of hydrolysates from SFL, attributing the effect to bioactive peptides [44,45]. However according to current results showing that antioxidant of SFL was parallel to polyphenol content, there might be increase in phenolic compounds such as flavonoids responsible for the enhanced antioxidant potential and bioconversion of garlic peel waste was suggested to be the reason behind this enhancement.
Fig. 1. Antioxidant activity of black soldier fly larvae extract (SFL) obtained from larvae fed with food waste (FW), garlic peel added food waste (G), or hydroponic growth media added food waste (H) evaluated by their ability to scavenge (A) DPPH and (B) NO radicals in cell-free environments.
Ascorbic acid was used as a positive control. a–kGroups with different superscript letters are significanly different, whereas same superscript letters mean no significant difference as revealed by Duncan’s multiple range post-hoc test (p < 0.05).
Anti-inflammatory activity
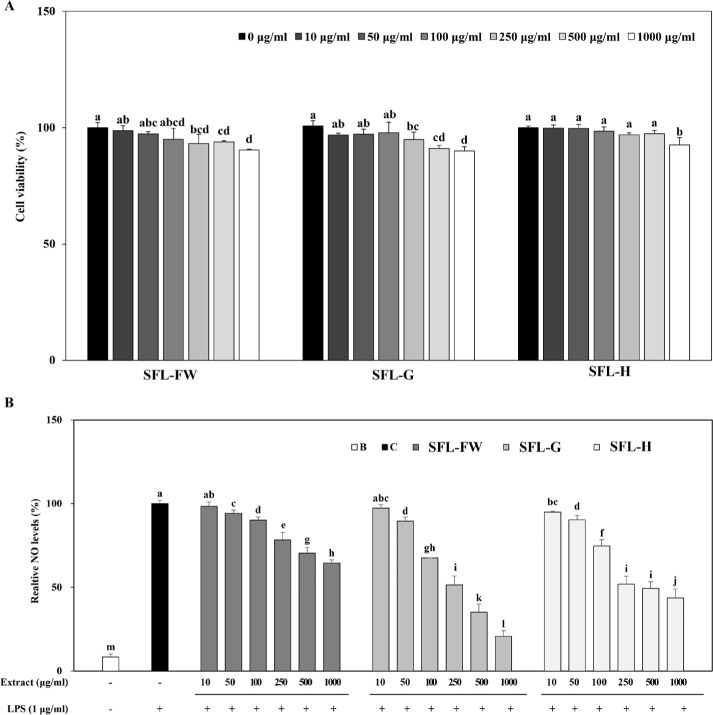
Next, anti-inflammatory effect of SFL was tested on LPS-induced RAW264.7 mouse macrophage cells. Prior to evaluate the effect of samples on NO production in inflammatory response-induced cells, cytotoxicity of SFLs were investigated. Results showed that up to 1,000 μg/mL, SFL treatment did not cause cell viability to drop below 90% (Fig. 2A). Therefore, the assay was carried out using this concentration as the upper limit.
Fig. 2. Effect of black soldier fly larvae extract (SFL) obtained from larvae fed with food waste (FW), garlic peel added food waste (G), or hydroponic growth media added food waste (H) on cell viability (A) and NO production (B) in RAW264.7 mouse macrophage cell line.
(A) Cells were treated with samples in given concentrations for 48 h and the viable cell levels were measured by MTT assay. Cell viability is given as a relative percentage of untreated (0 μg/mL) control group. (B) Inflammatory response in cells were induced by addition of lipopolysaccharides (LPS) 1 h prior to sample treatment. NO levels in cell culture medium were measured by Griess reaction. NO production levels were given as relative percentage of C. a-mGroups with different superscript letters are significanly different, whereas same superscript letters mean no significant difference as revealed by Duncan’s multiple range post-hoc test (p < 0.05). B, unstimulated untreated blank; C, LPS-stimulated untreated control.
LPS-induced inflammatory response in macrophages is known to elevate production of pro-inflammatory cytokines and release of NO as a result [46]. This was observed in the current results, where NO levels were increased 91.64% following LPS-stimulation (Fig. 2B). Treatment with three different SFLs were able to decrease LPS-induced NO levels in a dose-dependent manner. At the highest concentration treated (1,000 μg/mL), NO levels were 64.44%, 20.72% and 43.53% of untreated control group for SFL-FW, SFL-G and SFL-H respectively. In accordance with the antioxidant activity, SFL-G was observed to exert enhanced anti-inflammatory activity compared to SFL-FW. Results suggested that primarily garlic peel waste addition yielded beneficial effects on antioxidant and anti-inflammatory activities of SFL, further promoting its utilization as protein source for animal feed. Although current results were not enough to claim its anti-inflammatory effect in livestock, it was postulated that black soldier fly larvae reared on garlic peel waste added organic substrate would provide bioactive biomass which might reduce inflammatory responses in the livestock which it was fed.
Anti-adipogenesis activity
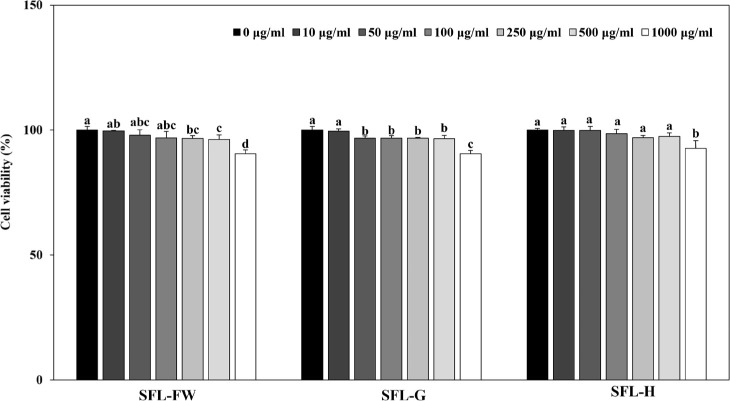
Finally, anti-adipogenic potential of SFLs were tested in 3T3-L1 mouse pre-adipocytes. Cells were induced to differentiate into mature adipocytes and accumulate lipid droplets and the effect of SFLs on decreasing lipid accumulation was investigated. Cytotoxicity analysis showed that up to 500 μg/mL, SFL treatment did not cause cell viability to drop below 90% (Fig. 3). Therefore, the assay was carried out using this concentration as the upper limit.
Fig. 3. Effect of black soldier fly larvae extract (SFL) obtained from larvae fed with food waste (FW), garlic peel added food waste (G), or hydroponic growth media added food waste (H) on viability of 3T3-L1 mouse pre-adipocytes.
Cells were treated with samples in given concentrations for 48 h and the viable cell levels were measured by MTT assay. Cell viability is given as a relative percentage of untreated (0 μg/ml) control group. a–dGroups with different superscript letters are significanly different, whereas same superscript letters mean no significant difference as revealed by Duncan’s multiple range post-hoc test (p < 0.05).
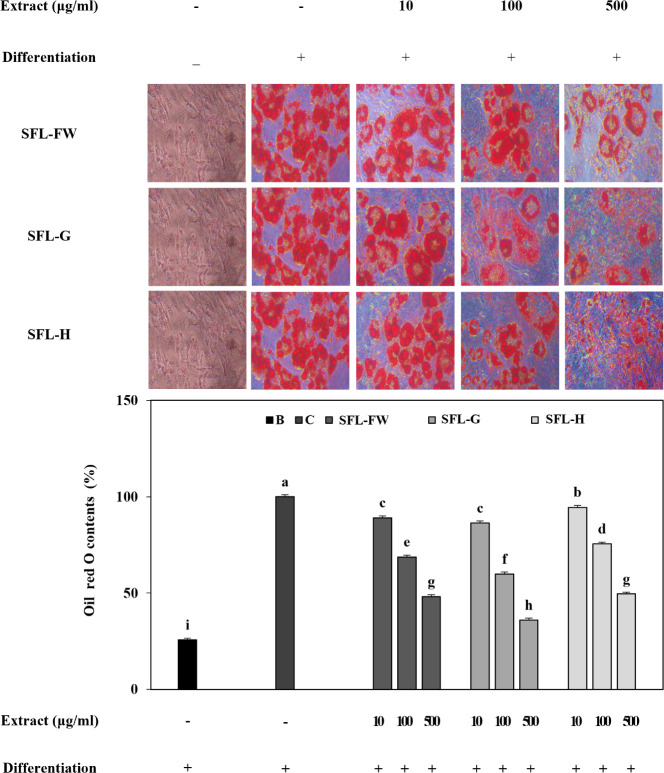
Both stained images of lipid droplets and measurement of lipid staining showed that presence of SFL significantly decreased the accumulated lipid droplets in adipocytes dose-dependently (Fig. 4). Among all tested SFLs, SFL-G was again the most active sample to decrease lipid accumulation in adipocytes. Compared to untreated control group, SFL-G group exhibited 64.02% less lipid whereas this decrease was 51.86% for SFL-FW and 50.45% for SFL-H at the concentration of 500 μg/mL. Results suggested that SFL with food waste feeding exhibited a lipid decreasing effect on adipocytes which was further enhanced by addition of garlic peel waste parallel to prior results.
Fig. 4. Effect of black soldier fly larvae extract (SFL) obtained from larvae fed with food waste (FW), garlic peel added food waste (G), or hydroponic growth media added food waste (H) on lipid accumulation in 3T3-L1 adipocytes.
Cells were induced to differentiate into adipocytes in the presence or absence of samples. At day 8 of differentiation, intracellular lipid droplets were stained by Oil Red O and the retained stain were measured. Staining levels were given as relative percentage of C. a-iGroups with different superscript letters are significanly different, whereas same superscript letters mean no significant difference as revealed by Duncan’s multiple range post-hoc test (p < 0.05). B, non-differentiated untreated blank; C, differentiated untreated control.
Studies already showed that when utilized as fish feed ingredient, SFL exerted beneficial effects on tissue fat composition of different fish such as juvenile mirror carp and juvenile Jian carp [47,48]. Current results suggested that SFL exerts inhibitory effects on lipid accumulation during adipogenic differentiation. In addition, alteration of black soldier fly larvae feed by adding different organic waste such as garlic peel significantly enhanced its effects on lipid profile. Overall, garlic peel waste addition to raw food waste was suggested to be a promising approach to yield notably more bioactive SFL which also contains higher amount of protein and polyphenols, and less fat.
CONCLUSION
Current results showed that rearing black soldier fly larvae on raw food waste added with garlic peel waste significantly increase its protein content while decreasing its fat content. Also, biomass obtained from larvae fed with garlic peel waste-added FW exhibited enhanced antioxidant, anti-inflammatory and anti-adipogenic potential in vitro compared to larvae wed with raw food waste only. Although, addition of hydroponic growth media waste did not alter the proximate composition and bioactivity as notable as garlic peel extract, current results provided valuable insights towards food waste composition that would result in value-added SFL. Despite the safety concerns for using insect proteins as animal feed due to the possibility of heavy metal accumulation, current results might provide insights towards futures studies. In conclusion, different compositions of food waste substrate for black soldier fly larvae as a means of converting organic waste into biomass is a promising solution for reducing wide range of organic waste and producing a sustainable source of high-quality protein.
Acknowledgements
Not applicable.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ015818042022)” Rural Development Administration, Korea.
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Oh JH, Park K, Kong CS.
Data curation: Yang J, Lee H, Choi MN, Jeon S, Park G, Kim J.
Formal analysis: Oh JH, Park K, Kong CS.
Methodology: Karadeniz F, Park K, Kong CS.
Software: Oh JH, Karadeniz F.
Validation: Park K, Kong CS.
Investigation: Oh JH, Yang J, Lee H, Choi MN, Jeon S, Park G, Kim J.
Writing - original draft: Oh JH, Karadeniz F.
Writing - review & editing: Oh JH, Karadeniz F, Yang J, Lee H, Choi MN, Jeon S, Park G, Kim J, Park K, Kong CS.
Ethics approval and consent to participate
This article does not require IRB/IACUC approval because there are no human and animal participants.
REFERENCES
- 1.Al-Obadi M, Ayad H, Pokharel S, Ayari MA. Perspectives on food waste management: prevention and social innovations. Sustain Prod Consum. 2022;31:190–208. doi: 10.1016/j.spc.2022.02.012. [DOI] [Google Scholar]
- 2.van Dijk M, Morley T, Rau ML, Saghai Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat Food. 2021;2:494–501. doi: 10.1038/s43016-021-00322-9. [DOI] [PubMed] [Google Scholar]
- 3.da Silva RFB, Viña A, Moran EF, Dou Y, Batistella M, Liu J. Socioeconomic and environmental effects of soybean production in metacoupled systems. Sci Rep. 2021;11:18662. doi: 10.1038/s41598-021-98256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komarek AM, Dunston S, Enahoro D, Godfray HCJ, Herrero M, Mason-D’Croz D, et al. Income, consumer preferences, and the future of livestock-derived food demand. Glob Environ Change. 2021;70:102343. doi: 10.1016/j.gloenvcha.2021.102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasco L, Acuti G, Bani P, Dalle Zotte A, Danieli PP, De Angelis A, et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital J Anim Sci. 2020;19:360–72. doi: 10.1080/1828051X.2020.1743209. [DOI] [Google Scholar]
- 6.Song XP, Hansen MC, Potapov P, Adusei B, Pickering J, Adami M, et al. Massive soybean expansion in South America since 2000 and implications for conservation. Nat Sustain. 2021;4:784–92. doi: 10.1038/s41893-021-00729-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macchi L, Decarre J, Goijman AP, Mastrangelo M, Blendinger PG, Gavier-Pizarro GI, et al. Trade-offs between biodiversity and agriculture are moving targets in dynamic landscapes. J Appl Ecol. 2020;57:2054–63. doi: 10.1111/1365-2664.13699. [DOI] [Google Scholar]
- 8.Chia SY, Tanga CM, van Loon JJA, Dicke M. Insects for sustainable animal feed: inclusive business models involving smallholder farmers. Curr Opin Environ Sustain. 2019;41:23–30. doi: 10.1016/j.cosust.2019.09.003. [DOI] [Google Scholar]
- 9.Kim TK, Yong HI, Kim YB, Kim HW, Choi YS. Edible insects as a protein source: a review of public perception, processing technology, and research trends. Food Sci Anim Resour. 2019;39:521–40. doi: 10.5851/kosfa.2019.e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mancini S, Sogari G, Espinosa Diaz S, Menozzi D, Paci G, Moruzzo R. Exploring the future of edible insects in Europe. Foods. 2022;11:455. doi: 10.3390/foods11030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Żuk-Gołaszewska K, Gałęcki R, Obremski K, Smetana S, Figiel S, Gołaszewski J. Edible insect farming in the context of the EU regulations and marketing—an overview. Insects. 2022;13:446. doi: 10.3390/insects13050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer-Rochow VB, Gahukar RT, Ghosh S, Jung C. Chemical composition, nutrient quality and acceptability of edible insects are affected by species, developmental stage, gender, diet, and processing method. Foods. 2021;10:1036. doi: 10.3390/foods10051036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangopadhyay D, Ray M, Sinha S. Comparison of amino acid profiles and vitamin contents of male and female prepupae and pupae of eri silkworm, Samia ricini. J Food Compos Anal. 2022;113:104723. doi: 10.1016/j.jfca.2022.104723. [DOI] [Google Scholar]
- 14.Weru J, Chege P, Kinyuru J. Nutritional potential of edible insects: a systematic review of published data. Int J Trop Insect Sci. 2021;41:2015–37. doi: 10.1007/s42690-021-00464-0. [DOI] [Google Scholar]
- 15.Altmann BA, Wigger R, Ciulu M, Mörlein D. The effect of insect or microalga alternative protein feeds on broiler meat quality. J Sci Food Agric. 2020;100:4292–302. doi: 10.1002/jsfa.10473. [DOI] [PubMed] [Google Scholar]
- 16.Toral PG, Hervás G, González-Rosales MG, Mendoza AG, Robles-Jiménez LE, Frutos P. Insects as alternative feed for ruminants: comparison of protein evaluation methods. J Anim Sci Biotechnol. 2022;13:21. doi: 10.1186/s40104-021-00671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim CH, Ryu J, Lee J, Ko K, Lee J, Park KY, et al. Use of black soldier fly larvae for food waste treatment and energy production in Asian countries: a review. Processes. 2021;9:161. doi: 10.3390/pr9010161. [DOI] [Google Scholar]
- 18.Mak TMW, Xiong X, Tsang DCW, Yu IKM, Poon CS. Sustainable food waste management towards circular bioeconomy: policy review, limitations and opportunities. Bioresour Technol. 2020;297:122497. doi: 10.1016/j.biortech.2019.122497. [DOI] [PubMed] [Google Scholar]
- 19.Molina-Peñate E, Artola A, Sánchez A. Organic municipal waste as feedstock for biorefineries: bioconversion technologies integration and challenges. Rev Environ Sci Biotechnol. 2022;21:247–67. doi: 10.1007/s11157-021-09605-w. [DOI] [Google Scholar]
- 20.Sharma P, Bano A, Singh SP, Atkinson JD, Lam SS, Iqbal HMN, et al. Biotransformation of food waste into biogas and hydrogen fuel – a review. Int J Hydrogen Energy. 2024;52:46–60. doi: 10.1016/j.ijhydene.2022.08.081. [DOI] [Google Scholar]
- 21.Fowles TM, Nansen C. Insect-based bioconversion: value from food waste. In: Närvänen E, Mesiranta N, Mattila M, Heikkinen A, editors. Food waste management. Cham: Palgrave Macmillan; 2020. pp. 321–46. p. [DOI] [Google Scholar]
- 22.Varelas V. Food wastes as a potential new source for edible insect mass production for food and feed: a review. Fermentation. 2019;5:81. doi: 10.3390/fermentation5030081. [DOI] [Google Scholar]
- 23.Yu M, Li Z, Chen W, Rong T, Wang G, Ma X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J Anim Sci Biotechnol. 2019;10:50. doi: 10.1186/s40104-019-0358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beesigamukama D, Mochoge B, Korir NK, Fiaboe KKM, Nakimbugwe D, Khamis FM, et al. Low-cost technology for recycling agro-industrial waste into nutrient-rich organic fertilizer using black soldier fly. Waste Manag. 2021;119:183–94. doi: 10.1016/j.wasman.2020.09.043. [DOI] [PubMed] [Google Scholar]
- 25.Pas C, Brodeur D, Deschamps MH, Lebeuf Y, Adjalle K, Barnabé S, et al. Valorization of pretreated biogas digestate with black soldier fly (Hermetia illucens, L; Diptera: Stratiomyidae) larvae. J Environ Manage. 2022;319:115529. doi: 10.1016/j.jenvman.2022.115529. [DOI] [PubMed] [Google Scholar]
- 26.Amrul NF, Kabir Ahmad I, Ahmad Basri NE, Suja F, Abdul Jalil NA, Azman NA. A review of organic waste treatment using black soldier fly (Hermetia illucens) Sustainability. 2022;14:4565. doi: 10.3390/su14084565. [DOI] [Google Scholar]
- 27.Rehman KU, Hollah C, Wiesotzki K, Rehman RU, Rehman AU, Zhang J, et al. Black soldier fly, Hermetia illucens as a potential innovative and environmentally friendly tool for organic waste management: a mini-review. Waste Manag Res. 2023;41:81–97. doi: 10.1177/0734242X221105441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abd El-Hack ME, Shafi ME, Alghamdi WY, Abdelnour SA, Shehata AM, Noreldin AE, et al. Black soldier fly (Hermetia illucens) meal as a promising feed ingredient for poultry: a comprehensive review. Agriculture. 2020;10:339. doi: 10.3390/agriculture10080339. [DOI] [Google Scholar]
- 29.Smetana S, Schmitt E, Mathys A. Sustainable use of Hermetia illucens insect biomass for feed and food: attributional and consequential life cycle assessment. Resour Conserv Recycl. 2019;144:285–96. doi: 10.1016/j.resconrec.2019.01.042. [DOI] [Google Scholar]
- 30.Bessa LW, Pieterse E, Marais J, Dhanani K, Hoffman LC. Food safety of consuming black soldier fly (Hermetia illucens) larvae: microbial, heavy metal and cross-reactive allergen risks. Foods. 2021;10:1934. doi: 10.3390/foods10081934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makkar HPS, Tran G, Heuzé V, Ankers P. State-of-the-art on use of insects as animal feed. Anim Feed Sci Technol. 2014;197:1–33. doi: 10.1016/j.anifeedsci.2014.07.008. [DOI] [Google Scholar]
- 32.Purschke B, Scheibelberger R, Axmann S, Adler A, Jäger H. Impact of substrate contamination with mycotoxins, heavy metals and pesticides on the growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017;34:1410–20. doi: 10.1080/19440049.2017.1299946. [DOI] [PubMed] [Google Scholar]
- 33.Diener S, Zurbrügg C, Tockner K. Bioaccumulation of heavy metals in the black soldier fly, Hermetia illucens and effects on its life cycle. J Insects Food Feed. 2015;1:261–70. doi: 10.3920/JIFF2015.0030. [DOI] [Google Scholar]
- 34.Kallel F, Ellouz Chaabouni S. Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: a review. Environ Prog Sustain Energy. 2017;36:1765–77. doi: 10.1002/ep.12649. [DOI] [Google Scholar]
- 35.Kumar RR, Cho JY. Reuse of hydroponic waste solution. Environ Sci Pollut Res. 2014;21:9569–77. doi: 10.1007/s11356-014-3024-3. [DOI] [PubMed] [Google Scholar]
- 36.Janssen RH, Vincken JP, van den Broek LAM, Fogliano V, Lakemond CMM. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J Agric Food Chem. 2017;65:2275–8. doi: 10.1021/acs.jafc.7b00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim TK, Lee JH, Yong HI, Kang MC, Cha JY, Chun JY, et al. Effects of defatting methods on the physicochemical properties of proteins extracted from Hermetia illucens larvae. Foods. 2022;11:1400. doi: 10.3390/foods11101400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Hajj R, Mhemdi H, Besombes C, Allaf K, Lefrançois V, Vorobiev E. Edible insects’ transformation for feed and food uses: an overview of current insights and future developments in the field. Processes. 2022;10:970. doi: 10.3390/pr10050970. [DOI] [Google Scholar]
- 39.Van Moll L, De Smet J, Paas A, Tegtmeier D, Vilcinskas A, Cos P, et al. In vitro evaluation of antimicrobial peptides from the black soldier fly (Hermetia Illucens) against a selection of human pathogens. Microbiol Spectr. 2022;10:e01664–21. doi: 10.1128/spectrum.01664-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia J, Ge C, Yao H. Antimicrobial peptides from black soldier fly (Hermetia illucens) as potential antimicrobial factors representing an alternative to antibiotics in livestock farming. Animals. 2021;11:1937. doi: 10.3390/ani11071937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei XJ, Kim TH, Park JH, Kim IH. Evaluation of supplementation of defatted black soldier fly (Hermetia illucens) larvae meal in beagle dogs. Ann Anim Sci. 2019;19:767–77. doi: 10.2478/aoas-2019-0021. [DOI] [Google Scholar]
- 42.Chen X, Jin J, Hou F, Song B, Li Z, Zhao Y. Effects of black soldier fly larvae oil on growth performance, immunity and antioxidant capacity, and intestinal function and microbiota of broilers. J Appl Poult Res. 2022;31:100292. doi: 10.1016/j.japr.2022.100292. [DOI] [Google Scholar]
- 43.Debelo H, Li M, Ferruzzi MG. Processing influences on food polyphenol profiles and biological activity. Curr Opin Food Sci. 2020;32:90–102. doi: 10.1016/j.cofs.2020.03.001. [DOI] [Google Scholar]
- 44.Riolo K, Rotondo A, La Torre GL, Marino Y, Franco GA, Crupi R, et al. Cytoprotective and antioxidant effects of hydrolysates from black soldier fly (Hermetia illucens) Antioxidants. 2023;12:519. doi: 10.3390/antiox12020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batish I, Brits D, Valencia P, Miyai C, Rafeeq S, Xu Y, et al. Effects of enzymatic hydrolysis on the functional properties, antioxidant activity and protein structure of black soldier fly (Hermetia illucens) protein. Insects. 2020;11:876. doi: 10.3390/insects11120876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin CY, Kao SH, Hung LC, Chien HJ, Wang WH, Chang YW, et al. Lipopolysaccharide-induced nitric oxide and prostaglandin E2 production is inhibited by tellimagrandin II in mouse and human macrophages. Life. 2021;11:411. doi: 10.3390/life11050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X, Ji H, Yu H, Zhou J. Influence of dietary black soldier fly (Hermetia illucens Linnaeus) pulp on growth performance, antioxidant capacity and intestinal health of juvenile mirror carp (Cyprinus carpio var. specularis) Aquac Nutr. 2020;26:432–43. doi: 10.1111/anu.13005. [DOI] [Google Scholar]
- 48.Zhou JS, Liu SS, Ji H, Yu HB. Effect of replacing dietary fish meal with black soldier fly larvae meal on growth and fatty acid composition of Jian carp (Cyprinus carpio var. Jian) Aquac Nutr. 2018;24:424–33. doi: 10.1111/anu.12574. [DOI] [Google Scholar]