Abstract
This study aimed to compare the changes in the bioactivities of peptide extracts (< 10 kDa) obtained from Jeju black pigs (JBP) and three-way crossbred pigs (Landrace × Yorkshire × Duroc, LYD) before and after digestion. The results showed that the loin peptide extracts of JBP maintained high 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity after in vitro digestion. However, the iron chelating activity and antihypertensive activity of all peptide extracts were decreased. This study suggested that the peptide extracts produced through alkaline-AK digestion have sufficiently high antioxidant and antihypertensive activities; however, these activities were reduced after in vitro digestion. Meanwhile, the JBP loin and ham peptide extracts promoted high superoxide dismutase (SOD) activity than that of LYD when administered to mice. Furthermore, the ham peptide extracts of JBP showed a relatively high antihypertensive activity in mice. Therefore, it is deemed that these peptide extracts from JBP are more bioactive than that of LYD, and can be used as bioactive materials.
Keywords: Jeju black pigs, Peptide extracts, Antioxidant activity, Antihypertensive activity
INTRODUCTION
Currently, the Korean pork market is dominated by three-way crossbred pigs (Landrace × Yorkshire × Duroc; LYD). On the other hand, the Jeju black pig (JBP) accounts for only 1.2% of the Korean pork market; however, Korean consumers prefer JBP owing to its higher muscle fat content and redness compared with LYD [1]. In addition, JBP has a higher essential fatty acid content and better overall taste compared with LYD [2]. Because JBP, whose breeding size continues to increase, is recognized as high-quality pork and domestic consumers increasingly prefer it, comparing JBP with LYD to identify differentiated characteristics is essential. Furthermore, there is a need to identify the excellence of JBP, which accounts for only 1.2% of the Korean pork market. Previous studies have compared the carcass and meat characteristics of JBP and LYD [1,2]; however, comparative studies on the changes in the bioactivities of pork peptides due to various physiological processes, including digestion, are insufficient.
In general, pork contains bioactive peptides that regulate various biological actions and have beneficial health effects [3,4]. Peptides that exhibit various bioactive functions, such as antioxidant, antihypertension, antithrombosis, and antibacterial functions, have been derived from the myofibrillar proteins of pigs [5–8]. These bioactive peptides can be efficiently extracted from proteins using enzymes such as papain, bromelain, ficin, and alkaline protease [9–11]. Interestingly, peptides with a small molecular weight (< 10 kDa) have higher antioxidant and antihypertensive activities than peptides with a large molecular weight [12,13]. However, research on the digestion-induced changes in the bioactivities of pork peptides is insufficient. Therefore, this study was conducted to compare and analyze the in vitro-digestion-induced changes in the antioxidant and antihypertensive activities of peptide extracts (< 10 kDa) derived (using alkaline-AK enzymes) from the loin and ham myofibrillar proteins of JBP and LYD. This study also aimed to ascertain and compare the benefits of these peptides to experimental animals (mice) by in vivo experiment.
MATERIALS AND METHODS
Chemical materials
α-Amylase (hog pancreas), bile extract (porcine), lipase (porcine pancreas), mucin (porcine stomach type II), pepsin (porcine gastric mucosa), uric acid, 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhudrazyl (DPPH), potassium persulfate, and 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt hydrate (ferrozine) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Bovine serum albumin was obtained from Biosesang (Seongnam, Korea) and pancreatin (porcine pancreas) was purchased from Tokyo Chemical Industry (Tokyo, Japan).
Experimental animals
All procedures related to animal testing were approved by the Institutional Animal Care and Use Committee (IACUC) of the Chung-Ang University Research Institute (Approval number: 2021-00038) and conducted at the CK-II Specialized Animal Experiment Center, Chung-Ang University. A total of 30 ICR mice (female, eight-week-old) were purchased from Orient Bio (Seongnam, Korea). During the two-week adaptation period, a pellet-type general feed was provided ad libitum and the breeding environment was maintained at 22 ± 2°C temperature, 60 ± 5% humidity, and a 12 h photoperiod (07:00–19:00).
Peptide extraction
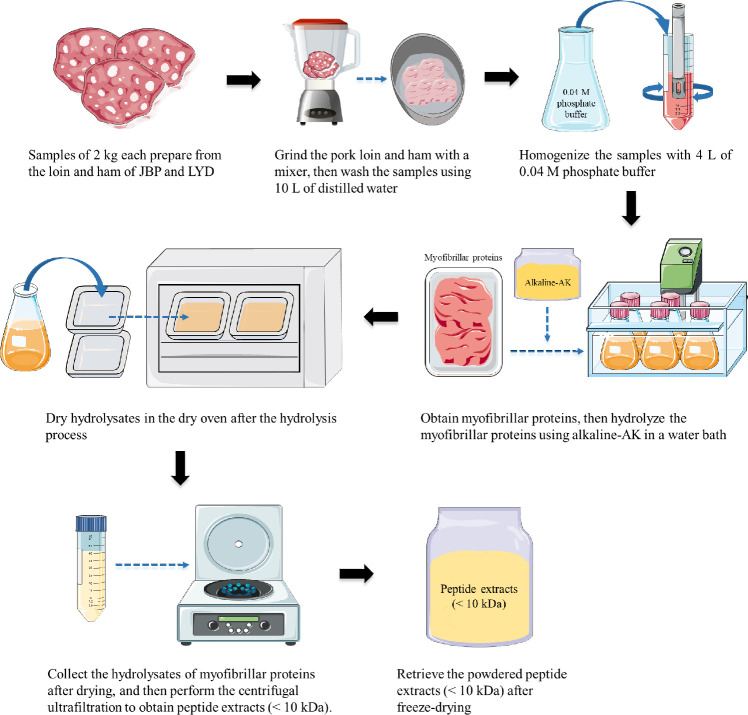
Fig. 1 shows the process for obtaining peptide extracts from the loin and ham of JBP and LYD. JBP loin and ham were obtained from pigs raised in Jeju Island, and trademarked JBP products were purchased and used. LYD loin and ham were purchased from three wolesale stores (Asan and Goyang, Korea). Each sample was sampled at least 5 individuals for meat part. After grinding 2 kg of each sample for 1 min, total 10 L of distilled water compare to 2 kg of each sample was added and the samples were washed 10 times to remove blood and fat as much as possible. 400 g of the sample was homogenized using 4 L of 0.04 M phosphate buffer (PBS, pH 7.4) and then centrifuged at 1,977×g and 4°C for 15 min. The myofibrillar protein thus obtained was hydrolyzed using 0.2% alkaline-AK at 60°C and pH 11 for 2 h. Alkaline-AK (180–200 KU/g solid) was obtained by fermenting soybean meal inoculated with Bacillus methylotrophicus and then extracting from the fermented soybean meal. The protein hydrolysate was heated at 80°C for 15 min to deactivate the enzyme activity and dried at 55°C for 24 h. After drying, a peptide extract of < 10 kDa was obtained through a central filter unit (Amicon® Ultra; Merck Millipore, Burlington, MA, USA). Finally, the peptide extract was freeze-dried at −70°C for 72 h.
Fig. 1. Schematic diagram of the procedure for obtaining myofibrillar proteins and peptide extracts (< 10 kDa) from JBP and LYD.
JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc).
Analysis of digestibility
In vitro digestion
The in vitro digestion experiment was performed using the method given by Lee et al. [14], with slight modifications. Table 1 shows the constituents and concentrations of digestive enzymes and inorganic and organic solvents used for conducting the in vitro digestion experiment [14,15]. To each peptide extract sample (120 mg), 2 mL of saliva was added and the mixture was stirred in a water bath (37°C, 150 rpm) for 5 min. Subsequently, 4 mL of gastric juice was added to the mixture and allowed to react for 2 h under the same conditions. Then, 4 mL of the small intestine solution and 2 mL of the bile solution were added, respectively, and allowed to react in a water bath at 37°Cand 150 rpm for 2 h to end the in vitro digestion process. In all in vitro digestion experiments, a control containing only digestive enzymes and a control containing only samples was prepared by replacing the sample or the digestive enzymes with distilled water. After the digestion process, all samples were centrifuged for 20 min at 13,000×g and 4°C, and the supernatant was taken and used as the final sample.
Table 1. Constituents and concentrations of synthetic digestive juices used in the In vitro digestion model1).
| Saliva (mouth step) | Gastric juice (stomach step) | Duodenal juice (small intestine step) | Bile juice (small intestine step) | |
|---|---|---|---|---|
| Inorganic and organic components | 1.7 mL NaCl2) (175.3
g/L)3) 8 mL Urea (25 g/L) 15 mg Uric acid |
6.5 mL HCl (37 g/L) mL CaCl2·2H2O (22.2 g/L) 1 g Bovine serum albumin |
6.3 mL KCl (89.6
g/L) 9 mL CaCl2·2H2O (22.2 g/L) 1 g Bovine serum albumin |
69.3 mL NaHCO3 (84.7
g/L) 10 mL CaCl2·2H2O (22.2 g/L) 1.8 g Bovine serum albumin 30 g Bile |
| Enzymes | 290 mg
α-Amylase 25 mg Mucin |
2.5 g Pepsin 3 g Mucin |
9 g Pancreatin 1.5 g Lipase |
|
| pH | 6.8 ± 0.2 | 1.50 ± 0.02 | 8.0 ± 0.2 | 7.0 ± 0.2 |
After mixing all components (inorganic components, organic components, and enzymes), the volume was made up to 500 mL with distilled water. If necessary, the pH of the digestive juices was adjusted to the appropriate value.
The numbers indicate the concentrations of chemicals used to make the digestive juices.
The numbers in parentheses indicate the concentrations of inorganic or organic components per liter of distilled water.
In vivo digestion
A total of 30 eight-week-old female ICR mice were separated into each cages by mass-based randomize block design. After a two-week adaptation period, the in vivo digestion experiment was conducted for 21 d; thus, the total duration of the experiment was 35 d. Diets for the in vivo digestion experiments of peptide extracts were limited based on the daily feed intake (5 g) of general ICR mice, and peptide extracts (800 mg/kg) were administered orally once a day (at the same time every day).
Analysis of molecular weight
Gel permeation chromatography was conducted using an ACQUITY APC System (Waters Corporation, Milford, MA, USA) with an Xbridge Protein BEH SEC column (150 × 7.8 mm, 3.5 μm) (Waters Corporation). Deionized water (A) and MeOH (B) were used as the mobile phase solvents; the solvent composition was set to 90% A and 10% B. The flow rate was 0.7 mL/min, and a 10 μL sample was injected and analyzed for 15 min using a reactive index (RI) detector.
Analysis of free amino acid composition
Sample pretreatment for the analysis of free amino acid composition was performed using the method given by Enda et al. [16]. Briefly, 1 mL of a 5% TCA solution was added to 1 mL of each sample (diluted at an appropriate concentration) and vortexed, and the protein was precipitated by centrifuging at 13,572×g for 20 min. Thereafter, 2 mL of the supernatant was taken and 4 mL of n-hexane solution was added to it. The mixture was shaken for 10 min and then centrifuged at 1,977×g and 20°C for 20 min. After centrifugation, the lower layer solution was obtained, and the process of centrifuging at 20°C for 20 min at 13,572×g was repeated twice to remove the remaining n-hexane solution. Finally, all samples were filtered using a 0.20 μm syringe filter and analyzed using a Hitachi L-8900 amino acid analyzer (Hitachi High-Tech, Tokyo, Japan); the detailed analysis conditions are presented in Table 2.
Table 2. The conditions maintained in the amino acid analyzer for studying the changes in free amino acid composition.
| Parameter | Condition |
|---|---|
| Column | Hitachi HPLC packed ion exchange column (#2622PF) |
| Mobile phase | L-8900 buffer solution PF-1, 2, 3, 4, RG |
| Flow rate | Buffer solution: 0.35
mL/min Ninhydrin solution: 0.30 mL/min |
| Detection wavelength | 440 nm, 570 nm |
| Temperatures | Reaction coil:
135°C Column: 30°C–70°C |
| Injection volume | 20 μL |
In vitro analysis of bioavailability
Analysis of 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity
The ABTS assay was performed according to the method given by Re et al. [17], with slight modifications. The ABTS stock solution was prepared by dissolving 38.41 mg of 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (7 mM) and 6.615 mg of potassium persulfate (2.45 mM) in 10 mL of distilled water, mixing and incubating the mixture in a dark room for 12 h. The prepared ABTS stock solution was diluted with MeOH and used as the ABTS working solution; the absorbance of the diluted solution was 0.7 ± 0.02 at 734 nm. Twenty microliters of each sample and 180 μL of ABTS working solution were added to a 96-well plate and incubated in a dark room for 10 min. Subsequently, their absorbance was measured at 734 nm using a microplate reader (Spectramax® 190; Molecular Devices, San Jose, CA, USA). The ABTS radical scavenging activity was calculated as follows:
Analysis of 2,2-diphenyl-1-picrylhudrazyl radical scavenging activity
The DPPH assay was performed using the method given by Tepe et al. [18], with slight modifications. The DPPH solution was prepared by dissolving 2 mg of 2,2-diphenyl-1-picrylhydrazyl in 25 mL of MeOH. One hundred microliters of each sample and 100 μL of DPPH solution were added to a 96-well plate and incubated in a dark room for 30 min. Subsequently, their absorbance was measured at 517 nm. The DPPH radical scavenging activity was calculated as follows:
Analysis of iron chelating activity
The iron chelating assay was performed using the method given by Dinis et al. [19], with slight modifications. Distilled water was added to prepare 2 mM iron (II) chloride and 5 mM ferrozine. The prepared samples (200 μL) were placed in 1.5 mL tubes, and 200 μL of 2 mM iron (II) chloride and 40 μL of 5 mM ferrozine was sequentially added to the tubes. Thereafter, the tubes were vortexed and incubated at 20°C–25°C for 10 min. Finally, the absorbance of the samples was measured at 562 nm. The iron chelating activity was calculated as follows:
Analysis of reducing power
The reducing power assay was conducted according to the method given by Oyaizu [20]. Iron (III) chloride (1 mg/mL), TCA (100 mg/mL), and potassium hexacyanoferrate (III) (10 mg/mL) were prepared using distilled water as a solvent. The samples (100 μL) were taken in 1.5 mL tubes, and 100 μL each of 0.2 M sodium phosphate buffer (pH 6.6) and potassium hexacyanoferrate (III) was added to the tubes and incubated in a dark room at 50°C for 20 min. Subsequently, 100 μL of TCA was added and the tubes were centrifuged at 9,425×g for 1 min. The supernatant (200 μL) obtained was mixed with 200 μL of distilled water and 40 μL of iron (III) chloride, and the absorbance of the solution was measured at 700 nm.
Analysis of angiotensin-converting enzyme (ACE) inhibitory activity
This experiment was conducted using the method given by Cushman & Cheung [21], with some modifications. To prepare the ACE solution, 0.1 M sodium borate buffer (pH 8.3) was prepared by properly mixing sodium tetraborate and boric acid, and 0.5 M sodium chloride was added to it. Subsequently, lung acetone powder from rabbit was extracted by stirring at a concentration of 50 mg/mL (w/v) for 24 h at 4°C and centrifuging at 4°C and 9,425×g for 30 min to obtain the supernatant. The ACE substrate was prepared using 0.1 M sodium borate buffer (pH 8.3) containing 8.3 mM N-Hippuryl-His-Leu hydrate. Each sample (50 µL) was taken in a 2 mL tube (distilled water was taken instead of the sample in the control), and 1 M HCl was added initially to prepare additional samples and controls that stopped the reaction. ACE substrate (50 µL) was added to each of the prepared samples and reacted for 10 min at 37°C. Thereafter, 50 µL of ACE solution (25 mM/mL) was added to the reaction solution and incubated at 37°C for 30 min. Initially, 250 µL of 1 M HCl was added to stop the reaction in the samples in which 1 M HCl was not previously added; furthermore, 500 µL of ethyl acetate was added and the mixture was vortexed for 1 min. Subsequently, the mixture was centrifuged at 1,977×g for 10 min and then 200 µL of the supernatant was taken and dried at 60°C for 30 min. Finally, 1 mL of distilled water was added to the dried sample to prepare the final sample. The absorbance of the final sample was measured at 228 nm using a UV/Vis spectrophotometer (Cary® 300; Agilent, Santa Clara, CA, USA). The ACE inhibitory activity was calculated as follows:
In vivo analysis of bioavailability
Collection of plasma and serum from mice
After completing the peptide extract feeding experiments, all experimental animals (mice) were sacrificed using CO2 gas, and blood was collected through cardiac puncture method. For analyzing the antioxidant enzyme activities, the collected blood was placed in a plasma separation gel tube and centrifuged for 10 min at 2,000×g and 4°C, and the obtained plasma was used as a sample. For ACE activity analysis, serum obtained by centrifuging blood in a serum separation gel tube for 15 at 2,000×g and 4°C was used as a sample.
Analysis of catalase activity
The catalase (CAT) activity of each sample was measured using an OxiTec™ Catalase assay kit (Biomax, Seoul, Korea). Briefly, each 25 μL sample was added to a separate well of a 96-well plate, 25 μL of 40 μM H2O2 solution was added to it, and the plate was incubated at 20°C–25°C for 30 min. Thereafter, 50 μL of Oxi-Probe/horseradish peroxidase (HRP) working solution was added to each well, the plate was incubated at 20°C–25°C for 30 min, and the absorbance was measured at 570 nm. One unit (U) of catalase activity refers to the amount of enzyme that will decompose 1 μM of H2O2 per min at pH 7.0 and 25°C.
Analysis of peroxidase activity
The peroxidase (POD) activity was analyzed using an OxiTec™ Hydrogen peroxide/Peroxidase assay kit (Biomax). Briefly, each 50 μL sample was added to a separate well of a 96-well plate and 50 μL of Oxi-Probe/H2O2 working solution was added to it. Thereafter, the plate was incubated at 20°C–25°C for 30 min and then the absorbance was measured at 560 nm. One unit of HRP activity refers to the amount of enzyme that catalyzes the production of 1 mg of purpurogallin from pyrogallol in 20 s at pH 6.0 and 20°C.
Analysis of superoxide dismutase activity
The superoxide dismutase (SOD) activity was analyzed using an OxiTec™ SOD assay kit (Biomax). Briefly, each prepared sample and reagent was reacted at 37°C for 30 min, and then the absorbance of samples and blanks was measured at 450 nm using a microplate reader. The SOD activity was calculated as follows:
Analysis of angiotensin converting enzyme activity
An ACE activity assay kit (Elabscience, Houston, TX, USA) was used to measure the ACE activity of the samples. The absorbance of samples and blanks was measured at 340 nm using a UV/Vis spectrophotometer. One unit (U) refers to the amount of 1 μM of substrate catalyzed by 1 L of sample per min at 37°C. ACE activity (U/L) was calculated using the following equation: where ε is the absorbance coefficient, d is the path length of the cuvette, and DF is the dilution factor. The ACE activity was calculated as follows:
Statistical analysis
All statistical analyses were conducted using the IBM SPSS Statistics 26 program (IBM, Armonk, NY, USA). The differences between the groups were analyzed using Student’s t-test and one-way analysis of variance (ANOVA). Using the t-test, the average difference between the cuts within the same breed, or the breeds within the same cut, or before and after in vitro digestion was compared at a significance level of p < 0.05. In the case of the one-way ANOVA, the average difference between the control group and loin group of JBP and LYD, or ham group of JBP and LYD (In vivo analysis of bioavailability) were compared at a significance level of p < 0.05. Pairwise comparisons were performed using a post-hoc test (Student–Newman–Keuls; SNK) at a significance level of p < 0.05.
RESULTS AND DISCUSSION
Analysis of the molecular weight of peptides
A common pattern was observed in the hydrolysis of peptide extracts into low-molecular-weight peptides after in vitro digestion (Table 3). The proportion of 200–3,000 Da peptides in the JBP loin and ham peptide extracts before in vitro digestion was approximately 85% and 88%, respectively, and that in the LYD loin and ham peptide extracts was approximately 87% and 79%, respectively. However, after in vitro digestion, the proportion of 200–3,000 Da peptides in the JBP loin and ham peptide extracts decreased to approximately 67% and 66%, respectively, and that in the LYD loin and ham peptide extracts decreased to approximately 65% and 63%, respectively. Accordingly, it was confirmed that the molecular weight distribution ratio of peptides with < 200 Da after in vitro digestion decreased by approximately 13%–25% compared to that before digestion in both JBP and LYD peptide extracts.
Table 3. The molecular weight distribution of loin and ham peptide extracts from JBP and LYD before and after In vitro digestion.
| Contents | Loin | Ham | ||||||
|---|---|---|---|---|---|---|---|---|
| JBP | LYD | JBP | LYD | |||||
| Before digestion | After digestion | Before digestion | After digestion | Before digestion | After digestion | Before digestion | After digestion | |
| Mn1) (Da) | 691 | 287 | 376 | 318 | 437 | 303 | 716 | 287 |
| > 10,000 Da (%) | 1.25 | 0.61 | 0.28 | 0.91 | - | 1.10 | 1.82 | 0.73 |
| 5,000–10,000 Da (%) | 5.55 | 2.46 | 1.73 | 3.74 | 0.22 | 4.28 | 7.12 | 3.16 |
| 3,000–5,000 Da (%) | 6.42 | 3.40 | 2.97 | 5.49 | 1.42 | 5.88 | 9.57 | 4.68 |
| 200–3,000 Da (%) | 84.93 | 66.62 | 86.65 | 64.85 | 88.38 | 65.84 | 78.54 | 63.32 |
| < 200 Da (%) | 1.85 | 26.91 | 8.37 | 25.01 | 9.98 | 22.89 | 2.96 | 28.11 |
The number averaged molecular weight
JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc).
These results are believed to be the consequences of peptide bond decomposition and the formation of peptides with smaller molecular weights due to hydrolysis by digestive enzymes during in vitro digestion. The results of this study are similar to those obtained by Paolella et al. [22] and Gallego et al. [23] in studies comparing and analyzing the molecular weight distribution of peptides produced after in vitro digestion of pork proteins. According to previous studies, most peptides that are derived from pork proteins and have antioxidant activity have a molecular weight of approximately 303–1,275 Da [3,8,24]. In addition, pork-derived peptides that exhibit ACE inhibitory activity have a molecular weight of approximately 520–950 Da [5,25–28].
Analysis of free amino acid composition
Table 4 shows the results of the analysis of changes in free amino acid composition after in vitro digestion of peptide extracts. The total content of free amino acids detected after in vitro digestion of JBP and LYD ham peptide extracts was 154.91 mg/g and 160.01 mg/g, respectively, which is higher than that detected after in vitro digestion of JBP and LYD loin peptide extracts. In addition, the main free amino acids of JBP and LYD peptide extracts before in vitro digestion were leucine, glutamic acid, isoleucine, and tyrosine, whereas those detected after in vitro digestion were arginine, leucine, lysine, phenylalanine and tyrosine.
Table 4. Changes in the free amino acid composition of loin and ham peptide extracts from JBP and LYD after In vitro digestion.
| Amino acid | Free amino acid content (mg/g dw) | |||||||
|---|---|---|---|---|---|---|---|---|
| Loin | Ham | |||||||
| JBP | LYD | JBP | LYD | |||||
| Before digestion | After digestion | Before digestion | After digestion | Before digestion | After digestion | Before digestion | After digestion | |
| Aspartic acid | 0.20 | 0.13 | 0.39 | 0.28 | 0.15 | 0.08 | 0.34 | 0.26 |
| Threonine | 0.46 | 2.62 | 0.55 | 2.50 | 0.53 | 2.74 | 0.47 | 2.70 |
| Serine | 0.53 | 1.70 | 0.62 | 1.65 | 0.55 | 1.79 | 0.53 | 1.78 |
| Asparagine | 0.77 | 1.93 | 0.97 | 2.03 | 0.79 | 2.09 | 0.92 | 2.32 |
| Glutamic acid | 2.61 | 3.00 | 4.64 | 4.64 | 3.07 | 3.50 | 4.31 | 5.19 |
| Glutamine | 0.27 | 4.26 | 0.33 | 3.55 | 0.41 | 4.60 | 0.53 | 4.52 |
| Glycine | 0.26 | 0.31 | 0.29 | 0.32 | 0.28 | 0.36 | 0.24 | 0.38 |
| Alanine | 1.21 | 3.68 | 1.47 | 3.63 | 1.38 | 3.78 | 1.46 | 3.70 |
| Valine | 0.59 | 2.91 | 0.88 | 2.95 | 0.67 | 3.05 | 0.74 | 3.10 |
| Methionine | 0.84 | 6.26 | 1.25 | 5.90 | 1.07 | 6.29 | 1.30 | 6.66 |
| Isoleucine | 1.72 | 5.33 | 2.18 | 5.15 | 1.77 | 5.66 | 2.03 | 5.85 |
| Leucine | 3.72 | 24.83 | 5.47 | 23.93 | 4.66 | 26.19 | 5.44 | 26.68 |
| Tyrosine | 1.56 | 16.70 | 1.77 | 15.31 | 1.58 | 17.47 | 1.88 | 17.38 |
| Phenylalanine | 0.95 | 16.11 | 1.10 | 15.03 | 0.99 | 16.54 | 1.12 | 16.82 |
| Tryptophan | ND | 2.71 | ND | 2.50 | ND | 2.90 | ND | 2.72 |
| Ornithine | 0.32 | 0.51 | 0.59 | 0.55 | 0.31 | 0.51 | 0.50 | 0.67 |
| Lysine | 1.16 | 22.17 | 1.83 | 20.99 | 1.31 | 23.67 | 1.40 | 24.84 |
| Histidine | 0.11 | 2.41 | 0.16 | 2.19 | 0.12 | 2.50 | 0.12 | 2.52 |
| Arginine | 1.20 | 27.85 | 1.89 | 28.60 | 1.43 | 30.09 | 1.80 | 30.78 |
| Proline | 0.34 | 1.14 | 0.32 | 0.96 | 0.43 | 1.10 | 0.35 | 1.14 |
| Carnosine | ND | ND | ND | ND | ND | ND | ND | ND |
| Total | 18.82 | 146.56 | 26.70 | 142.66 | 21.50 | 154.91 | 25.48 | 160.01 |
dw, dry weight; JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc); ND, not detected.
The composition of free amino acids before and after in vitro digestion is believed to have changed due to various factors, including the hydrolysis of peptides and structural changes in amino acids during digestion [29]. According to previous studies, peptides have smaller molecular weights as the enzymatic hydrolysis process progresses, and relatively more free amino acids are produced [30]. Therefore, these results appear to be the consequences of the decomposition of low-molecular-weight peptides in the peptide extracts (obtained from myofibrillar proteins using alkaline-AK enzymes) into large amounts of free amino acids by in vitro digestive enzymes.
According to Xu et al. [31], arginine, lysine, tyrosine, tryptophan, methionine, and histidine have higher antioxidant activities compared with all other amino acids. Arginine significantly increases the activities of SOD, glutathione S-transferase (GST), and glutathione peroxidase in the blood and liver of mice under oxidative stress, whereas lysine upregulates Nrf2, a transcription factor involved in regulating antioxidant gene expression [32,33].
In vitro analysis of bioavailability
In vitro analysis of antioxidative activity
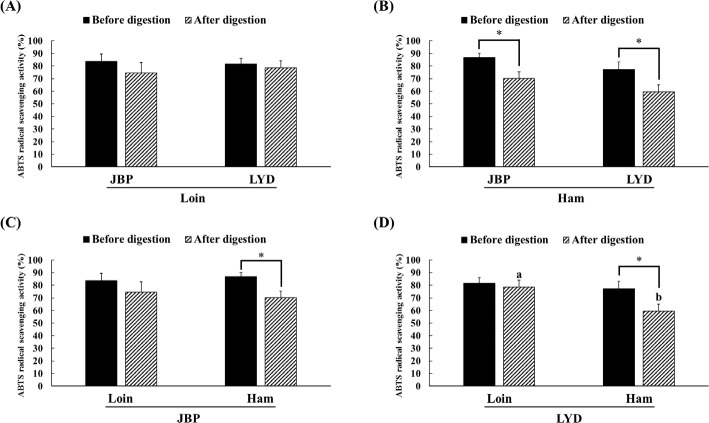
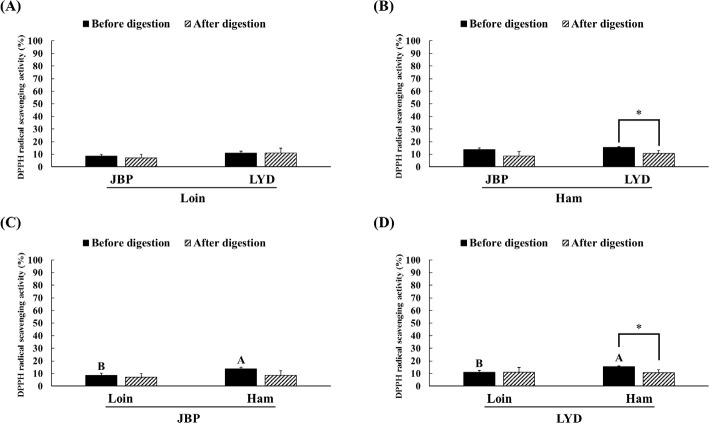
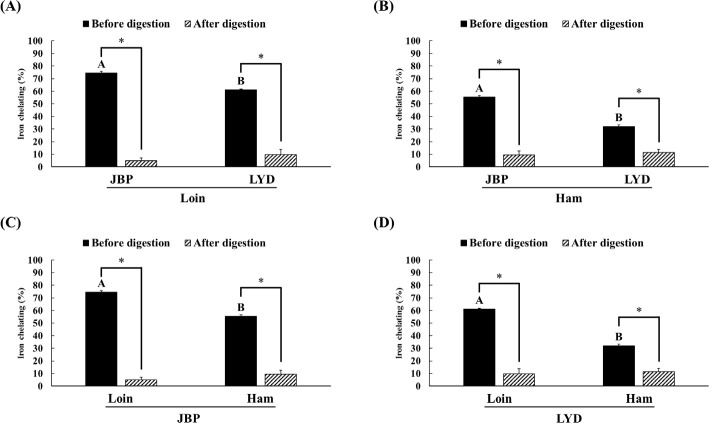
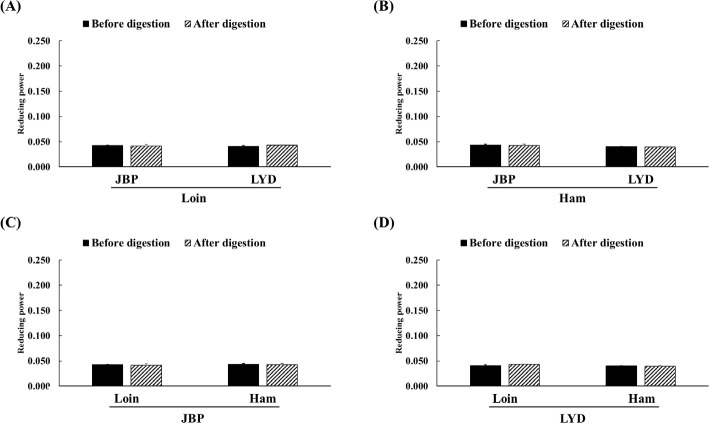
The JBP and LYD loin peptide extracts showed no significant difference in ABTS radical scavenging activity before and after in vitro digestion (Fig. 2A). However, the ABTS radical scavenging activity of the JBP and LYD ham peptide extracts decreased by approximately 17% and 18%, respectively, after in vitro digestion (p < 0.05) (Fig. 2B). In addition, there was no significant difference between the ABTS radical scavenging activity of the JBP loin peptide extracts after in vitro digestion (Fig. 2C); however, the ABTS radical scavenging activity of the LYD loin peptide extract was significantly higher than that of the LYD ham peptide extract (p < 0.05) (Fig. 2D). Furthermore, there was no significant difference in the DPPH radical scavenging activity of all peptide extracts after in vitro digestion except LYD ham peptide extract (Fig. 3). Before in vitro digestion, the iron chelating activity of JBP loin and ham peptide extracts was approximately 74% and 55%, respectively, which was significantly higher than that of LYD loin and ham peptide extracts (p < 0.05) (Fig. 4). However the iron chelating activity was reduced after in vitro digestion (p < 0.05), and there was no significant difference between after in vitro digestion of JBP and LYD loin and ham peptide extracts (Fig. 4). Furthermore, the reducing power assay confirmed that there was no significant difference in reducing power before and after in vitro digestion in all treatments (Fig. 5).
Fig. 2. Comparison of the 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity between (A) the loin peptide extracts from JBP and LYD, (B) the ham peptide extracts from JBP and LYD, (C) the loin and ham peptide extracts from JBP, and (D) the loin and ham peptide extracts from LYD.
Data are presented as mean ± standard deviation. a,bLowercase letters indicate a significant difference in the ABTS radical scavenging activity based on breeds and within cuts after digestion (p < 0.05). A single asterisk indicates a significant difference in the ABTS radical scavenging activity before and after in vitro digestion (p < 0.05). JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc).
Fig. 3. Comparison of the 2,2-diphenyl-1-picrylhudrazyl (DPPH) radical scavenging activity between (A) the loin peptide extracts from JBP and LYD, (B) the ham peptide extracts from JBP and LYD, (C) the loin and ham peptide extracts from JBP, and (D) the loin and ham peptide extracts from LYD.
Data are presented as mean ± standard deviation. A,BUppercase letters indicate a significant difference in the DPPH radical scavenging activity based on breeds and within cuts before digestion (p < 0.05). A single asterisk indicates a significant difference in the DPPH radical scavenging activity before and after in vitro digestion (p < 0.05). JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc).
Fig. 4. Comparison of the iron chelating activity between (A) the loin peptide extracts from JBP and LYD, (B) the ham peptide extracts from JBP and LYD, (C) the loin and ham peptide extracts from JBP, and (D) the loin and ham peptide extracts from LYD Data are presented as mean ± standard deviation.
A,BUppercase letters indicate a significant difference in iron chelating activity based on breeds and within cuts before digestion (p < 0.05). A single asterisk indicates a significant difference in iron chelating activity before and after in vitro digestion (p < 0.05). JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc).
Fig. 5. Comparison of the reducing power between (A) the loin peptide extracts from JBP and LYD, (B) the ham peptide extracts from JBP and LYD, (C) the loin and ham peptide extracts from JBP, and (D) the loin and ham peptide extracts from LYD.
Data are presented as mean ± standard deviation. JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc).
According to previous studies, the antioxidant activity of the peptides detected after enzymatic hydrolysis is higher than that of the proteins before decomposition because enzymatic hydrolysis increases antioxidant activities such as free radical removal, reactive oxygen species inactivation, and metal ion chelating by decomposing the protein’s peptide bond [34,35]. In addition, peptides with a small molecular weight of < 10 kDa have higher antioxidant activity than peptides with a larger molecular weight [12]. However, in this study, the structure and function of low-molecular-weight peptides changed due to pH changes and hydrolysis by digestive enzymes during in vitro digestion, reducing the antioxidant activities of peptide extracts [36]. Although it was thought that peptides extracted from pork can increase antioxidant activities by exposing the hydrophobic amino acid residues of the peptide through hydrolysis by pepsin during in vitro digestion, it has been confirmed that antioxidant activities are reduced when the hydrophobic amino acid residues of the peptide are exposed [37,38]. In addition, peptides can be reduced in bioactivity by oxidation, deamidization, or hydrolysis due to pH changes in the digestive system [39]. The pH change may affect the functional properties of the peptide by modifying one or more amino acids. For example, glutamine and asparagine are destroyed under acidic pH conditions, whereas cystine, serine, and threonine are destroyed under alkaline pH conditions [40].
Meanwhile, the results of this study showed that the proportion of peptides with a molecular weight of < 200 Da increased after in vitro digestion, which is relatively small compared to the molecular weight of most peptides with antioxidant activities [3,8,24]. In addition, free amino acids may have different antioxidant activities owing to the structural differences in amino acid side chains, and amino acids with side chains containing alkyl groups such as leucine, isoleucine, and phenylalanine have low antioxidant capacity [31]. Thus, free amino acids such as arginine, lysine, and tyrosine, which were detected in large quantities after in vitro digestion in the present study, were judged to exhibit high antioxidant activities, whereas leucine and phenylalanine were judged to have significantly low antioxidant activities [31].
Therefore, in the case of ABTS radical scavenging activity, it is judged that bioactive peptides and large amounts of free amino acids contribute to antioxidant activity and can exhibit high radical elimination even after in vitro digestion [41]. The ABTS assay is thought to show significantly higher free radical scavenging activity than the DPPH assay because ABTS radicals are more reactive and are less affected by pH than DPPH radicals [42]. In the case of the iron chelating activity, peptides with amino acid residues that can be chelated with Fe2+ are mostly hydrolyzed through the in vitro digestion process and lose their activity [34,43]. Regarding the reducing power, peptides that can act as electron donors are decomposed and lose their activity due to enzymatic hydrolysis; so, it is expected that the reducing power decreases after in vitro digestion [44].
Analysis of angiotensin-converting enzyme inhibitory activity
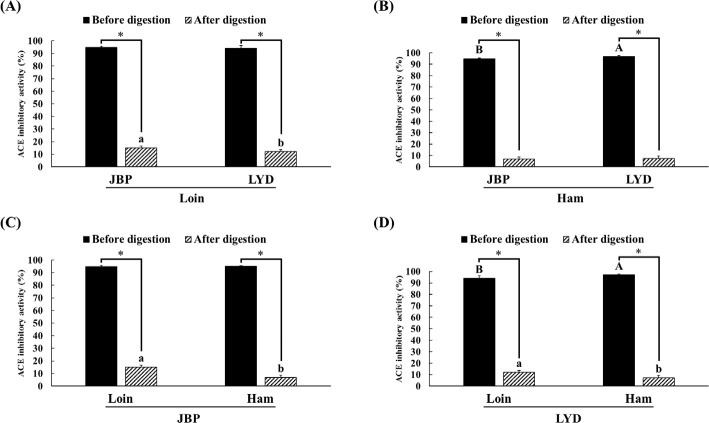
The ACE inhibitory activity of the loin and ham peptide extracts of JBP and LYD was approximately 94%–97% before in vitro digestion; however, after in vitro digestion, the ACE inhibitory activity was reduced (p < 0.05) (Fig. 6). Nevertheless, the ACE inhibitory activity of the loin peptide extract of JBP was approximately 3% higher than that of the LYD (p < 0.05) (Fig. 6).
Fig. 6. Comparison of the angiotensin-converting enzyme (ACE) inhibitory activity between (A) the loin peptide extracts from JBP and LYD, (B) the ham peptide extracts from JBP and LYD, (C) the loin and ham peptide extracts from JBP, and (D) the loin and ham peptide extracts from LYD.
Data are presented as mean ± standard deviation. A,BUppercase letters indicate a significant difference in the ACE inhibitory activity based on breeds and within cuts before digestion (p < 0.05). a,bLowercase letters indicate a significant difference in the ACE inhibitory activity based on breeds and within cuts after digestion (p < 0.05). A single asterisk indicates a significant difference in the ACE inhibitory activity before and after in vitro digestion (p < 0.05). JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc).
ACE has been confirmed to have an important effect on the renin-angiotensin system (RAS) that regulates blood pressure [45]. ACE converts an inactive decapeptide, angiotensin I, into an octapeptide with vascular contraction activity, angiotensin II, and inactivates bradykinin, which mediates vascular expansion; thus, high ACE activity causes hypertension [46,47]. Therefore, intensive research has been conducted on bioactive peptides derived from food proteins that can suppress ACE activity to reduce high blood pressure while avoiding the side effects caused by synthetic drugs [48]. According to previous papers, peptides derived through enzymatic hydrolysis of pork-derived proteins exhibit strong ACE inhibitory activity [5,25–28]. In addition, peptides with a molecular weight of < 10 kDa were found to have a higher antihypertensive activity than peptides with larger molecular weights [13]. However, these peptides can exhibit ACE inhibitory effects only if they maintain their active form and reach the bloodstream; in this study, it is judged that the peptides were decomposed due to the action of digestive enzymes, resulting in reduced ACE inhibitory activity [23,49]. Most of the dipeptides and tripeptides, which can inhibit ACE activity and also block the active site of ACE, are hydrolyzed into amino acids by cytoplasmic peptides in the small intestine, and only some of them maintain their peptide forms without breaking down into amino acids [50,51].
In vivo analysis of bioavailability
Analysis of antioxidative activity
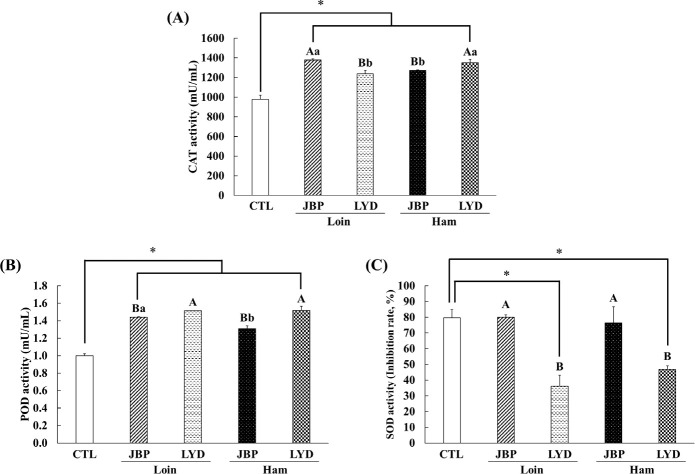
The CAT activity of the groups fed with JBP and LYD loin and ham was approximately 260–400 mU/mL higher than the control group (p < 0.05) (Fig. 7A). Also, the CAT activity of the groups fed with JBP loin and LYD ham was higher than the JBP ham and LYD loin group, respectively (p < 0.05) (Fig. 7A). The POD activity of the groups fed with JBP and LYD loin and ham was approximately 0.31–0.52 mU/mL higher than the control group (p < 0.05) (Fig. 7B). The POD activity of the groups fed with LYD loin and ham was significantly higher than that of the JBP group (p < 0.05) (Fig. 7B). The SOD activity of the groups fed with JBP loin and ham was 80.01 ± 1.51% and 76.49 ± 10.19%, respectively, which was relatively high compare to LYD group (p < 0.05) (Fig. 7C). However, JBP group was not significantly different to the control group (Fig. 7C).
Fig. 7. Dietary effects of the loin and ham peptide extracts from JBP and LYD on the (A) catalase (CAT), (B) peroxidase (POD), (C) superoxidase dismutase (SOD) activities in mice blood Data are presented as mean ± standard deviation.
A,BUppercase letters indicate a significant difference in the antioxidant enzyme activities between the breeds within the same cut (p < 0.05). a,bLowercase letters indicate a significant difference in the antioxidant enzyme activities between the cuts within the same breed (p < 0.05). A single asterisk indicates a significant difference in the ACE activity between the control and treatment groups (p < 0.05). CTL, control; JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc).
Antioxidant enzymes such as CAT, POD, and SOD play an important role in protecting the body from oxidative stress caused by free radicals [52]. SOD, an antioxidant enzyme that catalyzes the displacement of free radical O2- to H2O2 and O2, can protect biomolecules from oxidative damage by active oxygen species. In addition, it is judged that the large amount of free amino acids detected after digestion can enhance antioxidant activities [53,54]. For example, arginine has been found to increase the expression of antioxidant-related genes and proteins, leucine increases the activity of antioxidant enzymes such as GST and total superoxide dismutase (T-SOD), and lysine promotes the expression of antioxidant-related genes [33,55,56]. Therefore, when peptide extracts obtained from the loin and ham myofibrillar protein of JBP are consumed, CAT and POD activities increase and SOD activity is triggered to promote antioxidant activity.
Analysis of angiotensin-converting enzyme activity
The antihypertensive activity was analyzed by measuring the ACE activity after feeding mice with the different peptide extracts; the results of the analysis are presented in Fig. 8. The ACE activity of the groups fed with JBP and LYD loin and ham was higher than that of the control group (p < 0.05). The ACE activity of the groups fed with JBP ham and LYD loin was 287.87 ± 8.26 U/L and 297.28 ± 3.09 U/L, respectively, which was significantly lower than that of the groups fed with JBP loin and LYD ham (p < 0.05).
Fig. 8. Dietary effects of the loin and ham peptide extracts from JBP and LYD on the ACE activity in mice blood.
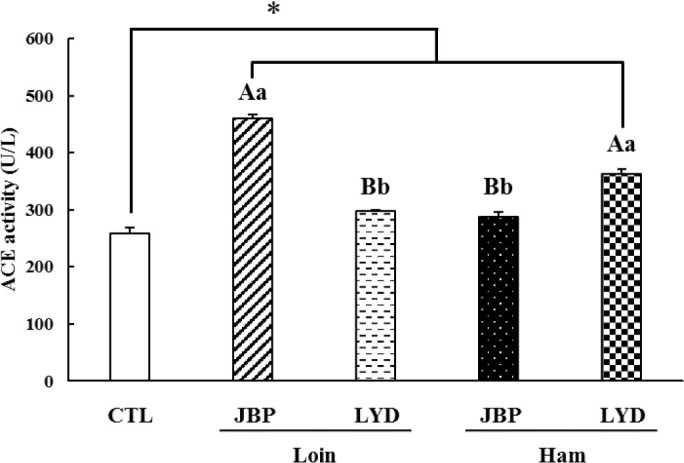
Data are presented as mean ± standard deviation. A,BUppercase letters indicate a significant difference in the ACE activity between breeds within the same cut (p < 0.05). a,bLowercase letters indicate a significant difference in the ACE activity between cuts within the same breed (p < 0.05). A single asterisk indicates a significant difference in the ACE activity between the control and treatment groups (p < 0.05). CTL, control; JBP, Jeju black pigs; LYD, three-way crossbred pigs (Landrace × Yorkshire × Duroc).
In this study, the ACE inhibitory activity of peptide extracts obtained from the loin and ham myofibrillar proteins of JBP and LYD was more than 94% before in vitro digestion (Fig. 6). However, the activity of peptides that exhibit ACE inhibitory activity is believed to decrease in peptide extracts owing to enzymatic hydrolysis, changes in pH, and the intestinal residence time during the digestive process [23,39,57]. Previously, peptides were reported to have low bioavailability when administered orally owing to their weak intestinal mucosal permeability, which can vary depending on the difference in mucosal thickness and surface area of the gastrointestinal tract [57, 58]. In addition, there is little action of protein hydrolyzing enzymes in the large intestine, making it easy to absorb peptides, but peptides can be decomposed by bacteria in the large intestine [59]. According to Lee et al. [60], peptides obtained using alkaline-AK can have relatively reduced digestive stability due to the influence of intestinal microbiome. Thus, various factors in the intestine affect the digestion, absorption, and activity of peptides, resulting in results that may vary from those of the in vitro experiments in this study.
Meanwhile, peptides derived from animal protein are believed to impact ACE activity due to the toxins produced from amino acid decomposition metabolites through fermentation by intestinal microbiome during digestion [61,62]. For example, indoxyl sulfate, a toxin produced from the metabolites of an intestinal microbiome, can activate RAS [63]. When RAS is activated, the activity of ACE is promoted and the amount of angiotensin II increases, which results in vascular contraction and high blood pressure [64,65]. In addition, trimethylamine N-oxide (TMAO), which is related to high blood pressure, also promotes an increase in ACE activity [66,67]. It is predicted that the ACE activity of the groups fed with different peptide extracts increased due to these factors. Nevertheless, the ACE activity of the groups fed with JBP ham and LYD loin was lower than those of the groups fed with JBP loin and LYD ham; thus, JBP ham peptide extract believed to have a relatively high ACE inhibitory activity.
CONCLUSION
In this study, changes in the molecular weight distribution, free amino acid composition, and antioxidant and antihypertensive activities of peptide extracts (< 10 kDa) of JBP and LYD after in vitro digestion were compared and analyzed. In addition, the changes in antioxidant and antihypertensive activities of the peptide extracts and their benefits were confirmed through animal experiments. The loin peptide extract of JBP maintained a relatively high ABTS radical scavenging activity after in vitro digestion. The iron chelating activity of JBP loin and ham peptide extracts was significantly higher than that of LYD loin and ham peptide extracts before in vitro digestion. However, the iron chelating activity of all peptide extracts decreased after in vitro digestion, and there was no significant difference. Although the ACE inhibitory activity of all peptide extracts decreased, the ACE inhibitory activity of JBP loin peptide extract was higher than that of the LYD loin peptide extract. These results are believed to be the consequences of changes in peptide structure and function due to hydrolysis by digestive enzymes and change in pH. Both JBP and LYD peptide extracts promoted antioxidant enzyme activities in the experimental animals; among the different peptide extracts, the JBP loin and ham peptide extracts showed significantly high SOD activity than that of LYD. In addition, the JBP ham peptide extracts showed a relatively higher ACE inhibitory activity in the case of in vivo experiment. Therefore, it is deemed that the peptide extracts of JBP are more bioactive than that of LYD, and can be used as bioactive materials; however, additional research is needed to improve their bioavailability.
Acknowledgements
Not applicable.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry(IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs(MAFRA)(322008-5). This research was supported by the Chung-Ang University Graduated Research Scholarship in 2023.
Availability of data and material
Upon reasonable request, the datasets of this study can be available from the corresponding author.
Authors’ contributions
Conceptualization: Hur SJ.
Data curation: Jeong JW, Mariano E, Lee J, Han D, Kim JS.
Formal analysis: Jeong JW, Lee DY, Lee SY, Yun SH.
Methodology: Jeong JW, Kim JH.
Software: Choi Y.
Validation: Jeong JW, Park J, Choi Y, Han D.
Investigation: Jeong JW, Kim JH, Yun SH.
Writing - original draft: Jeong JW, Mariano E.
Writing - review & editing: Jeong JW, Mariano E, Lee DY, Lee SY, Kim JH, Yun SH, Lee J, Park J, Choi Y, Han D, Kim JS, Hur SJ.
Ethics approval and consent to participate
All procedures related to animal testing were approved by the Institutional Animal Care and Use Committee (IACUC) of the Chung-Ang University Research Institute (approval number: 2021-00038).
REFERENCES
- 1.Jin SK, Kim CW, Song YM, Jang WH, Kim YB, Yeo JS, et al. Physicochemical characteristics of longissimus muscle between the Korean native pig and Landrace. Korean J Food Sci Anim Resour. 2001;21:142–8. [Google Scholar]
- 2.Kim GW, Kim HY, Chun JY. Different characteristics between improved breeds and Jeju black pigs; 2016 KFN International Symposium and Annual Meeting; Jeju, Korea. 2016. pp. 320–1. In. p. [Google Scholar]
- 3.Arihara K. Functional properties of bioactive peptides derived from meat proteins. In: Nollet LML, Toldrá F, editors. Advanced technologies for meat processing. Boca Raton, FL: CRC Press; 2006. pp. 245–74. p. [DOI] [Google Scholar]
- 4.Sánchez A, Vázquez A. Bioactive peptides: a review. Food Qual Saf. 2017;1:29–46. doi: 10.1093/fqsafe/fyx006. [DOI] [Google Scholar]
- 5.Katayama K, Mori T, Kawahara S, Miake K, Kodama Y, Sugiyama M, et al. Angiotensin-I converting enzyme inhibitory peptide derived from porcine skeletal muscle myosin and its antihypertensive activity in spontaneously hypertensive rats. J Food Sci. 2007;72:S702–6. doi: 10.1111/j.1750-3841.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 6.Keska P, Stadnik J. Antimicrobial peptides of meat origin - an in silico and in vitro analysis. Protein Pept Lett. 2017;24:165–73. doi: 10.2174/0929866523666161220113230. [DOI] [PubMed] [Google Scholar]
- 7.Kęska P, Rohn S, Halagarda M, Wójciak KM. Peptides from different carcass elements of organic and conventional pork—potential source of antioxidant activity. Antioxidants. 2020;9:835. doi: 10.3390/antiox9090835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saiga A, Tanabe S, Nishimura T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J Agric Food Chem. 2003;51:3661–7. doi: 10.1021/jf021156g. [DOI] [PubMed] [Google Scholar]
- 9.Ryan JT, Ross RP, Bolton D, Fitzgerald GF, Stanton C. Bioactive peptides from muscle sources: meat and fish. Nutrients. 2011;3:765–91. doi: 10.3390/nu3090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma M, Gat Y, Arya S, Kumar V, Panghal A, Kumar A. A review on microbial alkaline protease: an essential tool for various industrial approaches. Ind Biotechnol. 2019;15:69–78. doi: 10.1089/ind.2018.0032. [DOI] [Google Scholar]
- 11.Singh PK, Shrivastava N, Ojha BK. Enzymes in the meat industry. In: Kuddus M, editor. Enzymes in food biotechnology: production, applications, and future prospects. London: Academic Press; 2019. pp. 111–28. editor. p. [DOI] [Google Scholar]
- 12.Ajibola CF, Fashakin JB, Fagbemi TN, Aluko RE. Effect of peptide size on antioxidant properties of African yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fractions. Int J Mol Sci. 2011;12:6685–702. doi: 10.3390/ijms12106685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Ruiz J, Dávila-Ortíz G, Chel-Guerrero L, Betancur-Ancona D. Angiotensin I-converting enzyme inhibitory activity in peptide fractions from hard-to-cook bean hydrolysates. J Biotechnol. 2010;150:309–10. doi: 10.1016/j.jbiotec.2010.09.283. [DOI] [Google Scholar]
- 14.Lee SJ, Lee SY, Chung MS, Hur SJ. Development of novel in vitro human digestion systems for screening the bioavailability and digestibility of foods. J Funct Foods. 2016;22:113–21. doi: 10.1016/j.jff.2016.01.005. [DOI] [Google Scholar]
- 15.Hur SJ, Lim BO, Decker EA, McClements DJ. In vitro human digestion models for food applications. Food Chem. 2011;125:1–12. doi: 10.1016/j.foodchem.2010.08.036. [DOI] [Google Scholar]
- 16.Enda H, Sagane Y, Nakazawa Y, Sato H, Yamazaki M. Data on free amino acid contents in Japanese basket clams (Corbicula japonica) from Lake Abashiri and Abashirigawa River. Data Br. 2018;16:639–43. doi: 10.1016/j.dib.2017.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 18.Tepe B, Sokmen M, Akpulat HA, Daferera D, Polissiou M, Sokmen A. Antioxidative activity of the essential oils of Thymus sipyleus subsp. sipyleus var. sipyleus and Thymus sipyleus subsp. sipyleus var. rosulans. J Food Eng. 2005;66:447–54. doi: 10.1016/j.jfoodeng.2004.04.015. [DOI] [Google Scholar]
- 19.Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161–9. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- 20.Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 21.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–48. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 22.Paolella S, Falavigna C, Faccini A, Virgili R, Sforza S, Dall’Asta C, et al. Effect of dry-cured ham maturation time on simulated gastrointestinal digestion: characterization of the released peptide fraction. Food Res Int. 2015;67:136–44. doi: 10.1016/j.foodres.2014.10.026. [DOI] [Google Scholar]
- 23.Gallego M, Mauri L, Aristoy MC, Toldrá F, Mora L. Antioxidant peptides profile in dry-cured ham as affected by gastrointestinal digestion. J Funct Foods. 2020;69:103956. doi: 10.1016/j.jff.2020.103956. [DOI] [Google Scholar]
- 24.Xing LJ, Hu YY, Hu HY, Ge QF, Zhou GH, Zhang WG. Purification and identification of antioxidative peptides from dry-cured Xuanwei ham. Food Chem. 2016;194:951–8. doi: 10.1016/j.foodchem.2015.08.101. [DOI] [PubMed] [Google Scholar]
- 25.Escudero E, Sentandreu MA, Arihara K, Toldrá F. Angiotensin I-converting enzyme inhibitory peptides generated from in vitro gastrointestinal digestion of pork meat. J Agric Food Chem. 2010;58:2895–901. doi: 10.1021/jf904204n. [DOI] [PubMed] [Google Scholar]
- 26.Escudero E, Toldrá F, Sentandreu MA, Nishimura H, Arihara K. Antihypertensive activity of peptides identified in the in vitro gastrointestinal digest of pork meat. Meat Sci. 2012;91:382–4. doi: 10.1016/j.meatsci.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Muguruma M, Ahhmed AM, Katayama K, Kawahara S, Maruyama M, Nakamura T. Identification of pro-drug type ACE inhibitory peptide sourced from porcine myosin B: evaluation of its antihypertensive effects in vivo. Food Chem. 2009;114:516–22. doi: 10.1016/j.foodchem.2008.09.081. [DOI] [Google Scholar]
- 28.Nakashima Y, Arihara K, Sasaki A, Mio H, Ishikawa S, Itoh M. Antihypertensive activities of peptides derived from porcine skeletal muscle myosin in spontaneously hypertensive rats. J Food Sci. 2002;67:434–7. doi: 10.1111/j.1365-2621.2002.tb11424.x. [DOI] [Google Scholar]
- 29.Diep TT, Yoo MJY, Rush E. Effect of in vitro gastrointestinal digestion on amino acids, polyphenols and antioxidant capacity of tamarillo yoghurts. Int J Mol Sci. 2022;23:2526. doi: 10.3390/ijms23052526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng TM, Chen MT. Changes of protein in natto (a fermented soybean food) affected by fermenting time. Food Sci Technol Res. 2010;16:537–42. doi: 10.3136/fstr.16.537. [DOI] [Google Scholar]
- 31.Xu N, Chen G, Liu H. Antioxidative categorization of twenty amino acids based on experimental evaluation. Molecules. 2017;22:2066. doi: 10.3390/molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Serie MM, El-Gamal BA, El-Kersh MA, El-Saadani MA. Investigation into the antioxidant role of arginine in the treatment and the protection for intralipid-induced non-alcoholic steatohepatitis. Lipids Health Dis. 2015;14:128. doi: 10.1186/s12944-015-0124-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XY, Liu Y, Jiang WD, Jiang J, Wu P, Zhao J, et al. Co- and post-treatment with lysine protects primary fish enterocytes against Cu-induced oxidative damage. PLOS ONE. 2016;11:e0147408. doi: 10.1371/journal.pone.0147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias RJ, Kellerby SS, Decker EA. Antioxidant activity of proteins and peptides. Crit Rev Food Sci Nutr. 2008;48:430–41. doi: 10.1080/10408390701425615. [DOI] [PubMed] [Google Scholar]
- 35.Park SY, Chin KB. Antioxidant activities of pepsin hydrolysates of water- and salt-soluble protein extracted from pork hams. Int J Food Sci Technol. 2011;46:229–35. doi: 10.1111/j.1365-2621.2010.02454.x. [DOI] [Google Scholar]
- 36.Segura-Campos M, Chel-Guerrero L, Betancur-Ancona D, Hernandez-Escalante VM. Bioavailability of bioactive peptides. Food Rev Int. 2011;27:213–26. doi: 10.1080/87559129.2011.563395. [DOI] [Google Scholar]
- 37.Acquah C, Di Stefano E, Udenigwe CC. Role of hydrophobicity in food peptide functionality and bioactivity. J Food Bioact. 2018;4:88–98. doi: 10.31665/JFB.2018.4164. [DOI] [Google Scholar]
- 38.Zhu CZ, Zhang WG, Kang ZL, Zhou GH, Xu XL. Stability of an antioxidant peptide extracted from Jinhua ham. Meat Sci. 2014;96:783–9. doi: 10.1016/j.meatsci.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Chen MC, Sonaje K, Chen KJ, Sung HW. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials. 2011;32:9826–38. doi: 10.1016/j.biomaterials.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 40.Korhonen H, Pihlanto-Leppäla A, Rantamäki P, Tupasela T. Impact of processing on bioactive proteins and peptides. Trends Food Sci Technol. 1998;9:307–19. doi: 10.1016/S0924-2244(98)00054-5. [DOI] [Google Scholar]
- 41.Clausen MR, Skibsted LH, Stagsted J. Characterization of major radical scavenger species in bovine milk through size exclusion chromatography and functional assays. J Agric Food Chem. 2009;57:2912–9. doi: 10.1021/jf803449t. [DOI] [PubMed] [Google Scholar]
- 42.Shalaby EA, Shanab SMM. Antioxidant compounds, assays of determination and mode of action. Afr J Pharm Pharmacol. 2013;7:528–39. doi: 10.5897/AJPP2013.3474. [DOI] [Google Scholar]
- 43.Zhu CZ, Zhang WG, Zhou GH, Xu XL. Identification of antioxidant peptides of Jinhua ham generated in the products and through the simulated gastrointestinal digestion system. J Sci Food Agric. 2016;96:99–108. doi: 10.1002/jsfa.7065. [DOI] [PubMed] [Google Scholar]
- 44.Kęska P, Stadnik J. Effect of in vitro gastro-pancreatic digestion on antioxidant activity of low-molecular-weight (<3.5 kDa) peptides from dry-cured pork loins with probiotic strains of LAB. Int J Food Sci Technol. 2021;56:6268–78. doi: 10.1111/ijfs.15066. [DOI] [Google Scholar]
- 45.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol. 2007;27:144–52. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Mora L, Gallego M, Toldrá F. ACEI-inhibitory peptides naturally generated in meat and meat products and their health relevance. Nutrients. 2018;10:1259. doi: 10.3390/nu10091259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuo JL, Ferrao FM, Zheng Y, Li XC. New frontiers in the intrarenal renin-angiotensin system: a critical review of classical and new paradigms. Front Endocrinol. 2013;4:166. doi: 10.3389/fendo.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toldrá F, Reig M, Aristoy MC, Mora L. Generation of bioactive peptides during food processing. Food Chem. 2018;267:395–404. doi: 10.1016/j.foodchem.2017.06.119. [DOI] [PubMed] [Google Scholar]
- 49.Vercruysse L, Van Camp J, Morel N, Rougé P, Herregods G, Smagghe G. Ala-Val-Phe and Val-Phe: ACE inhibitory peptides derived from insect protein with antihypertensive activity in spontaneously hypertensive rats. Peptides. 2010;31:482–8. doi: 10.1016/j.peptides.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 50.Hernández-Ledesma B, del Mar Contreras M, Recio I. Antihypertensive peptides: production, bioavailability and incorporation into foods. Adv Colloid Interface Sci. 2011;165:23–35. doi: 10.1016/j.cis.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu M. Food-derived peptides and intestinal functions. BioFactors. 2004;21:43–7. doi: 10.1002/biof.552210109. [DOI] [PubMed] [Google Scholar]
- 52.Woo JY, Gu W, Kim KA, Jang SE, Han MJ, Kim DH. Lactobacillus pentosus var. plantarum C29 ameliorates memory impairment and inflammaging in a D-galactose-induced accelerated aging mouse model. Anaerobe. 2014;27:22–6. doi: 10.1016/j.anaerobe.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Boguszewska D, Grudkowska M, Zagdańska B. Drought-responsive antioxidant enzymes in potato (Solanum tuberosum L.) Potato Res. 2010;53:373–82. doi: 10.1007/s11540-010-9178-6. [DOI] [Google Scholar]
- 54.Vlasova II. Peroxidase activity of human hemoproteins: keeping the fire under control. Molecules. 2018;23:2561. doi: 10.3390/molecules23102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X, Xiang L, Jia G, Liu G, Zhao H, Huang Z. Effects of dietary leucine on antioxidant activity and expression of antioxidant and mitochondrial-related genes in longissimus dorsi muscle and liver of piglets. Anim Sci J. 2019;90:990–8. doi: 10.1111/asj.13249. [DOI] [PubMed] [Google Scholar]
- 56.Liang M, Wang Z, Li H, Cai L, Pan J, He H, et al. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem Toxicol. 2018;115:315–28. doi: 10.1016/j.fct.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 57.Hamman JH, Enslin GM, Kotzé AF. Oral delivery of peptide drugs: barriers and developments. BioDrugs. 2005;19:165–77. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- 58.Kompella UB, Lee VHL. Delivery systems for penetration enhancement of peptide and protein drugs: design considerations. Adv Drug Deliv Rev. 2001;46:211–45. doi: 10.1016/S0169-409X(00)00137-X. [DOI] [PubMed] [Google Scholar]
- 59.Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6:33–66. [PubMed] [Google Scholar]
- 60.Lee SY, Lee DY, Hur SJ. Changes in the stability and antioxidant activities of different molecular weight bioactive peptide extracts obtained from beef during in vitro human digestion by gut microbiota. Food Res Int. 2021;141:110116. doi: 10.1016/j.foodres.2021.110116. [DOI] [PubMed] [Google Scholar]
- 61.Jaworska K, Koper M, Ufnal M. Gut microbiota and renin-angiotensin system: a complex interplay at local and systemic levels. Am J Physiol Gastrointest Liver Physiol. 2021;321:G355–66. doi: 10.1152/ajpgi.00099.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mafra D, Borges NA, de Franca Cardozo LFM, Anjos JS, Black AP, Moraes C, et al. Red meat intake in chronic kidney disease patients: two sides of the coin. Nutrition. 2018;46:26–32. doi: 10.1016/j.nut.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 63.Akbar MF. Inhibition of indoxyl sulfate-induced intrarenal renin-angiotensin system activation: targeting the aryl hydrocarbon receptor. Transl Clin Pharmacol. 2017;25:114–6. doi: 10.12793/tcp.2017.25.3.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aiemratchanee P, Panyawechamontri K, Phaophu P, Reamtong O, Panbangred W. In vitro antihypertensive activity of bioactive peptides derived from porcine blood corpuscle and plasma proteins. Int J Food Sci Technol. 2021;56:2315–24. doi: 10.1111/ijfs.14853. [DOI] [Google Scholar]
- 65.Volpe M, Tocci G, Pagannone E. Activation of the renin-angiotensin-aldosterone system in heart failure. Ital Heart J. 2005;6:16S–23S. [PubMed] [Google Scholar]
- 66.Ge X, Zheng L, Zhuang R, Yu P, Xu Z, Liu G, et al. The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose–response meta-analysis. Adv Nutr. 2020;11:66–76. doi: 10.1093/advances/nmz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu M, Han Q, Yang J. Trimethylamine-N-oxide (TMAO) increased aquaporin-2 expression in spontaneously hypertensive rats. Clin Exp Hypertens. 2019;41:312–22. doi: 10.1080/10641963.2018.1481420. [DOI] [PubMed] [Google Scholar]