Abstract
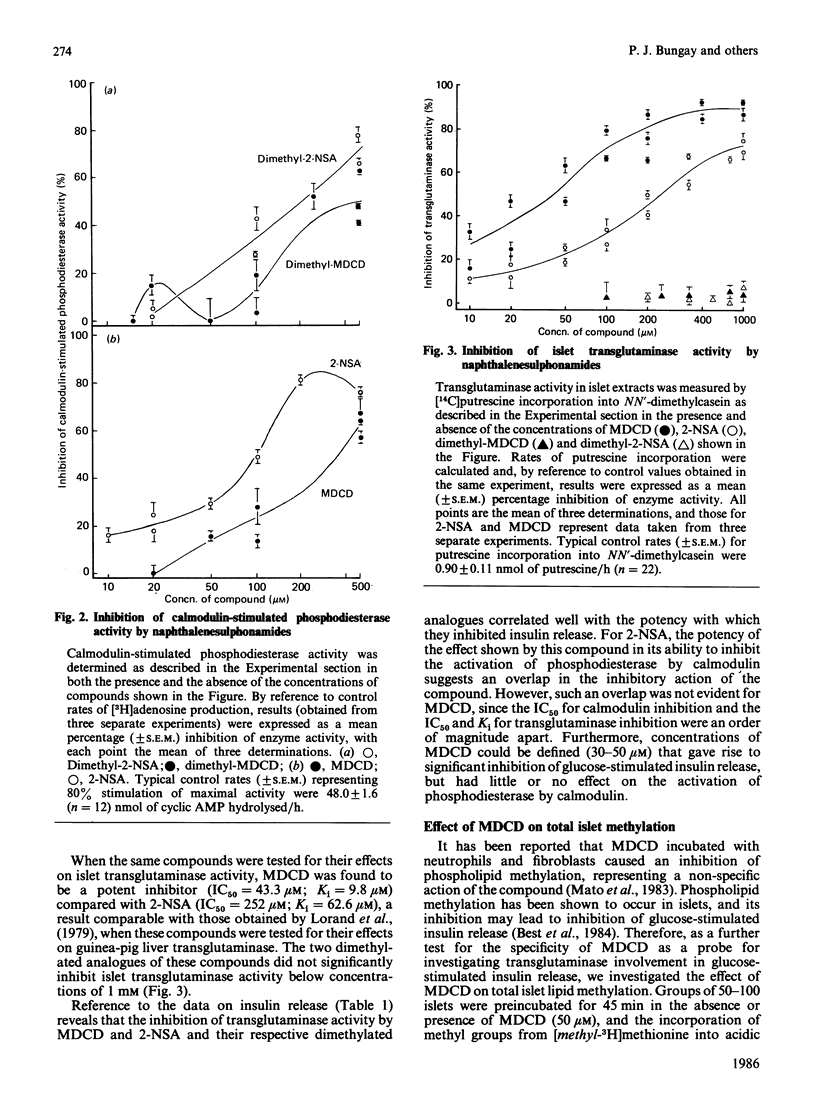
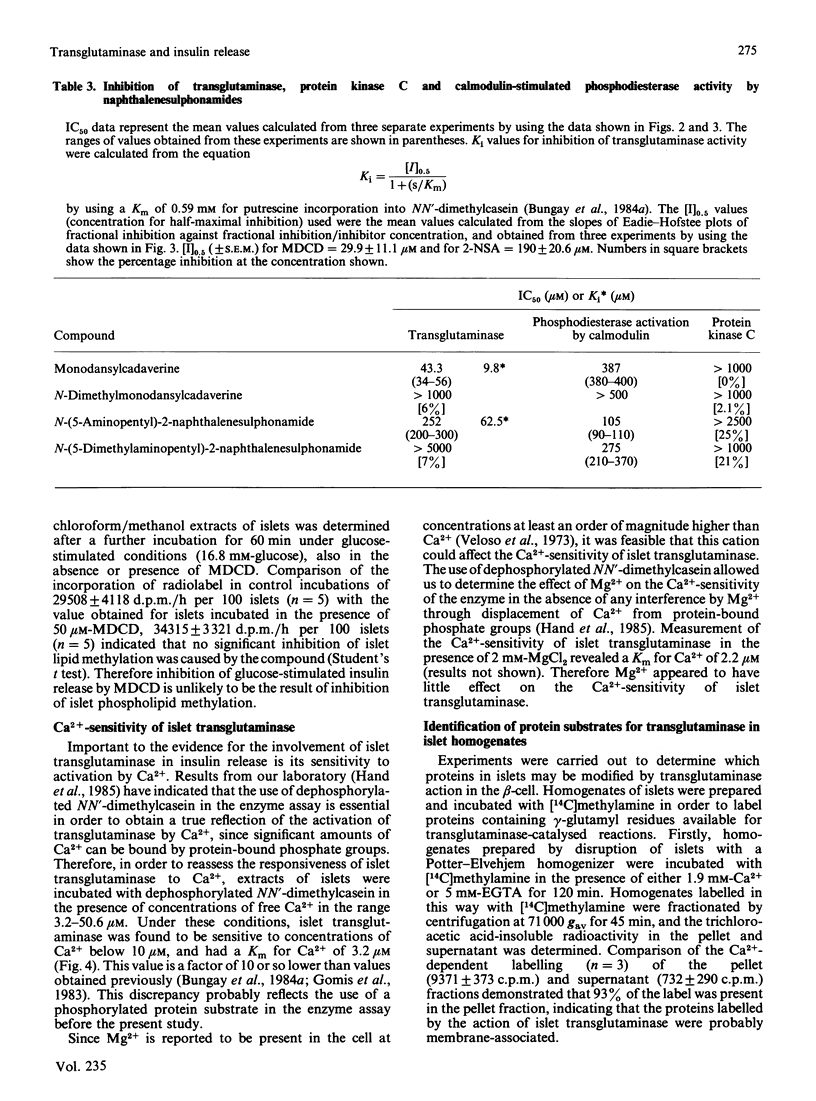
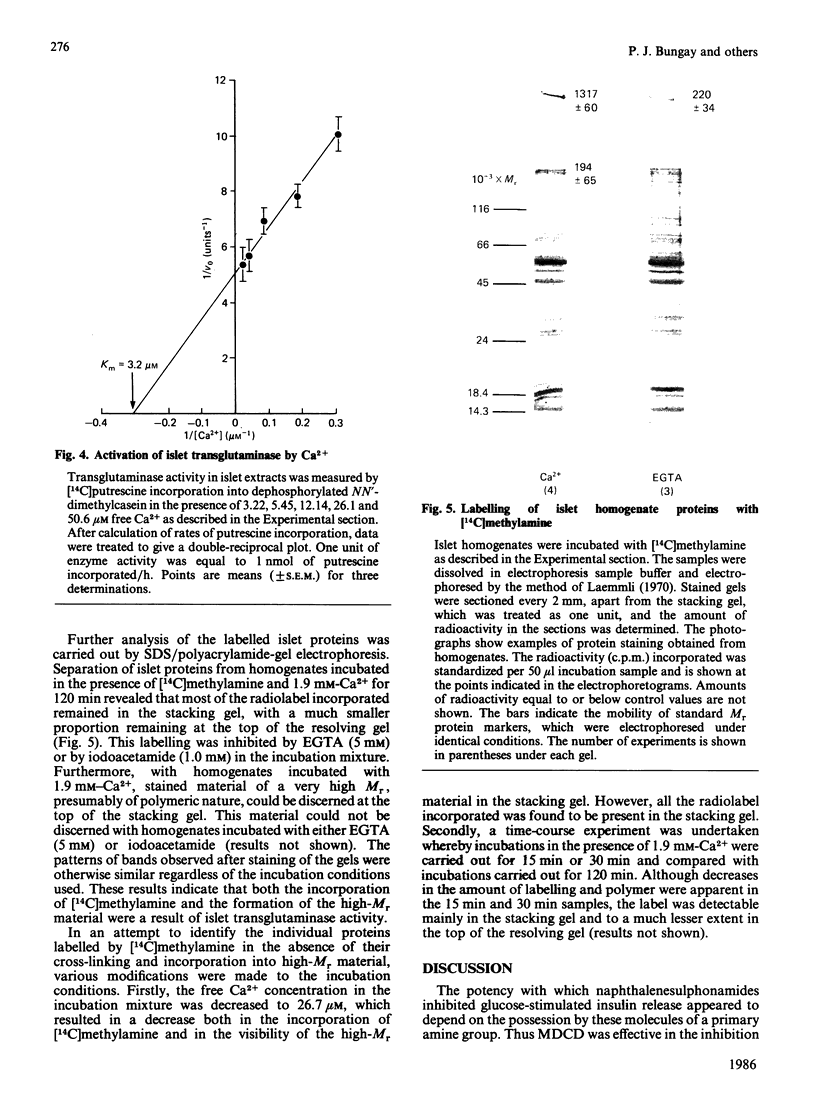
Preincubation of rat islets of Langerhans with the potent inhibitors of islet transglutaminase activity, monodansylcadaverine (30-100 microM) and N-(5-aminopentyl)-2-naphthalenesulphonamide (100-200 microM), led to significant inhibition of glucose-stimulated insulin release from islets. In contrast, the respective N'-dimethylated derivatives of these two compounds, which did not inhibit islet transglutaminase activity, were much less effective as inhibitors of glucose-stimulated insulin release. None of the compounds inhibited rat spleen protein kinase C activity at concentrations which gave rise to inhibition of glucose-stimulated insulin release. When tested for their effects on calmodulin-stimulated bovine heart phosphodiesterase activity, of the compounds that inhibited insulin release, only monodansylcadaverine did not act as an effective antagonist of calmodulin at concentrations (up to 50 microM) that gave rise to significant inhibition of glucose-stimulated insulin release. Furthermore, at 50 microM, monodansylcadaverine did not inhibit methylation of islet lipids. The inhibition of glucose-stimulated insulin release by monodansylcadaverine is therefore likely to be attributable to its interference with islet transglutaminase activity. The sensitivity of islet transglutaminase to activation by Ca2+ was investigated by using a modified assay incorporating dephosphorylated NN'-dimethylcasein as a substrate protein. The Km for Ca2+ obtained (approx. 3 microM) was an order of magnitude lower than previously reported for the islet enzyme [Bungay, Potter & Griffin (1984) Biochem. J. 219, 819-827]. Mg2+ (2 mM) was found to have little effect on the sensitivity of the enzyme to Ca2+. Investigation of the endogenous substrate proteins of islet transglutaminase by using the Ca2+-dependent incorporation of [14C]methylamine into proteins of islet homogenates demonstrated that most of the incorporated radiolabel was present in cross-linked polymeric aggregates which did not traverse 3% (w/v) acrylamide gels. The radiolabelled polymeric aggregates were present in 71 000 g-sedimented material of homogenates, and their formation was transglutaminase-mediated. These findings provide new evidence for the involvement of islet transglutaminase in the membrane-mediated events necessary for glucose-stimulated insulin release.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best L., Lebrun P., Saceda M., Garcia-Morales P., Hubinont C., Juvent M., Herchuelz A., Malaisse-Lagae F., Valverde I., Malaisse W. J. Impairment of insulin release by methylation inhibitors. Biochem Pharmacol. 1984 Jul 1;33(13):2033–2039. doi: 10.1016/0006-2952(84)90570-7. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Dowben R. M., Matacic S., Loewy A. G. Isopeptide bonds in membrane proteins from eukaryotic cells. Biochim Biophys Acta. 1973 Jan 2;291(1):149–155. doi: 10.1016/0005-2736(73)90070-9. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J., Hawkins M., Swanson P., Griffin M., Lorand L. An immunochemical approach for the analysis of membrane protein alterations in Ca2+-loaded human erythrocytes. J Supramol Struct Cell Biochem. 1981;16(3):289–301. doi: 10.1002/jsscb.1981.380160309. [DOI] [PubMed] [Google Scholar]
- Bungay P. J., Potter J. M., Griffin M. A role for polyamines in stimulus-secretion coupling in the pancreatic beta-cell. Biosci Rep. 1984 Oct;4(10):869–877. doi: 10.1007/BF01138169. [DOI] [PubMed] [Google Scholar]
- Bungay P. J., Potter J. M., Griffin M. The inhibition of glucose-stimulated insulin secretion by primary amines. A role for transglutaminase in the secretory mechanism. Biochem J. 1984 May 1;219(3):819–827. doi: 10.1042/bj2190819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. D., Holbrook J. J. Calcium and the assays of human plasma clotting factor XIII. Biochem J. 1974 Jul;141(1):71–78. doi: 10.1042/bj1410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. J., Davies D. R., Levitzki A., Maxfield F. R., Milhaud P., Willingham M. C., Pastan I. H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980 Jan 10;283(5743):162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- Deleers M., Mahy M., Malaisse W. J. Glucose increases cytosolic Ca2+ activity in pancreatic islet cells. Biochem Int. 1985 Jan;10(1):97–103. [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Fésüs L., Sándor M., Horváth L. I., Bagyinka C., Erdei A., Gergely J. Immune-complex-induced transglutaminase activation: its role in the Fc-receptor-mediated transmembrane effect on peritoneal macrophages. Mol Immunol. 1981 Jul;18(7):633–638. doi: 10.1016/0161-5890(81)90034-1. [DOI] [PubMed] [Google Scholar]
- Gagliardino J. J., Harrison D. E., Christie M. R., Gagliardino E. E., Ashcroft S. J. Evidence for the participation of calmodulin in stimulus-secretion coupling in the pancreatic beta-cell. Biochem J. 1980 Dec 15;192(3):919–927. doi: 10.1042/bj1920919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis R., Sener A., Malaisse-Lagae F., Malaisse W. J. Transglutaminase activity in pancreatic islets. Biochim Biophys Acta. 1983 Nov 8;760(3):384–388. doi: 10.1016/0304-4165(83)90378-1. [DOI] [PubMed] [Google Scholar]
- Günzler V., Schopf R. E., Hanauske-Abel H. M., Schulte-Wissermann H. Transglutaminase and polyamine dependence of effector functions of human immunocompetent cells. The effect of specific inhibitors on lymphocyte proliferation and granulocyte chemiluminescence. FEBS Lett. 1982 Dec 27;150(2):390–396. doi: 10.1016/0014-5793(82)80774-6. [DOI] [PubMed] [Google Scholar]
- Hand D., Bungay P. J., Elliott B. M., Griffin M. Activation of transglutaminase at calcium levels consistent with a role for this enzyme as a calcium receptor protein. Biosci Rep. 1985 Dec;5(12):1079–1086. doi: 10.1007/BF01119629. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Determination of total serum insulin (IRI) in insulin-treated diabetic patients. Diabetologia. 1972 Aug;8(4):260–266. doi: 10.1007/BF01225569. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Tanaka T. Naphthalenesulfonamides as calmodulin antagonists. Methods Enzymol. 1983;102:185–194. doi: 10.1016/s0076-6879(83)02019-4. [DOI] [PubMed] [Google Scholar]
- Julian C., Speck N. A., Pierce S. K. Primary amines inhibit the triggering of B lymphocytes to antibody synthesis. J Immunol. 1983 Jan;130(1):91–96. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Lorand L., Parameswaran K. N., Stenberg P., Tong Y. S., Velasco P. T., Jönsson N. A., Mikiver L., Moses P. Specificity of guinea pig liver transglutaminase for amine substrates. Biochemistry. 1979 May 1;18(9):1756–1765. doi: 10.1021/bi00576a019. [DOI] [PubMed] [Google Scholar]
- Lord J. M., Ashcroft S. J. Identification and characterization of Ca2+-phospholipid-dependent protein kinase in rat islets and hamster beta-cells. Biochem J. 1984 Apr 15;219(2):547–551. doi: 10.1042/bj2190547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Neil S., Walker S. W., Senior H. J., Bleehen S. S., Tomlinson S. Effects of extracellular calmodulin and calmodulin antagonists on B16 melanoma cell growth. J Invest Dermatol. 1984 Jul;83(1):15–19. doi: 10.1111/1523-1747.ep12261637. [DOI] [PubMed] [Google Scholar]
- Mato J. M., Pencev D., Vasanthakumar G., Schiffmann E., Pastan I. Inhibitors of endocytosis perturb phospholipid metabolism in rabbit neutrophils and other cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1929–1932. doi: 10.1073/pnas.80.7.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Strominger J. L. Transglutaminase modifies the carboxy-terminal intracellular region of HLA-A and -B antigens. Nature. 1981 Feb 26;289(5800):819–821. doi: 10.1038/289819a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B. Cytosolic free Ca2+ in insulin secreting cells and its regulation by isolated organelles. Experientia. 1984 Oct 15;40(10):1052–1060. doi: 10.1007/BF01971451. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A., Gomis R., Lebrun P., Herchuelz A., Malaisse-Lagae F., Malaisse W. J. Methylamine and islet function: possible relationship to Ca2+-sensitive transglutaminase. Mol Cell Endocrinol. 1984 Jul;36(3):175–180. doi: 10.1016/0303-7207(84)90033-9. [DOI] [PubMed] [Google Scholar]
- Van Leuven F., Cassiman J. J., Van Den Berghe H. Primary amines inhibit recycling of alpha 2M receptors in fibroblasts. Cell. 1980 May;20(1):37–43. doi: 10.1016/0092-8674(80)90232-9. [DOI] [PubMed] [Google Scholar]
- Veloso D., Guynn R. W., Oskarsson M., Veech R. L. The concentrations of free and bound magnesium in rat tissues. Relative constancy of free Mg 2+ concentrations. J Biol Chem. 1973 Jul 10;248(13):4811–4819. [PubMed] [Google Scholar]
- Wani M. C., Campbell H. F., Brine G. A., Kepler J. A., Wall M. E., Levine S. G. Plant antitumor agents. IX. The total synthesis of dl-camptothecin. J Am Chem Soc. 1972 May 17;94(10):3631–3632. doi: 10.1021/ja00765a064. [DOI] [PubMed] [Google Scholar]
- Wise B. C., Glass D. B., Chou C. H., Raynor R. L., Katoh N., Schatzman R. C., Turner R. S., Kibler R. F., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II. Substrate specificity and inhibition by various agents. J Biol Chem. 1982 Jul 25;257(14):8489–8495. [PubMed] [Google Scholar]
- Wise B. C., Raynor R. L., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. I. Purification and general properties. J Biol Chem. 1982 Jul 25;257(14):8481–8488. [PubMed] [Google Scholar]
- Wollheim C. B., Pozzan T. Correlation between cytosolic free Ca2+ and insulin release in an insulin-secreting cell line. J Biol Chem. 1984 Feb 25;259(4):2262–2267. [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]



