Abstract
In the last few years, next-generation sequencing (NGS) has emerged as a technology for laboratory diagnosis of many culture-negative infections and slow-growing microorganisms. In this study, we describe the use of metagenomic NGS (mNGS) for rapid diagnosis of T. marneffei infection in a 37-year-old renal transplant recipient who presented with chronic pneumonia syndrome. Bronchoalveolar lavage for mNGS was positive for T. marneffei sequence reads. Prolonged incubation of the bronchoalveolar lavage revealed T. marneffei colonies after 6 days of incubation. Analysis of 23 cases of T. marneffei infections in renal transplant recipients from the literature revealed that the number of cases ranged from 1 to 4 cases per five years from 1990 to 2020; but increased rapidly to 9 cases from 2021 to 2023, with 7 of them diagnosed by NGS. Twenty of the 23 cases were from T. marneffei-endemic areas [southern part of mainland China (n = 9); Hong Kong (n = 4); northeastern India (n = 2); Indonesia (n = 1) and Taiwan (n = 4)]. For the 3 patients from non-T. marneffei-endemic areas [United Kingdom (n = 2) and Australia (n = 1)], they had travel histories to China and Vietnam respectively. The time taken for diagnosis by mNGS [median 1 (range 1 to 2) day] was significantly shorter than that for fungal culture [median 6 (range 3 to 15) days] (P = 0.002). mNGS is useful for picking up more cases of T. marneffei infections in renal transplant recipients as well as providing a rapid diagnosis. Talaromycosis is an emerging fungal infection in renal transplant recipients.
Keywords: Talaromyces marneffei, Renal transplant, Next-generation sequencing, Rapid diagnosis
Introduction
Talaromyces (Penicillium) marneffei is the most important pathogenic thermally dimorphic fungus causing systemic mycosis in Southeast Asia [1–3]. T. marneffei infection is endemic in tropical regions, especially Thailand, Vietnam, northeastern India, Southern China, Hong Kong, Taiwan, Laos, Malaysia, Myanmar, Cambodia and Laos [4]. Bamboo rats (Rhizomys spp. and Cannomys spp.) and soil from their burrows are considered to be important enzootic and environmental reservoirs of T. marneffei, respectively [5–7]. Historically, T. marneffei infection in human has been considered to be almost exclusively associated with acquired immunodeficiency syndrome (AIDS) caused by human immunodeficiency virus (HIV) infection [4, 8]. In some regions such as Hong Kong and southern China, T. marneffei infection has long been considered as one of the top three AIDS-defining opportunistic infections, alongside tuberculosis and cryptococcosis [9, 10].
In recent years, improved treatment of HIV infection with highly active antiretroviral therapy and control of the HIV/AIDS epidemic with other measures have led to a change in the epidemiology of T. marneffei infection, with an increasing number and proportion of cases being reported in non-HIV-infected patients who had other immunocompromising conditions [11–14]. For example, we have described the first case of fatal T. marneffei infection in a patient with underlying autoimmune hepatitis a few years ago [11]. However, making a diagnosis of T. marneffei infection requires a high index of suspicion, ordering the appropriate laboratory tests and alerting the clinical microbiology laboratory on such a suspicion, which is sometimes not easy particularly in geographical regions that are not endemic with this fungus. This is because isolating T. marneffei from clinical specimens often takes at least five days and most cultures will be discarded by that time.
In the last few years, next-generation sequencing (NGS) has emerged as a technology for laboratory diagnosis of many culture-negative infections [15, 16]. We have recently reported its application in confirming the first case of listeria meningitis in a patient with autoantibody against interferon gamma and fatal Nocardia kroppenstedtii bacteremic pneumonia and empyema thoracis as well as understanding the spectrum of Q fever, Whipple disease, fungal infections and culture-negative meningitis and encephalitis [17–21]. In this study, we describe the use of metagenomic NGS (mNGS) for rapid diagnosis of T. marneffei infection in a renal transplant recipient. The epidemiology of this clinical entity as well as the usefulness of mNGS for its rapid diagnosis in comparison to traditional fungal culture are also analyzed and discussed.
Materials and Methods
Ethical Statement
This study was approved by the Institutional Review Board of The University of Hong Kong—Shenzhen Hospital ([2022]120), and informed consent was signed.
Index Patient
The index patient in this study was hospitalized in The University of Hong Kong—Shenzhen Hospital, Shenzhen, China. This 1400-bed multi-specialty hospital was established in 2012 and provides primary to tertiary medical services to the residents of Shenzhen city in both inpatient and outpatient settings. Supported through the policy from the government of Shenzhen, the hospital is established as a reform model medical institution in China, and many new medical technologies can be introduced to the hospital first.
Microbiological Tests
Clinical specimens collected from the index patient were handled according to the standard operating protocols [22]. For fungal culture, respiratory specimens, including bronchoalveolar lavage fluid (BAL), tracheal aspirate and sputum, as well as abscess aspirate were each inoculated onto two Sabouraud dextrose agar (SDA) plates and one triphenyltetrazolium chloride (TTC) SDA plate. One of the SDA plates and the TTC SDA plate were incubated at 37 °C, and the other SDA plate incubated at 25 °C. The agar plates were examined daily for the first 5 days, and then twice weekly afterwards. All suspected colonies were identified based on morphological characteristics, conventional biochemical methods and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) Microflex LT/SH (Bruker Daltonics, Bremen, Germany) and the spectra analyzed with IVD MALDI Biotyper 4.2.100.19 and reference library DB-11897_4274 (Bruker Daltonics) [23, 24].
Next-Generation Sequencing
BAL collected from the index patient was sent to GensKey Laboratory (Beijing, China) for NGS analysis. Interpretation of the sequencing results and determination on whether the sequences were from pathogens, latent infections or contaminants were performed by correlating them with the clinical details, laboratory data and radiological findings of the patient.
Epidemiology of T. marneffei Infections in Renal Transplant Recipients
“Talaromyces marneffei” and “renal transplant” were used as keywords for PubMed search on 30th November, 2023. All the 20 articles were retrieved and the clinical and laboratory data of the 22 cases of talaromycosis in renal transplant recipients described in these 20 articles were analyzed [25–44].
Statistical Analysis
The time between collection of specimen and diagnosis using NGS and culture were compared using Mann–Whitney U test.
Results
Index Patient
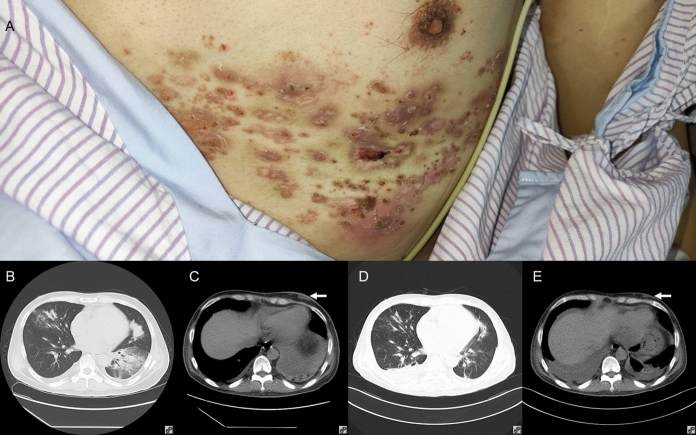
A 37-year-old Chinese man was admitted because of fever, cough and shortness of breath for one day. Three years ago, the patient underwent cadaveric renal transplantation because of chronic glomerulonephritis. Since then, he received tacrolimus 0.5 mg twice daily, mycophenolate 540 mg twice daily and prednisone 10 mg once daily as anti-rejection therapy. Six months ago, he had SARS coronavirus-2 infection, developed acute renal failure and was put on hemodialysis for two months. Two weeks ago, he had herpes zoster of the left T5-T7 regions and was put on valacyclovir. One day ago, he developed gradual onset of fever, cough and shortness of breath. His body temperature was 42 °C. The heart rate was 112/min and respiratory rate 17/min. Crusting due to herpes zoster as well as painless subcutaneous nodules was observed on the left chest wall (Fig. 1A). His SaO2 was 97.6% on room air. The total white cell count was 2.09 × 109/L, with a neutrophil count of 1.68 × 109/L, a lymphocyte count of 0.26 × 109/L and a monocyte count of 0.13 × 109/L. The patient’s hemoglobin level was 60 g/L and his platelet count was 87 × 109/L. The serum creatinine level was 606 μmol/L. Liver enzymes and total bilirubin levels were normal. C-reactive protein was 133.37 mg/L, and procalcitonin was 7.42 ng/mL. Computed tomography (CT) scan of the thorax revealed multiple patchy infiltrates and consolidation with air bronchograms in both lungs, bilateral pleural effusion and multiple subcutaneous nodular foci of low density in the left thoracic wall (Fig. 1B and C). Blood culture was performed. Blood was also collected for serum galactomannan antigen and 1,3-β-D-glucan tests. Bronchoscopy was also performed and BAL was collected for bacterial, fungal and mycobacterial culture as well as mNGS. Empirical intravenous piperacillin-tazobactam, doxycycline, cotrimoxazole, voriconazole, micafungin, valacyclovir and immunoglobulin were commenced. Tacrolimus and mycophenolate were stopped and prednisone was switched to methylprednisolone, 40 mg once daily. Hemodialysis was also commenced.
Fig. 1.
Clinical and radiological assessment of the index patient. Panel A: Clinical photo on admission, showing subcutaneous nodules on left chest wall and crusting due to healing herpes zoster. Panel B: Thoracic computerized tomography (CT) on admission, showing multiple patchy infiltrates and consolidation with air bronchograms in both lungs and bilateral pleural effusion. Panel C: Mediastinum window of thoracic CT on admission, showing multiple subcutaneous nodules of low density in the left chest wall (arrow). Panel D: Thoracic CT two weeks after commencement of antifungal therapy, showing resolving lung infiltrates and consolidation, and residual pleural effusion. Panel E: Mediastinum window of thoracic CT two weeks after commencement of antifungal therapy, showing shrinking of the subcutaneous nodules in left chest wall (arrow)
After 10 h of incubation, blood culture was positive for Acinetobacter pittii. The optical density index of serum galactomannan was 0.86 (normal range: < 0.5). Serum 1,3-β-D-glucan was < 37.5 pg/mL. BAL for mNGS was positive for sequences of A. pittii, T. marneffei, and other oropharyngeal microbes and latent herpesviruses (Table 2). A. pittii, Staphylococcus aureus, Streptococcus mitis and Streptococcus milleri were recovered from BAL. Since BAL was positive for T. marneffei sequences, prolonged incubation of the BAL-inoculated plates was performed. The antimicrobial regimen was modified to intravenous meropenem for A. pittii bacteremia, intravenous voriconazole for T. marneffei infection and oral cotrimoxazole for Pneumocystis jirovecii prophylaxis. Fever and shortness of breath gradually subsided. After a total of six days of incubation, T. marneffei with characteristic thermal dimorphism and diffusible red pigment production by the mycelial form was isolated from the BAL. CT scan of the thorax performed two weeks after commencement of voriconazole showed that the lung infiltrates and consolidation were resolved and the subcutaneous nodules have also shrunk significantly (Fig. 1D and E). The patient was discharged with oral voriconazole and cotrimoxazole. There was no relapse of the illness up to the time of writing, six months after discharge.
Table 2.
Microorganisms detected in bronchoalveolar lavage fluid of the index patient by mNGS
| Microorgansims | Number of reads |
|---|---|
| Acinetobacter pittii | 1,649,958 |
| Streptococcus constellatus | 68,863 |
| Staphylococcus aureus | 61,410 |
| Talaromyces marneffei | 200 |
| Varicella zoster virus | 21,541 |
| Herpes simplex virus-1 | 3693 |
| Epstein-Barr virus | 773 |
| Human herpes virus-7 | 273 |
| Human herpes virus-6B | 12 |
| Torque teno virus | 66 |
| Cytomegalovirus | 121 |
| Streptococcus salivarius | 598,829 |
| Streptococcus oralis | 184,690 |
| Prevotella melaninogenica | 702,644 |
| Actinomyces graevenitzii | 739,902 |
| Peptostreptococcus stomatis | 364,570 |
| Neisseria elongate | 54,683 |
| Alloprevotella tannerae | 214,813 |
| Gemella morbillorum | 142,707 |
| Porphyromonas gingivalis | 118,646 |
| Solobacterium moorei | 147,988 |
| Candida parapsilosis | 24 |
Epidemiology of T. marneffei Infections in Renal Transplant Recipients
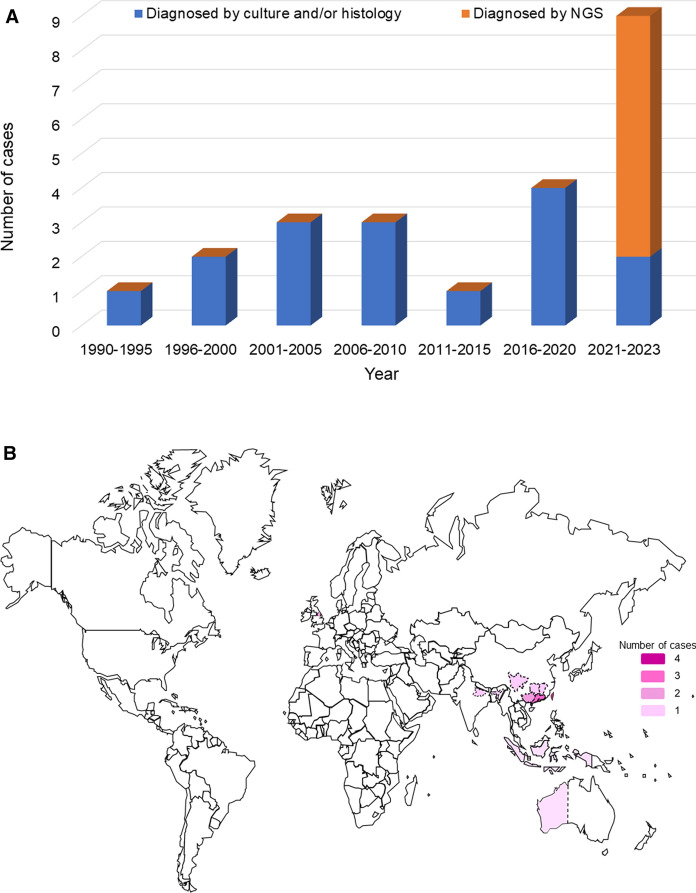
Including the index case of the present report, a total of 23 cases of T. marneffei infections in renal transplant recipients were identified in the literature (Table 1, Fig. 2A). These 23 cases were from the southern part of mainland China (n = 9), Taiwan (n = 4), Hong Kong (n = 4), India (n = 2), the United Kingdom (n = 2), Indonesia (n = 1) and Australia (n = 1) (Fig. 2B). Clinical details were available for 21 cases (Table 1). The male-to-female ratio is 15:6, and the median age is 47 (range 27–67) years. Thirteen (61.9%) of the 21 cases had disseminated T. marneffei infection, with pneumonia present in nine (42.9%) patients (Table 1).
Table 1.
Clinical and laboratory characteristics of renal transplant recipients with Talaromyces marneffei infection
| Patient number | Year | Sex/Age | Geographical region | Clinical syndrome(s) of T. marneffei infection | Diagnostic methods for detection of T. marneffei | Interval between specimen collection and diagnosis of T. marneffei infection (days) | Antifungal therapy | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| NGS | Culture | ||||||||
| 1 [25] | 1990 | F/27 | Hong Kong | Disseminated T. marneffei infection | Blood, urine, sputum, and marrow for culture | N/A | Not mentioned | Amphotericin B, 5-fluorocytosine | Improved |
| 2 [26] | 1998 | M/43 | Taiwan | Disseminated T. marneffei infection | Ascites and blood for culture | N/A | Not mentioned | Amphotericin B | Succumbed |
| 3 [27] | 1999 | M/33 | Taiwan | Disseminated T. marneffei infection complicated with intestinal mycoses, septic shock | Blood for culture | N/A | 8 | None | Succumbed |
| 4 [28] | 2003 | M/47 | Taiwan | Disseminated T. marneffei infection complicated with skin abscess, osteomyelitis | Blood and debrided specimens for culture | N/A | Not mentioned | Amphotericin B, itraconazole | Improved |
| 5 [29] | 2004 | M/38 | Hong Kong | Disseminated T. marneffei infection complicated with pneumonia, lymphadenitis | Blood and bone marrow for culture | N/A | 15 | Amphotericin B, itraconazole | Improved |
| 6 [30] | 2004 | M/46 | Indonesia | Disseminated T. marneffei infection | Skin lesion for culture | N/A | Not mentioned | Not mentioned | Succumbed |
| 7 [31] | 2008 | Not mentioned | Hong Kong | Not mentioned | Not mentioned | N/A | Not mentioned | Not mentioned | Not mentioned |
| 8 [31] | 2008 | Not mentioned | Hong Kong | Not mentioned | Not mentioned | N/A | Not mentioned | Not mentioned | Not mentioned |
| 9 [32] | 2010 | F/42 | Taiwan | T. marneffei osteomyelitis | Bone tissue for culture | N/A | Not mentioned | Amphotericin B, itraconazole | Improved |
| 10 [33] | 2011 | M/67 | Western Australia | Disseminated T. marneffei infection complicated with peritonitis secondary to perforated sigmoid colon diverticulum | Blood and peritoneal fluid for culture | N/A | 3 | Amphotericin B, itraconazole | Improved |
| 11 [34] | 2017 | M/51 | Guangdong | Disseminated T. marneffei infection | Blood for culture | N/A | 3 | Amphotericin B, itraconazole | Improved |
| 12 [35] | 2018 | F/53 | Greater Manchester | Lung mass due to T. marneffei | Lung mass tissue for culture | N/A | Not mentioned | Amphotericin B, itraconazole | Improved |
| 13 [36] | 2020 | F/53 | Greater Manchester | Lung mass and lymphadenitis due to T. marneffei | Lung mass and lymph node biopsy for culture | N/A | Not mentioned | Amphotericin B, itraconazole | Improved |
| 14 [37] | 2020 | M/56 | Assam | Lung mass due to T. marneffei | Lung mass tissue for culture | N/A | 7 | Amphotericin B, itraconazole | Improved |
| 15 [38] | 2021 | M/34 | Sichuan | Disseminated T. marneffei infection complicated with pneumonia | BAL for NGS and culture, blood for culture | 3 | Not mentioned | Posaconazole | Improved |
| 16 [39] | 2021 | F/47 | Hunan | Disseminated T. marneffei infection complicated with urinary tract infection | Blood and urine for culture | N/A | 6 | Voriconazole, itraconazole | Improved |
| 17 [40] | 2022 | M/51 | Jiangxi | T. marneffei pneumonia | BAL for NGS and culture | 2 | 7 | Voriconazole | Improved |
| 18 [41] | 2022 | M/54 | Guangdong | T. marneffei laryngitis | BAL for NGS and culture, sputum for culture | 1 | 7 | Voriconazole | Improved |
| 19 [42] | 2022 | F/41 | Bihar | T. marneffei skin abscess and skull osteomyelitis | Scalp lesion aspiration for culture | N/A | 6 | Itraconazole | Improved |
| 20 [43] | 2022 | M/61 | Guangxi | T. marneffei pneumonia | BAL for NGS | Not mentioned | N/A | Voriconazole, caspofungin | Succumbed |
| 21 [43] | 2022 | M/55 | Guangxi | Disseminated T. marneffei infection | Blood for NGS | 1 | N/A | Voriconazole, caspofungin | Succumbed |
| 22 [44] | 2023 | M/31 | Hainan | Disseminated T. marneffei infection complicated with pneumonia, lymphadenitis | Blood and BAL for NGS, blood for culture | 1 | 7 | Voriconazole, amphotericin B | Improved |
| 23 [Present case] | 2023 | M/37 | Guangdong | Disseminated T. marneffei infection and pneumonia complicated with septic shock | BAL for NGS and culture | 1 | 6 | Voriconazole, micafungin, valacyclovir | Improved |
F female, M male, N/A not applicable, NGS next-generation sequencing
Fig. 2.
Temporal and geographical distribution of T. marneffei infections in renal transplant recipients. Panel A: Number of reported cases of T. marneffei infections in renal transplant recipients from 1990 to 2023. Panel B: Global distribution of reported cases of T. marneffei infections in renal transplant recipients. The number of patients is depicted in different grades of purple color
Rapid Diagnosis of T. marneffei Infections in Renal Transplant Recipients by NGS
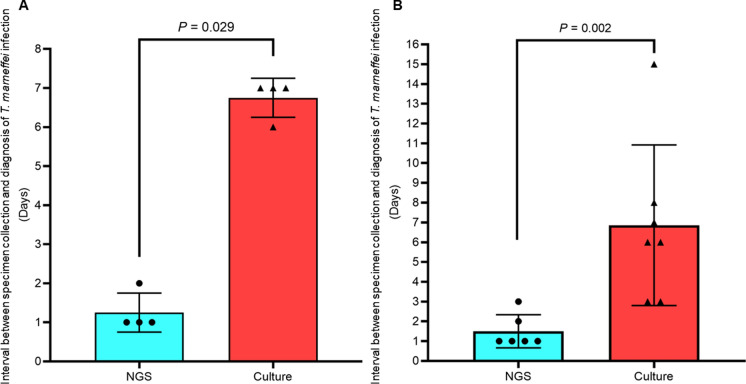
Among all the cases of T. marneffei infections in renal transplant recipients, seven were diagnosed by mNGS (Cases 15, 17, 18, 20, 21, 22 and 23, Table 1), with a median time between collection of specimen and diagnosis of 1 (range 1–2) day. In five of the seven cases, T. marneffei was also recovered from specimens sent for culture. The time between collection of specimen and positive culture result was mentioned in 4 cases, the median of which was 7 (range 6–7) days, which is significantly longer than that by mNGS (P = 0.029) (Fig. 3A). As for the other cases of T. marneffei infections in renal transplant recipients diagnosed by culture in the literature, the time between collection of specimen and positive culture result was mentioned in 7 cases, the median of which was 6 (range 3–15 days, which is also significantly longer than the cases diagnosed by mNGS (P = 0.002) (Fig. 3B).
Fig. 3.
Comparison of time taken for diagnosis of T. marneffei infection by mNGS and culture. Panel A: The 4 cases diagnosed by both mNGS and culture. Panel B: The 6 cases diagnosed by mNGS and the 7 cases diagnosed only by culture
Discussion
In this study, we describe an emergence of T. marneffei infections in renal transplant recipients and showed the role of mNGS in picking up difficult-to-diagnose cases. Among the 23 cases of T. marneffei infections in renal transplant recipients, 20 were from T. marneffei endemic areas (Fig. 2B). The nine patients from mainland China were from Guangdong (n = 3) (Cases 11, 18, and 23), Guangxi (n = 2) (Cases 20 and 21), Hainan (n = 1) (Case 22), Hunan (n = 1) (Case 16), Jiangxi (n = 1) (Case 17) and Sichuan (n = 1) (Case 15), which were all located in the southern part of China (Fig. 2B). The two patients from India were from Dibrugarh, Assam (Case 14) and Patna, Bihar (Case 19) respectively. These two regions are in northeastern India (Fig. 2B), where T. marneffei is endemic. For the three patients that were from non-T. marneffei-endemic areas, two were from the United Kingdom (Cases 12 and 13) and one from Australia (Case 10). It is notable that although the two patients from the United Kingdom resided in Manchester, they had travel histories to China. One had traveled to South China whereas the other to Beijing, Xi’an, Sichuan and Yunnan. As for the Australian from Perth, he also had travel histories to Hanoi, HaLong Bay and Sapa of Vietnam, where T. marneffei is endemic. It is interesting to note that the number of cases of T. marneffei infections in renal transplant recipients ranged from one to four cases per five years from 1990 to 2020, but increased rapidly in the last few years, with already nine cases reported from 2021 to 2023 (Fig. 2A). Seven of these nine cases were diagnosed by mNGS, indicating that this technology has resulted in an apparent emergence of this disease entity and that this infection has probably been underestimated previously. It is also important to note that among these seven cases, T. marneffei sequence reads were positive in the BAL and blood samples in six (Cases 15, 17, 18, 20, 22 and 23) and two (Cases 21 and 22) patients respectively. Although formal comparison between the sensitivities of different samples is yet to be performed, it seems that BAL is a reasonably promising specimen when the patient had pneumonia.
In addition to picking up more cases of T. marneffei infections in renal transplant recipients, NGS is also crucial in providing a rapid diagnosis for this clinical entity. Definitive laboratory diagnosis of T. marneffei infection was traditionally achieved by isolating the fungus in clinical specimens and recognizing its unique phenotypic characteristics of thermal dimorphism, diffusible red pigment production by its filamentous form as well as microscopic features of the yeast and filamentous forms, etc. [23]. Recently, we have demonstrated that MALDI-TOF MS is useful for the identification of T. marneffei cultures as [3, 24]. However, isolating the fungus from clinical samples usually take at least five days. For example, for the patients with T. marneffei infections in renal transplant recipients described and reviewed in the present study, a median of six or seven days was required (Fig. 3). On the other hand, when mNGS was used, the laboratory diagnosis was made within one day in most of the cases (Fig. 3). In fact, for the index case described in the present study, T. marneffei was isolated only because prolonged incubation of the BAL sample was requested due to the fact that T. marneffei sequences were observed in the BAL submitted for mNGS analysis. If the sample was not sent for mNGS analysis, the sample would probably have been discarded and the diagnosis markedly delayed or missed. It is important to note that during mNGS analysis, in addition to the microbes that cause the present clinical problem, many other colonizers and/or contaminants are usually also being sequenced. For example, in the present case, in addition to A. pittii (also recovered in blood culture) and T. marneffei, sequence reads of other oropharyngeal microbes and latent herpesviruses were also detected in the patient’s BAL by mNGS analysis (Table 2). Results of mNGS analysis must be interpreted together with the clinical context of the patient in order to determine which ones are the real causative agents.
A high index of suspicion is required for making an early diagnosis of T. marneffei infections in HIV-negative patients. The clinical presentation of T. marneffei infections is always non-specific, with fever, weight loss, lymphadenopathy and hepatosplenomegaly being some of the more common symptoms and signs. It makes it particularly difficult for clinicians who are not familiar with this fungal infection. For example, in the present patient, the skin lesion might have prompted some physicians to perform skin biopsy and examine it microscopically, which may reveal the characteristic yeast cells with septum formation and give a clue to the culprit [45]. In addition to the difficulty in isolating the fungus, it has been shown that T. marneffei infection often resulted in false-positive galactomannan antigen test [46], which will give the clinician a wrong impression that the patient may be suffering from aspergillosis, as invasive Aspergillus infections are as important as talaromycosis in these immunocompromised patients. For the underlying disease, traditionally this infection manifested mostly in HIV-positive patients. In recent years, as we have been using more immunosuppressive regimens, such as targeted therapy; and technology advancement has also resulted in the recognition of more primary immunodeficiency syndromes and their corresponding genetic origins, T. marneffei has emerged in certain groups of HIV-negative patients [11–14, 17]. As for the geographical distribution, T. marneffei infections occurred almost exclusively in Southeast Asia, where T. marneffei infection is regarded as an AIDS-defining condition in HIV-positive patients. As shown in the present study, only 3 of the 23 renal transplant recipients with T. marneffei infections were from non-endemic areas; but in fact, they all have travel histories to Southeast Asia [33, 35, 36]. As a result of all these factors, making a diagnosis of T. marneffei infection is particularly difficult in HIV-negative patients, especially if the individual is not from endemic regions. With globalization and more frequent travels, we anticipate that there will be more T. marneffei infections in the western world. A high index of suspicion and ordering the appropriate laboratory tests are essential for timely commencement of proper antifungal treatment for T. marneffei.
Acknowledgements
We are grateful to the staff at the Department of Infectious Diseases and Microbiology, The University of Hong Kong – Shenzhen Hospital for their technical support and assistance.
Author Contributions
Fanfan Xing, Susanna K. P. Lau and Patrick C. Y. Woo conceptualized and designed the study; Fanfan Xing, Shan Zou and Patrick C. Y. Woo performed data collection and curation; Fanfan Xing, Chaowen Deng and Patrick C. Y. Woo performed data analysis; Fanfan Xing, Simon K. F. Lo, Susanna K. P. Lau and Patrick C. Y. Woo audited the methodology; Simon K. F. Lo supervised the laboratory administration and resources; Susanna K. P. Lau and Patrick C. Y. Woo supervised the research; Fanfan Xing and Patrick C. Y. Woo wrote the original draft; Fanfan Xing, Chaowen Deng, Shan Zou, Chi-Ching Tsang, Simon K. F. Lo, Susanna K. P. Lau and Patrick C. Y. Woo reviewed, edited and approved the draft.
Funding
This work was partly supported by the Sanming Project of Medicine in Shenzhen [SZSM201911014], the Feature Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE-113-S-0023-A) in Taiwan, as well as the Early Career Researcher Award (2022/2023) from Tung Wah College, Hong Kong.
Declarations
Conflict of interest
Patrick C. Y. Woo has provided scientific advisory/laboratory services for Gilead Sciences, Incorporated; International Health Management Associates, Incorporated; Merck & Corporation, Incorporated; Micología Molecular S.L. and Pfizer, Incorporated. The funding sources had no role in study design, data collection, analysis, interpretation, or writing of the report. The authors alone are responsible for the content and the writing of the manuscript. The other authors report no conflict of interest.
Footnotes
Handling Editor: Cunwei Cao
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Susanna K. P. Lau, Email: skplau@hku.hk
Patrick C. Y. Woo, Email: pcywoo@hku.hk
References
- 1.Tsang CC, Lau SKP, Woo PCY. Sixty years from Segretain’s description: what have we learned and should learn about the basic mycology of Talaromyces marneffei? Mycopathologia. 2019;184(6):721–9. 10.1007/s11046-019-00395-y. [DOI] [PubMed] [Google Scholar]
- 2.Wong SSY, Siau H, Yuen KY. Penicilliosis marneffei–West meets East. J Med Microbiol. 1999;48(11):973–5. 10.1099/00222615-48-11-973. [DOI] [PubMed] [Google Scholar]
- 3.Lau SKP, Xing F, Tsang CC, Tang JYM, Tan YP, Ye H, Lau RWT, Chen JHK, Lo SKF, Woo PCY. Clinical characteristics, rapid identification, molecular epidemiology and antifungal susceptibilities of Talaromyces marneffei infections in Shenzhen, China. Mycoses. 2019;62(5):450–7. 10.1111/myc.12887. [DOI] [PubMed] [Google Scholar]
- 4.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in southeast Asia. Lancet. 1994;344(8915):110–3. 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 5.Ajello L, Padhye AA, Sukroongreung S, Nilakul CH, Tantimavanic S. Occurrence of Penicillium marneffei infections among wild bamboo rats in Thailand. Mycopathologia. 1995;131(1):1–8. 10.1007/BF01103897. (Erratum in: Mycopathologia 1996;135(3):195-7). [DOI] [PubMed] [Google Scholar]
- 6.Chariyalertsak S, Vanittanakom P, Nelson KE, Sirisanthana T, Vanittanakom N. Rhizomys sumatrensis and Cannomys badius, new natural animal hosts of Penicillium marneffei. J Med Vet Mycol. 1996;34(2):105–10. [PubMed] [Google Scholar]
- 7.Gugnani H, Fisher MC, Paliwal-Johsi A, Vanittanakom N, Singh I, Yadav PS. Role of Cannomys badius as a natural animal host of Penicillium marneffei in India. J Clin Microbiol. 2004;42(11):5070–5. 10.1128/JCM.42.11.5070-5075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao C, Xi L, Chaturvedi V. Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: Insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia. 2019;184(6):709–20. 10.1007/s11046-019-00410-2. [DOI] [PubMed] [Google Scholar]
- 9.Li PC, Yeoh EK. Current epidemiological trends of HIV infection in Asia. AIDS Clin Rev. 1992:1–23. [PubMed]
- 10.Chen M, Hu D, Li T, Zheng D, Liao W, Xia X, Cao C. The epidemiology and clinical characteristics of fungemia in a tertiary hospital in Southern China: a 6-year retrospective study. Mycopathologia. 2023;188(4):353–60. 10.1007/s11046-023-00757-7. [DOI] [PubMed] [Google Scholar]
- 11.Wong SCY, Sridhar S, Ngan AHY, Chen JHK, Poon RWS, Lau SKP, Woo PCY. Fatal Talaromyces marneffei infection in a patient with autoimmune hepatitis. Mycopathologia. 2018;183(3):615–8. 10.1007/s11046-017-0239-0. [DOI] [PubMed] [Google Scholar]
- 12.Chan JF, Chan TS, Gill H, Lam FY, Trendell-Smith NJ, Sridhar S, Tse H, Lau SK, Hung IF, Yuen KY, Woo PC. Disseminated Infections with Talaromyces marneffei in Non-AIDS patients given monoclonal antibodies against CD20 and kinase inhibitors. Emerg Infect Dis. 2015;21(7):1101–6. 10.3201/eid2107.150138. (Erratum in: Emerg Infect Dis. 2015;21(10):1877). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo PC, Lau SK, Lau CC, Chong KT, Hui WT, Wong SS, Yuen KY. Penicillium marneffei fungaemia in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. 2005;35(8):831–3. 10.1038/sj.bmt.1704895. [DOI] [PubMed] [Google Scholar]
- 14.Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5(3): e19. 10.1038/emi.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson MR, Naccache SN, Samayoa E, Biagtan M, Bashir H, Yu G, Salamat SM, Somasekar S, Federman S, Miller S, Sokolic R, Garabedian E, Candotti F, Buckley RH, Reed KD, Meyer TL, Seroogy CM, Galloway R, Henderson SL, Gern JE, DeRisi JL, Chiu CY. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–17. 10.1056/NEJMoa1401268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsang CC, Teng JLL, Lau SKP, Woo PCY. Rapid genomic diagnosis of fungal infections in the age of next-generation sequencing. J Fungi. 2021;7(8):636. 10.3390/jof7080636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing F, Hung DLL, Lo SKF, Chen S, Lau SKP, Woo PCY. Next-generation sequencing-based diagnosis of bacteremic Listeria monocytogenes meningitis in a patient with anti-interferon gamma autoantibodies: a case report. Infect Microb Dis. 2022;4(1):44–6. 10.1097/IM9.0000000000000080. [Google Scholar]
- 18.Xing F, Xia Y, Lu Q, Lo SKF, Lau SKP, Woo PCY. Rapid diagnosis of fatal Nocardia kroppenstedtii bacteremic pneumonia and empyema thoracis by next-generation sequencing: a case report. Front Med. 2023;10:1226126. 10.3389/fmed.2023.1226126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing F, Ye H, Deng C, Sun L, Yuan Y, Lu Q, Yang J, Lo SKF, Zhang R, Chen JHK, Chan JFW, Lau SKP, Woo PCY. Diverse and atypical manifestations of Q fever in a metropolitan city hospital: emerging role of next-generation sequencing for laboratory diagnosis of Coxiella burnetii. PLoS Negl Trop Dis. 2022;16(4): e0010364. 10.1371/journal.pntd.0010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing F, Lo SW, Liu M, Deng C, Ye H, Sun L, Yang J, Lo SKF, Lau SKP, Woo PCY. Emergence of Tropheryma whipplei detection in respiratory samples by next-generation sequencing: pathogen or innocent bystander? J Infect. 2023;86(2):154–225. 10.1016/j.jinf.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Xing F, Yang Q, Deng C, Sun L, Luo Z, Ye H, Yang J, Lo SKF, Lau SKP, Woo PCY. Clinical impact of next-generation sequencing on laboratory diagnosis of suspected culture-negative meningitis and encephalitis. J Infect. 2022;85(5):573–607. 10.1016/j.jinf.2022.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Carroll KC, Pfaller MA. Manual of clinical microbiology. 12th ed. Washington: ASM Press; 2019. [Google Scholar]
- 23.Woo PC, Lam CW, Tam EW, Lee KC, Yung KK, Leung CK, Sze KH, Lau SK, Yuen KY. The biosynthetic pathway for a thousand-year-old natural food colorant and citrinin in Penicillium marneffei. Sci Rep. 2014;4:6728. 10.1038/srep06728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau SK, Lam CS, Ngan AH, Chow WN, Wu AK, Tsang DN, Tse CW, Que TL, Tang BS, Woo PC. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for rapid identification of mold and yeast cultures of Penicillium marneffei. BMC Microbiol. 2016;16:36. 10.1186/s12866-016-0656-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan YF, Chow TC. Ultrastructural observations on Penicillium marneffei in natural human infection. Ultrastruct Pathol. 1990;14(5):439–52. 10.3109/01913129009007223. [DOI] [PubMed] [Google Scholar]
- 26.Hung CC, Hsueh PR, Chen MY, Hsiao CH, Chang SC, Luh KT. Invasive infection caused by Penicillium marneffei: an emerging pathogen in Taiwan. Clin Infect Dis. 1998;26(1):202–3. 10.1086/517068. [DOI] [PubMed] [Google Scholar]
- 27.Ko CI, Hung CC, Chen MY, Hsueh PR, Hsiao CH, Wong JM. Endoscopic diagnosis of intestinal penicilliosis marneffei: report of three cases and review of the literature. Gastrointest Endosc. 1999;50(1):111–4. 10.1016/s0016-5107(99)70359-7. [DOI] [PubMed] [Google Scholar]
- 28.Wang JL, Hung CC, Chang SC, Chueh SC, La MK. Disseminated Penicillium marneffei infection in a renal-transplant recipient successfully treated with liposomal amphotericin B. Transplantation. 2003;76(7):1136–7. 10.1097/01.TP.0000088667.02294.E7. [DOI] [PubMed] [Google Scholar]
- 29.Chan YH, Wong KM, Lee KC, Kwok PC, Chak WL, Choi KS, Chau KF, Li CS. Pneumonia and mesenteric lymphadenopathy caused by disseminated Penicillium marneffei infection in a cadaveric renal transplant recipient. Transpl Infect Dis. 2004;6(1):28–32. 10.1111/j.1399-3062.2004.00038.x. [DOI] [PubMed] [Google Scholar]
- 30.Liyan X, Changming L, Xianyi Z, Luxia W, Suisheng X. Fifteen cases of penicilliosis in Guangdong, China. Mycopathologia. 2004;158(2):151–5. 10.1023/b:myco.0000041842.90633.86. [DOI] [PubMed] [Google Scholar]
- 31.Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Med J. 2008;14(2):103–9. [PubMed] [Google Scholar]
- 32.Lin JN, Lin HH, Lai CH, Wang JL, Yu TJ. Renal transplant recipient infected with Penicillium marneffei. Lancet Infect Dis. 2010;10(2):138. 10.1016/S1473-3099(10)70005-0. [DOI] [PubMed] [Google Scholar]
- 33.Hart J, Dyer JR, Clark BM, McLellan DG, Perera S, Ferrari P. Travel-related disseminated Penicillium marneffei infection in a renal transplant patient. Transpl Infect Dis. 2012;14(4):434–9. 10.1111/j.1399-3062.2011.00700.x. [DOI] [PubMed] [Google Scholar]
- 34.Peng J, Chen Z, Cai R, Huang X, Lin L, Liang W, Xiong Z, Chen J, Chen H, Yang Y, Liu S, Jiang Q. Recovery from Talaromyces marneffei involving the kidney in a renal transplant recipient: a case report and literature review. Transpl Infect Dis. 2017. 10.1111/tid.12710. [DOI] [PubMed] [Google Scholar]
- 35.Ma W, Thiryayi SA, Holbrook M, Shelton D, Narine N, Sweeney LC, Augustine T, Bailey S, Al-Najjar H, Rana DN. Rapid on-site evaluation facilitated the diagnosis of a rare case of Talaromyces marneffei infection. Cytopathology. 2018;29(5):497–9. 10.1111/cyt.12563. [DOI] [PubMed] [Google Scholar]
- 36.Vergidis P, Rao A, Moore CB, Rautemaa-Richardson R, Sweeney LC, Morton M, Johnson EM, Borman AM, Richardson MD, Augustine T. Talaromycosis in a renal transplant recipient returning from South China. Transpl Infect Dis. 2021;23(1): e13447. 10.1111/tid.13447. [DOI] [PubMed] [Google Scholar]
- 37.Sethuraman N, Thirunarayan MA, Gopalakrishnan R, Rudramurthy S, Ramasubramanian V, Parameswaran A. Talaromyces marneffei outside endemic areas in India: an emerging infection with atypical clinical presentations and review of published reports from India. Mycopathologia. 2020;185(5):893–904. 10.1007/s11046-019-00420-0. [DOI] [PubMed] [Google Scholar]
- 38.Lang Q, Pasheed Chughtai A, Kong WF, Yan HY. Case report: successful treatment of pulmonary Talaromyces marneffei infection with posaconazole in a renal transplant recipient. Am J Trop Med Hyg. 2020;104(2):744–7. 10.4269/ajtmh.20-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Tang M, Sun S, Hu Q, Deng S. Successful treatment of Talaromyces marneffei infection in a kidney transplant recipient with voriconazole followed by itraconazole for the first time. J Mycol Med. 2022;32(1): 101214. 10.1016/j.mycmed.2021.101214. [DOI] [PubMed] [Google Scholar]
- 40.Cai DH, Wang J, Fang XL. Successful treatment of Talaromyces marneffei pneumonia in a HIV-negative renal transplantation recipient: a case report. Medicine (Baltimore). 2022;101(40): e30958. 10.1097/MD.0000000000030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan M, Zhang J. Disseminated Talaromyces marneffei infection mimicking laryngitis tuberculosis. Int J Infect Dis. 2022;120:168–9. 10.1016/j.ijid.2022.04.051. [DOI] [PubMed] [Google Scholar]
- 42.Gupta P, Kaur H, Kenwar DB, Gupta P, Agnihotri S, Rudramurthy SM. First case of subcutaneous infection by Talaromyces marneffei in a renal transplant recipient from India and review of literature. J Mycol Med. 2022;32(1): 101207. 10.1016/j.mycmed.2021.101207. [DOI] [PubMed] [Google Scholar]
- 43.Xing S, Zhang H, Qiu Y, Pan M, Zeng W, Zhang J. Clinical characteristics of transplant recipients infected with Talaromyces marneffei: 2 case reports and a literature review. Infect Drug Resist. 2022;15:2879–90. 10.2147/IDR.S363362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu L, Chen X, Yang X, Jiang H, Wang J, Chen S, Xu J. Disseminated Talaromyces marneffei infection after renal transplantation: A case report and literature review. Front Cell Infect Microbiol. 2023;13:1115268. 10.3389/fcimb.2023.1115268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai SK, Rauf NA, Preet KR, Tan LJ. Tzanck cytology smear in diagnosis of cutaneous talaromycosis (penicilliosis). Indian J Dermatol Venereol Leprol. 2023;89(2):233–6. 10.25259/IJDVL_268_20. [DOI] [PubMed] [Google Scholar]
- 46.Van Cutsem J, Meulemans L, Van Gerven F, Stynen D. Detection of circulating galactomannan by Pastorex Aspergillus in experimental invasive aspergillosis. Mycoses. 1990;33(2):61–9. 10.1111/myc.1990.33.2.61. [DOI] [PubMed] [Google Scholar]