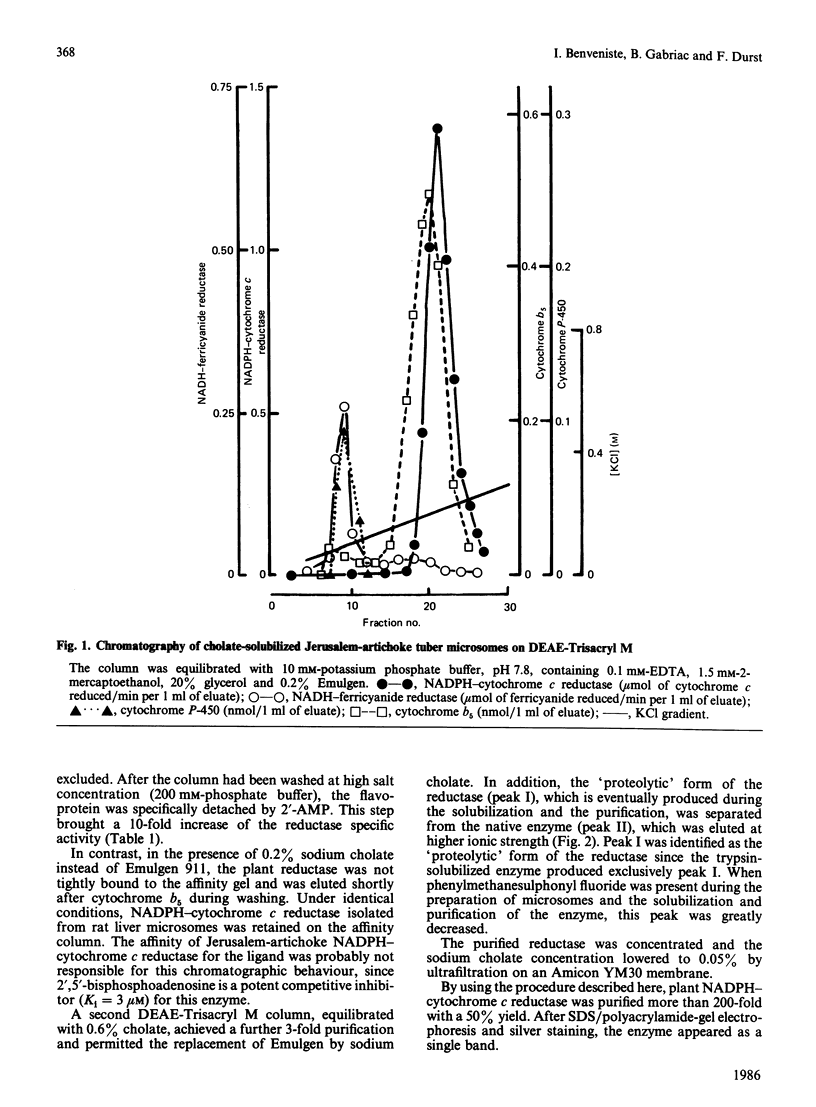
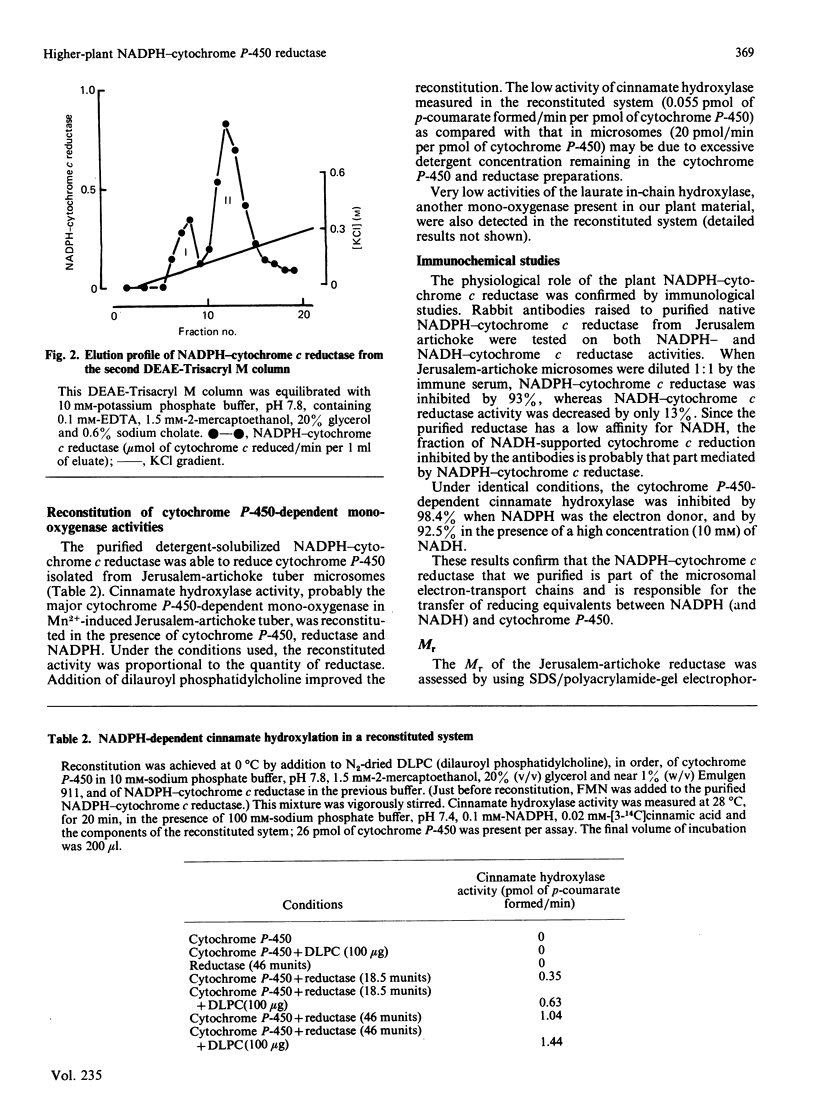
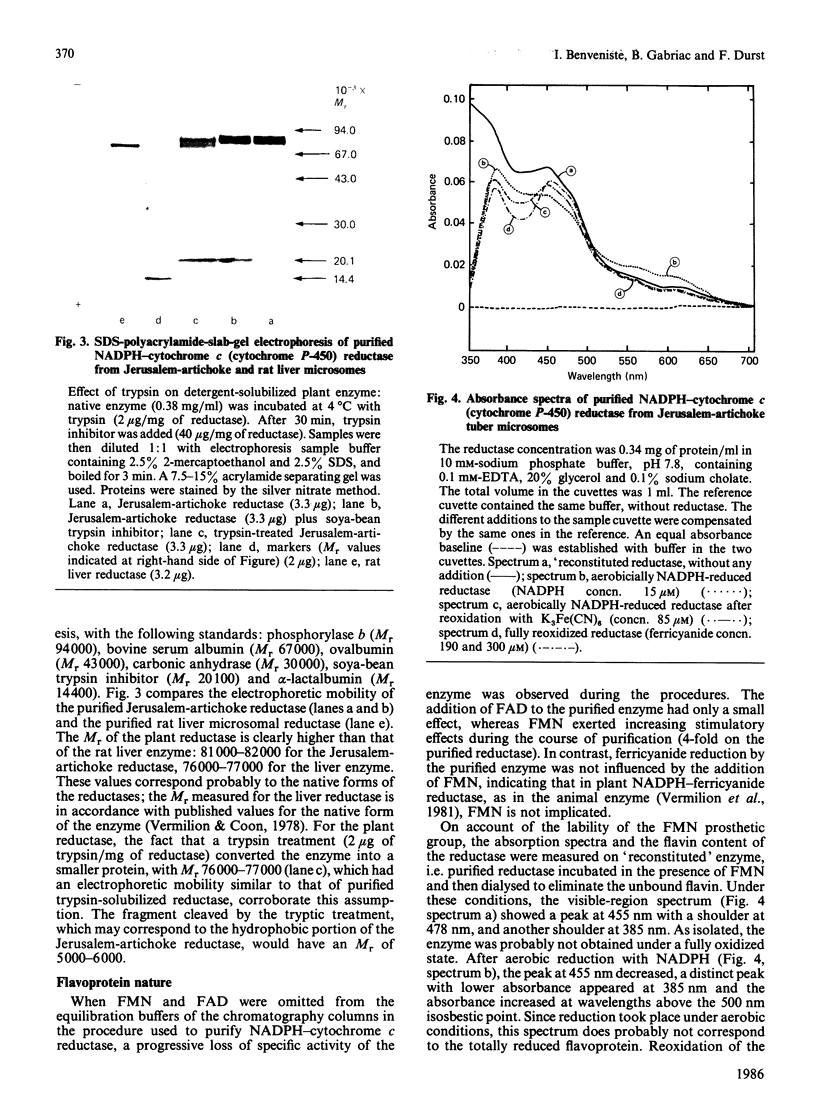
Abstract
NADPH-cytochrome P-450 (cytochrome c) reductase (EC 1.6.2.4) was solubilized by detergent from microsomal fraction of wounded Jerusalem-artichoke (Helianthus tuberosus L.) tubers and purified to electrophoretic homogeneity. The purification was achieved by two anion-exchange columns and by affinity chromatography on 2',5'-bisphosphoadenosine-Sepharose 4B. An Mr value of 82,000 was obtained by SDS/polyacrylamide-gel electrophoresis. The purified enzyme exhibited typical flavoprotein redox spectra and contained equimolar quantities of FAD and FMN. The purified enzyme followed Michaelis-Menten kinetics with Km values of 20 microM for NADPH and 6.3 microM for cytochrome c. In contrast, with NADH as substrate this enzyme exhibited biphasic kinetics with Km values ranging from 46 microM to 54 mM. Substrate saturation curves as a function of NADPH at fixed concentration of cytochrome c are compatible with a sequential type of substrate-addition mechanism. The enzyme was able to reconstitute cinnamate 4-hydroxylase activity when associated with partially purified tuber cytochrome P-450 and dilauroyl phosphatidylcholine in the presence of NADPH. Rabbit antibodies directed against plant NADPH-cytochrome c reductase affected only weakly NADH-sustained reduction of cytochrome c, but inhibited strongly NADPH-cytochrome c reductase and NADPH- or NADH-dependent cinnamate hydroxylase activities from Jerusalem-artichoke microsomal fraction.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama Y., Yoshida Y., Kubota S., Kumaoka H., Furumichi A. NADPH-cytochrome P-450 reductase of yeast microsomes. Arch Biochem Biophys. 1978 Jan 30;185(2):362–369. doi: 10.1016/0003-9861(78)90178-9. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Crankshaw D. L., Hetnarski K., Wilkinson C. F. Purification and characterization of NADPH--cytochrome c reductase from the midgut of the southern armyworm (Spodoptera eridania). Biochem J. 1979 Sep 1;181(3):593–605. doi: 10.1042/bj1810593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Strobel H. W. NADPH-cytochrome P-450 reductase from rat liver: purification by affinity chromatography and characterization. Biochemistry. 1977 Mar 22;16(6):1116–1123. doi: 10.1021/bi00625a014. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Faeder E. J., Siegel L. M. A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem. 1973 May;53(1):332–336. doi: 10.1016/0003-2697(73)90442-9. [DOI] [PubMed] [Google Scholar]
- Fan L. L., Masters B. S. Properties of purified kidney microsomal NADPH-cytochrome c reductase. Arch Biochem Biophys. 1974 Dec;165(2):665–671. doi: 10.1016/0003-9861(74)90295-1. [DOI] [PubMed] [Google Scholar]
- Fromm H. J. Use of competitive inhibitors to study substrate binding order. Methods Enzymol. 1979;63:467–486. doi: 10.1016/0076-6879(79)63020-3. [DOI] [PubMed] [Google Scholar]
- Fujita M., Oba K., Uritani I. Properties of a Mixed Function Oxygenase Catalyzing Ipomeamarone 15-Hydroxylation in Microsomes from Cut-Injured and Ceratocystis fimbriata-Infected Sweet Potato Root Tissues. Plant Physiol. 1982 Aug;70(2):573–578. doi: 10.1104/pp.70.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P., Martin M. V. Purification of cytochrome P-450, NADPH-cytochrome P-450 reductase, and epoxide hydratase from a single preparation of rat liver microsomes. Arch Biochem Biophys. 1980 Dec;205(2):365–379. doi: 10.1016/0003-9861(80)90119-8. [DOI] [PubMed] [Google Scholar]
- Hagmann M. L., Heller W., Grisebach H. Induction and characterization of a microsomal flavonoid 3'-hydroxylase from parsley cell cultures. Eur J Biochem. 1983 Aug 15;134(3):547–554. doi: 10.1111/j.1432-1033.1983.tb07601.x. [DOI] [PubMed] [Google Scholar]
- Hasson E. P., West C. A. Properties of the System for the Mixed Function Oxidation of Kaurene and Kaurene Derivatives in Microsomes of the Immature Seed of Marah macrocarpus: Electron Transfer Components. Plant Physiol. 1976 Oct;58(4):479–484. doi: 10.1104/pp.58.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwatashi A., Ichikawa Y. Physicochemical properties of reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase from bovine adrenocortical microsomes. Biochim Biophys Acta. 1979 Sep 29;580(1):44–63. doi: 10.1016/0005-2795(79)90196-x. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Mason H. S. Some properties of hepatic reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase. Biochemistry. 1973 Jun 5;12(12):2297–2308. doi: 10.1021/bi00736a018. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Rikans L. E. Kinetic properties of guinea pig liver microsomal NADPH-cytochrome P-450 reductase. Comp Biochem Physiol B. 1984;77(2):313–318. doi: 10.1016/0305-0491(84)90335-3. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E. Biopolyester membranes of plants: cutin and suberin. Science. 1980 May 30;208(4447):990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Kubota S., Yoshida Y., Kumaoka H., Furumichi A. Studies on the microsomal electron-transport system of anaerobically grown yeast. V. Purification and characterization of NADPH-cytochrome c reductase. J Biochem. 1977 Jan;81(1):197–205. doi: 10.1093/oxfordjournals.jbchem.a131436. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madyastha K. M., Coscia C. J. Detergent-solubilized NADPH-cytochrome c(P-450) reductase from the higher plant, Catharanthus roseus. Purification and characterization. J Biol Chem. 1979 Apr 10;254(7):2419–2427. [PubMed] [Google Scholar]
- Madyastha K. M., Meehan T. D., Coscia C. J. Characterization of a cytochrome P-450 dependent monoterpene hydroxylase from the higher plant Vinca rosea. Biochemistry. 1976 Mar 9;15(5):1097–1102. doi: 10.1021/bi00650a023. [DOI] [PubMed] [Google Scholar]
- Masters B. S., Bilimoria M. H., Kamin H., Gibson Q. H. The mechanism of 1- and 2-electron transfers catalyzed by reduced triphosphopyridine nucleotide-cytochrome c reductase. J Biol Chem. 1965 Oct;240(10):4081–4088. [PubMed] [Google Scholar]
- Mayer R. T., Durrant J. L. Preparation of homogenous NADPH cytochrome c (P-450) reductase from house flies using affinity chromatography techniques. J Biol Chem. 1979 Feb 10;254(3):756–761. [PubMed] [Google Scholar]
- Mayer R. T., Prough R. A. Purification and characterization of NADPH-cytochrome c (P450) reductase from the house fly, Musca domestica. Comp Biochem Physiol B. 1977;57(1):81–87. doi: 10.1016/0305-0491(77)90087-6. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- Potts J. R., Weklych R., Conn E. E., Rowell J. The 4-hydroxylation of cinnamic acid by sorghum microsomes and the requirement for cytochrome P-450. J Biol Chem. 1974 Aug 25;249(16):5019–5026. [PubMed] [Google Scholar]
- Reichhart D., Salaün J. P., Benveniste I., Durst F. Time Course of Induction of Cytochrome P-450, NADPH-Cytochrome c Reductase, and Cinnamic Acid Hydroxylase by Phenobarbital, Ethanol, Herbicides, and Manganese in Higher Plant Microsomes. Plant Physiol. 1980 Oct;66(4):600–604. doi: 10.1104/pp.66.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaün J. P., Benveniste I., Reichhart D., Durst F. A microsomal (cytochrome P-450)-linked lauric-acid-monooxygenase from aged Jerusalem-artichoke-tuber tissues. Eur J Biochem. 1978 Sep 15;90(1):155–159. doi: 10.1111/j.1432-1033.1978.tb12586.x. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Shephard E. A., Pike S. F., Rabin B. R., Phillips I. R. A rapid one-step purification of NADPH-cytochrome c (P-450) reductase from rat liver microsomes. Anal Biochem. 1983 Mar;129(2):430–433. doi: 10.1016/0003-2697(83)90573-0. [DOI] [PubMed] [Google Scholar]
- Soliday C. L., Kolattukudy P. E. Biosynthesis of Cutin omega-Hydroxylation of Fatty Acids by a Microsomal Preparation from Germinating Vicia faba. Plant Physiol. 1977 Jun;59(6):1116–1121. doi: 10.1104/pp.59.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermilion J. L., Ballou D. P., Massey V., Coon M. J. Separate roles for FMN and FAD in catalysis by liver microsomal NADPH-cytochrome P-450 reductase. J Biol Chem. 1981 Jan 10;256(1):266–277. [PubMed] [Google Scholar]
- Vermilion J. L., Coon M. J. Highly purified detergent-solubilized NADPH-cytochrome P-450 reductase from phenobarbital-induced rat liver microsomes. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1315–1322. doi: 10.1016/0006-291x(74)90341-6. [DOI] [PubMed] [Google Scholar]
- Vermilion J. L., Coon M. J. Purified liver microsomal NADPH-cytochrome P-450 reductase. Spectral characterization of oxidation-reduction states. J Biol Chem. 1978 Apr 25;253(8):2694–2704. [PubMed] [Google Scholar]
- Williams D. E., Becker R. R., Potter D. W., Guengerich F. P., Buhler D. R. Purification and comparative properties of NADPH-cytochrome P-450 reductase from rat and rainbow trout: differences in temperature optima between reconstituted and microsomal trout enzymes. Arch Biochem Biophys. 1983 Aug;225(1):55–65. doi: 10.1016/0003-9861(83)90006-1. [DOI] [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]