Abstract
Introduction
The shortage of general practitioners (GPs) and the increasing prevalence of type 2 diabetes create significant pressure on primary healthcare services. To enable that medical services are available to all that need it, innovative solutions are needed. One of those, a Short Message Service (SMS)-supported basal insulin titration service is investigated in this study. The primary objective was to determine the percentage of subjects who achieved stable fasting blood glucose (FBG) within their individual target range with this service after week 16.
Methods
This single-arm, 16-week study aimed to enroll 111 adults diagnosed with type 2 diabetes that needed insulin. The study subjects measured their FBG 4 consecutive days to establish a baseline, then received SMS prompts for daily FBG measurements and evening insulin injections until their FBG stabilized within the target range. Adjusting the insulin based on the FBG. Once stabilization was achieved, subjects continued with their optimal insulin dose for the remainder of the study. Sixteen weeks after the baseline, subjects measured FBG for 4 days before visit 4, where these values were read by the healthcare provider.
Results
Out of the planned 111 subjects, only 30 were enrolled, with one withdrawal prior to service activation. Challenges in subject recruitment were attributed to the COVID-19 outbreak, limited eligibility, competing studies, and new medications delaying insulin initiation. Subjects were on average 59.97 years old, had an HbA1c of 9.29% a FBG of 205.64 mg/dl, and had diabetes for 10 years. Among the 29 subjects who started the service, 72% achieved successful titration at visit 4, with a median time of 49 days. Notable improvements were observed in HbA1c levels (decreased by 1.58%) and FBG levels (decreased by 64 mg/dl) over the 16-week study period. No adverse events or device-related issues were reported.
Conclusions
Despite recruitment challenges, guided basal insulin titration holds promise for insulin therapy initiation in individuals. The findings emphasize the potential of tele-medical approaches, specifically through remote messaging, in managing diabetes and improving therapy adherence.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-024-01650-2.
Keywords: Basal therapy, Fasting glucose, Insulin titration, SMS, Telemedicine
Key Summary Points
| The shortage of general practitioners is impacting the accessibility and quality of primary healthcare services, highlighting the need to explore supportive services. |
| The hypothesis was that an SMS-supported titration of once-daily long-acting insulin is able to successfully titrate a bigger proportion of people compared to a historical control. |
| Study recruitment for people new to basal insulin therapy is challenging. |
| Guided insulin titration can potentially assist people with diabetes who are new to this therapy. |
Introduction
The primary healthcare system is under strain. The global healthcare landscape has been marked by a significant shortage of general practitioners (GPs) and increased demand due to an increase in chronic diseases [1–3]. As it is not likely that these factors will change, other means need to be explored to maintain the same quality of care. One such solution could be innovation in the delivery of care [4, 5].
In particular, type 2 diabetes and the initiation of insulin therapy impose significant pressure on primary healthcare services. The growing prevalence of type 2 diabetes worldwide has resulted in an increased demand for diabetes management and treatment [6]. As the disease progresses, many individuals with type 2 diabetes may eventually require insulin therapy to achieve glycemic control [7]. However, initiating insulin therapy is a complex process that necessitates comprehensive education, close monitoring, and ongoing support [7, 8]. This places a substantial burden on primary healthcare services. The initiation of insulin therapy requires frequent consultations and adjustments to insulin dosages, which can result in longer appointment times and an increased workload for primary care physicians. Furthermore, the management of insulin-related complications, such as hypoglycemia, adds further pressure to primary healthcare services.
Telemedicine represents a viable approach to tackle the challenge of increased workload. It leverages the widespread availability of mobile phones and enabling efficient remote communication between patients and healthcare providers. While obstacles exist, the potential benefits in terms of improved access to medical care, reduced strain on GPs, and enhanced healthcare delivery make telemedicine a promising avenue for addressing the shortage of GPs and improving healthcare outcomes on a global scale. Further research and practical implementation strategies are essential to fully harness the potential of this approach and ensure its successful integration into healthcare systems.
Tele-medical approaches are increasingly used in diabetes management to support therapy and to increase patient motivation and adherence to the therapy. Systematic reviews have shown the positive effect of telecare in people with type 2 diabetes (T2D) [9, 10]. Short text messages generally have a positive effect in T2D, including, but not limited to, basal, i.e., once-daily, long-acting, insulin titration [9, 11–21]. Automated text messages seem to be as effective as tailored text messages [9, 15], so that the personal effort may be reduced while still providing a sufficient level of care. The optimal support duration seems to be up to 3 months [9]. For longer durations, additional measures to increase people with diabetes (PwD) adherence and maintain PwD motivation may be required. Such measures are usually part of standard care as well. Tele-medical approaches for basal insulin titration have been shown to be non-inferior to standard care with varying impact. Several publications report improvement or at least non-inferiority to the standard approach [9, 11–21]; however, in at least one study, the comparison subject group, which was provided with a healthcare professional (HCP)-driven diabetes education program and basal insulin titration by certified diabetes educators, had better outcomes than the group using the tele-medical approach [22].
The central hypothesis behind the Short Message Service (SMS) service implementation in this study was to use telecommunication to streamline the insulin titration process for people with type 2 diabetes new to insulin. This could potentially yield multiple benefits for PwD and HCPs alike. Firstly, it was anticipated that subjects receiving SMS support would exhibit higher adherence to the prescribed insulin titration scheme, compared to those self-managing their titration. Consequently, this improved adherence was expected to lead to a higher frequency of subjects efficiently reaching their target fasting blood glucose (FBG) levels and achieving adequate glycemic control. Moreover, this, in turn, was projected to positively impact the measurement of glycated hemoglobin (HbA1c), providing a tangible outcome indicative of improved glycemic control and improvement in patient-reported outcomes. In conclusion, the study aimed to explore the efficacy of the SMS-supported titration of once-daily long-acting insulin.
Methods
Study Subjects and Design
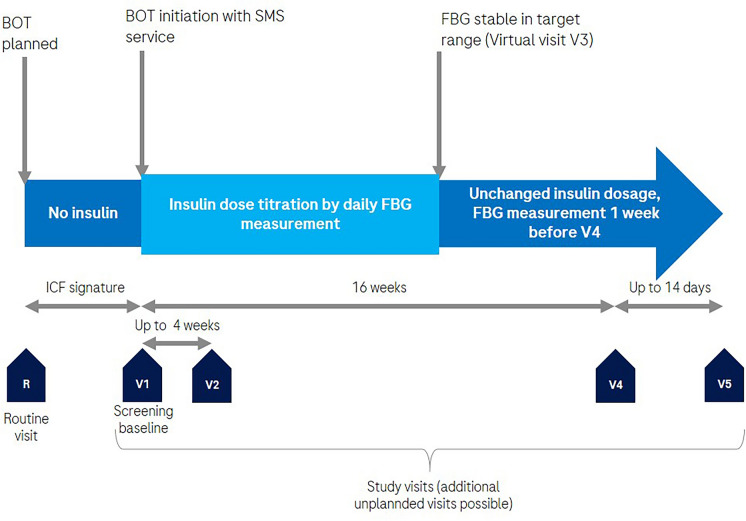
The study aimed to enroll a total of 111 subjects based on sample size calculation. Recruitment of participants took place from July 2020 to November 2021. With the last patient out in February 2022. Subjects were selected from the investigator's established patient population based on specific inclusion and exclusion criteria. Eligible subjects had to be at least 21 years old with type 2 diabetes, planning to start basal insulin titration. They were required to have an HbA1c level of ≥ 7.5% (58.5 mmol/mol) within the past 3 months and a FBG target range set to 140 mg/dl (7.8 mmol/l) or lower as determined by the investigator. An HbA1c > 7.5% in the last 3 months was included to target those individuals in need of basal therapy titration as a high HbA1c is one of the main reasons to initiate basal insulin and a target group for the SMS service. Additionally, they needed to possess and use a mobile phone with SMS capabilities and access to the mobile phone network while at home (Fig. 1).
Fig. 1.
Study overview. R on-site screening; V1 baseline visit with the handout of study supplies, start and training of insulin therapy and SMS service, HbA1c testing, and questionnaire administration. V2 Clarification of any questions and documenting any adverse events or device deficiencies, if any. V3 documentation of FBG stable in range, V4 HbA1c testing, data download, questionnaires, and documentation and documenting any adverse events or device deficiencies, V5 Documenting any adverse events or device deficiencies, if any. BOT basal oral-supported therapy. SMS Short Messaging Service; ICF informed consent form; FBG fasting blood glucose
Exclusion criteria included prior insulin therapy (except for gestational diabetes or < 1 week), current insulin therapy (e.g., prandial insulin, premixed insulin), impaired awareness of hypoglycemia with a history of regular hypoglycemia or recent hospitalization due to severe hypoglycemia within the previous 3 months, severe diabetes-related long-term complications (e.g., severe retinopathy, neuropathy, nephropathy requiring dialysis), pregnancy or plans to become pregnant, legal incompetence or limited legal competence, serious or unstable chronic physical or psychological conditions rendering subjects unable to understand the study's nature and scope or follow study procedures, and addiction to alcohol or other substances of abuse.
Subjects who met the inclusion criteria and did not meet any of the exclusion criteria were planned to be enrolled consecutively until the number of 111 subjects. Prior to study-related procedures, subjects signed the valid subject informed consent form approved by the Independent Ethics Committee.
Treatment
The study subject measured their FBG on 4 days after the baseline visit 1 without starting to inject insulin, thereby generating the mean baseline FBG (Fig. 1). The SMS service started on the fifth day after visit 1 requiring the subject to perform daily FBG measurements in the morning and injections of long-acting (basal) insulin in the evening. The subjects were asked by an SMS in the morning to enter their FBG value measured and by a SMS in the evening to enter their insulin dose injected into the responding SMS. This interaction between the subject and the SMS service was done until the FBG was stable in the target range according to the SMS service. The insulin titration scheme for this was preset based on the respective insulin manufacturers recommendation and could be changed at the investigator’s discretion. Once the optimal basal insulin dose had been found, the subject received a completion SMS. The subject continued to inject the final basal insulin dose every evening without performing regular FBG measurements in the morning. The insulin dose was not changed anymore. The SMS service stopped after the completion SMS. Regardless of whether the SMS service had been completed, visit 4 occurred 16 weeks ± 14 days after the baseline visit (visit 1). The subjects were reminded prior to visit 4 to measure their FBG on the 4 days before visit 4 in order to generate a mean FBG for this time point. These FBG values were not entered into the SMS service, but were automatically stored in the BG meter, which was downloaded by the HCP at visit 4. Over the entire study, subjects were allowed to perform BG measurements as recommended by their HCP using the BG meter supplied for the study. This specifically could include BG measurements to verify symptomatic hypoglycemia independent of the time of the day.
Outcomes
The primary objective of this study was to determine the percentage of subjects who achieved stable FBG within their individual target range after completing SMS-supported basal insulin titration by the latest at visit 4, which occurred at week 16 (± 14 days). The secondary objectives of the study included assessing various factors related to the treatment, such as the number of days until the FBG target range was initially reached, changes in HbA1c levels at visit 4 compared to baseline, FBG levels at visit 4 compared to baseline, total daily basal insulin dose at visit 4, the number of hypoglycemic events. Other secondary objectives were related to adherence to the SMS service requests, including response rate and response time to the service's SMS and changes in the following questionnaire scores: diabetes distress scale (DDS), Hospital Anxiety and Depression Scale (HADS), the Diabetes Medication System Rating Questionnaire (DMSRQ) and the 12-item Short-Form Health Survey (SF-12). Additionally, satisfaction regarding the use of the SMS service was assessed in a custom-made questionnaire. Finally, adverse events were recorded. The following adverse events were of special interest: symptomatic hypoglycemia episodes, hyperglycemia in combination with medical intervention, hypoglycemia in combination with medical intervention, ketosis, and ketoacidosis.
Statistical analysis
The analysis of the primary variable was based on a linear mixed probability model (LMM) for estimating the proportion of subjects in the target range with the study site as a random effect. The LMM is preferred over the generalized linear mixed model with logit link (GLMM) because of its greater numerical stability and direct interpretability. Therefore, the GLMM is reported as a sensitivity analysis only. The lower bound of the Wald-type one-sided 95% confidence interval (CI) for the model intercept, which measures the proportion of subjects in the target, was estimated.
All secondary endpoints were analyzed in an exploratory manner. The types of descriptive statistics used in this study are outlined in the following:
Type Categorical: Absolute (N) and relative frequencies (in %) per category together with 95% Wilson score confidence intervals. Where appropriate and present, the number of missing values as a “Missing” category was added.
Type Continuous: Number of observations (N), the number of missing values (missing), mean (mean), standard deviation (SD), median (median), quartiles (Q1/Q3), minimum and maximum (Min/Max) and a 95% confidence interval for the mean from t-distribution with N − 1 degrees of freedom (where reasonable).
Type Count: Total number of events (count), subject years under risk (subject years), incidence rate per subject year (rate), and a 95% confidence interval from Poisson distribution.
Riddle et al. [23] reported for their randomized controlled trial that 36.2% of the patients in the glargine group and 34.4% in the NPH insulin group (Neutral Protamine Hagedorn insulin) reached FBG levels ≤ 100 mg/dl (5.6 mmol/l) at the end of the study period (24 weeks). These proportions are in line with other trials, such as the randomized controlled trial of Hu et al. [24] who showed that 45.6% from their control group achieved FBG ≤ 7 mmol/l (≤ 126 mg/dl) after 12 weeks. However, this work was only reported in an abstract so that potential methodical issues cannot be analyzed. We therefore assume that 40% of patients might reach their individual FBG target range.
Sample size calculation is performed using the arcsine transformation, also termed angular transformation, which is A(p) = 2 arcsin arcsin √p, with A(p) being measured in radians. It is assumed that 15% of the patients drop out during follow-up. For the sake of comparison, sample size was also calculated using the standard approximation of the binomial to the normal distribution.
The following assumptions are used as basis for sample size calculation:
Usual care proportion of patients reaching the target level at the end of the study period: p0 = 0.4 and the SMS service p = 0.55.
Significance level: α = 0.05 one-sided.
Power: 1 − β = 0.9
Dropout: 0.15.
With these assumptions, a total of 111 patients are required for this study. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Landesärztekammer Baden-Württemberg (protocol code DC000058, EUDAMED-Nr.: CIV-20-01-031566) on 20-Apr-2020. Informed consent was obtained from all subjects involved in the study.
Results
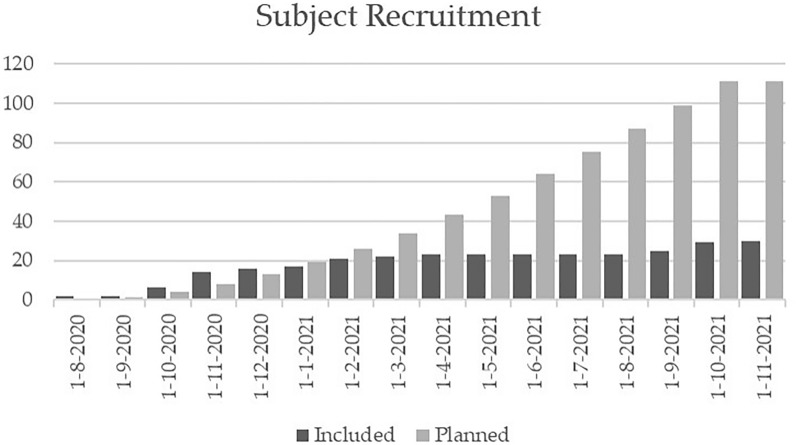
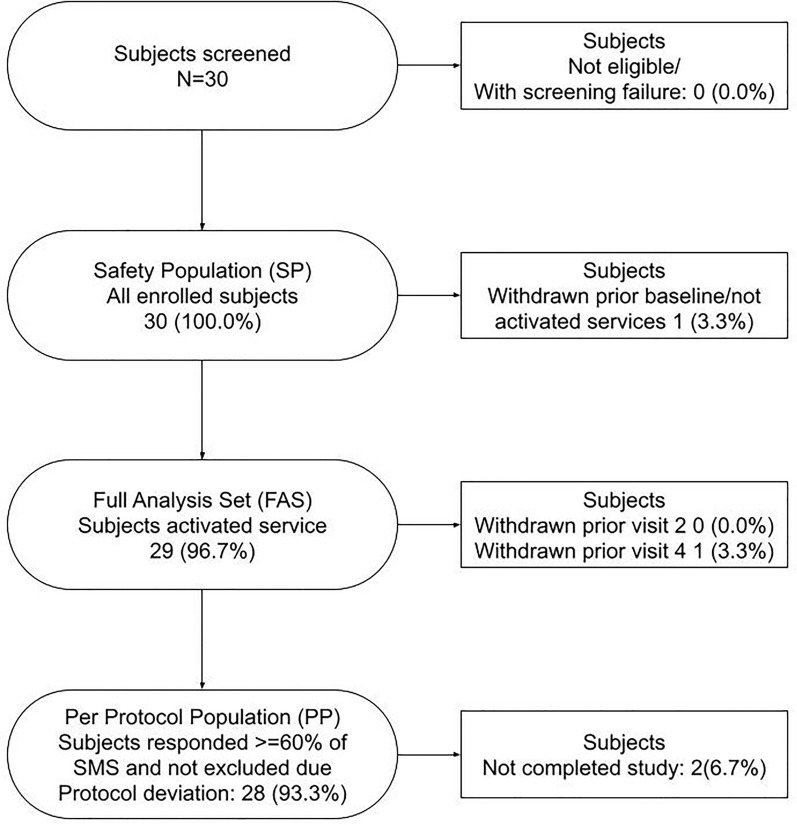
In total, 30 of the 111 planned subjects were included (Figs. 2, 3). Enrollments of subjects with type 2 diabetes who are new to insulin plateaued after about 5 months (Fig. 2). This led to the premature stop of the study due to difficulty recruiting. One subject withdrew from the study after the screening visit and one subject withdrew prior to visit 4. The results were based on subjects, who started the service with a total of 29 subjects (Table 1).
Fig. 2.
Subject recruitment planned versus included. Recruitment reached a plateau around February 2021
Fig. 3.
Disposition of subjects
Table 1.
Characteristics age, height, weight, BMI, HbA1c (central lab), baseline FBG, duration since diabetes diagnosis, and the SF-12 scores
| Item | Parameter | N (missing) | Mean (SD) | [CI] | Median | Q1–Q3 | Min–max |
|---|---|---|---|---|---|---|---|
| Baseline characteristics | Age in years | 29 (0) | 59.97 (10.3) | [56.1, 63.9] | 61.0 | 56.0–65.0 | 27–78 |
| Height in cm | 29 (0) | 171.59 (8.6) | [168.3, 174.9] | 172.0 | 166.0–177.0 | 157–186 | |
| Weight at baseline in kg | 29 (0) | 97.00 (21.1) | [89.0, 105.0] | 94.0 | 83.0–114.0 | 63–147 | |
| BMI at baseline in kg/m2 | 29 (0) | 32.88 (6.40) | [30.45, 35.32] | 32.80 | 29.10–36.80 | 21.6–47.5 | |
| Glycemic status | HbA1c in % at baseline | 29 (0) | 9.29 (1.39) | [8.76, 9.82] | 8.80 | 8.20–9.80 | 7.8–13.4 |
| Mean FBG in mg/dl at baseline | 27 (2) | 205.64 (48.78) | [186.34, 224.94] | 194.75 | 173.00–225.00 | 113.50–330.50 | |
| Diabetes history | Duration since diabetes diagnosis in years | 29 (0) | 10 (6) | [8, 13] | 9 | 6–13 | 2–23 |
| SF-12 | Mental health composite score | 28 (1) | 45.13 (12.32) | [40.35, 49.90] | 47.54 | 33.62–55.82 | 24.04–65.66 |
| Physical health composite score | 28 (1) | 40.34 (11.71) | [35.80, 44.88] | 40.86 | 30.62–51.91 | 20.26–58.10 |
N number of subjects, SD standard deviation, [CI] 95% confidence interval for the mean (t-distribution with N − 1 degrees of freedom), Q1/Q3 first/third quartile, Min/Max minimum/maximum
The assumption before the study was that 40% of the subjects might reach their individual FBG target range during usual care and that 55% of the subjects who use the SMS service would reach their target. In total, 21 of the 29 subjects who started the service were successfully titrated at visit 4. Comparing the lower bound of the 95% one-sided confidence interval for the fixed intercept of the LMM, i.e., the proportion of people with a successful titration, we can see that with 0.59 it is higher than the anticipated 0.4. The estimated intercept of 0.72 is also above the assumed success proportion of 0.55, which was the basis for the sample size calculation. The proportion of 0.72 means that 72% of the enrolled subjects who activated the service were stable in their FBG target range at the latest at visit 4 (16 weeks ± 14 days). The sensitivity analysis based on the GLMM results in a lower CI bound of 0.57 and an intercept estimate of 0.72. The titrations of the eight individuals that did not reach their FBG within the target range were either stopped by their HCPs (5) or reached the maximum duration (3). The median number of days to achieve individual initial FBG within the target range was 49 with a 95% CI of [31–60] days. Twenty-three out of 29 achieved one mean FBG in range as calculated by the SMS service (Table 2).
Table 2.
Primary objective analysis
| Model | Estimated intercept (SE) | One-sided 95% Wald-type CI for model intercept (lower bound) | Number of coerced centers | Number of titration successes | Total N | Missing (%) |
|---|---|---|---|---|---|---|
| Linear mixed effect | 0.72 (0.08) | 0.59a | 4 | 21 | 29 | 0 (0.0%) |
| Generalized linear mixed effects | 0.72 (0.09) | 0.57 | 4 | 21 | 29 | 0 (0.0%) |
The titration success of study subjects after the SMS-supported basal insulin titration (at latest at visit 4) was the primary endpoint of this study and was evaluated as the proportion of successfully titrated subjects compared to the historic control proportion of 0.4 according to literature search. The decision was based on the comparison of the lower one-sided 95% confidence interval (CI) bound for the fixed intercept to the control value based on the linear mixed effects model (LMM). Significant superiority of the SMS-supported titration over the control was shown if the lower one-sided 95% CI bound is greater than 0.4. Generalized linear mixed effects model (GLMM) result was reported as sensitivity analysis only. LMM/GLMM model with fixed intercept and random intercept per center. The center random intercepts are estimated (close) to zero due to insufficient data amount as premature recruitment stop results in low sample size. The centers with less or equal to three subjects are coerced to one
aSuperiority of SMS service over the historically reported control is shown
Total N number of subjects who started the service, SE standard error
HbA1c and mean fasting FBG at visit 4 were both reduced with 95% CIs for the change below zero (Table 3). The average insulin dose at visit 4 was 42.1 IU. The lowering of the glucose level was accompanied by occasional hypoglycemic events. Over the course of the study, there were six events of self-monitored BG < 70 mg/dl, which amounts to 1.19 events per subject year (95% CI for the rate of [0.44, 2.60]). Of the total 29 subjects, two self-monitored 2–3 hypoglycemic events, and one subject self-monitored only a single hypoglycemic event. A total of eight adverse events relating to six subjects were reported (e.g., diarrhea, bronchiolitis, upper respiratory infection) in the safety population (all enrolled subjects). Two of the subjects had at least one AE with moderate severity and four subjects had at least one AE with mild severity. No adverse events were related to the medical procedure or medical device. There were no adverse device effects (ADEs) and no withdrawals of the investigational device. Finally, there were no adverse events in the categories of special interest.
Table 3.
HbA1c, mean fasting blood glucose (FBG), and insulin level at baseline, at visit 4, the HbA1c lowered as well as the FBG with on average 42.1 units of insulin used at the end of the study
| Item | Parameter | Baseline | Visit 4 | Change |
|---|---|---|---|---|
| HbA1c in % | N (missing) | 29 (0) | 29 (0) | 29 (0) |
| Mean (SD) | 9.29 (1.39) | 7.72 (1.08) | − 1.58 (1.09) | |
| [CI] | [8.76–9.82] | [7.31–8.13] | [− 1.99 to − 1.16] | |
| N (missing) | 27 (2) | 24 (5) | 23 (6) | |
| Mean FBG in mg/dl | Mean (SD) | 205.64 (48.78) | 136.53 (23.51) | − 63.78 (44.18) |
| [CI] | [186.34–224.94] | [126.60–146.45] | [− 82.88 to − 44.68] | |
| Insulin dose in IU | N (missing) | NA | 29 (0) | NA |
| Mean (SD) | NA | 42.1 (25.0) | NA | |
| [CI] | NA | [32.6 – 51.6] | NA |
N number of subjects, SD standard deviation, [CI] 95% confidence interval for the mean (t-distribution with N − 1 degrees of freedom
The DDS, HADS, DMSRQ, and DF-12 all showed slight improvement in distress, anxiety and depression, satisfaction with global judgments of the medication system, and mental/physical health, respectively. For the diabetes distress, all of the mean scores were reduced at visit 4 with the regimen-related distress score changing from moderate distress to no distress with a 95% CI for the change in score of [− 1.09, − 0.01] (Supplementary Table 1). The HADS mainly showed a reduction in mean anxiety scores with a slight difference in depression (Supplementary Table 2). The DMSRQ showed improvement in the subscales SMBG burden, efficacy, and treatment preference (Supplementary Table 3).
On average, study subjects reacted promptly to the SMS, for those that were designed to be replied to (supplementary Table 4). The highest median reaction time for a subject was 50 min with regard to the FBG value confirmation, but, on average, subjects reacted within 6–8 min (all SMS, FBG, and insulin dose confirmation). The subjects showed an average reaction adherence of more than 96% to the SMS that were designed to be answered. The correctness of FBG values sent by the subjects is about 87% on average and the compliance with the advised insulin dose ranges from 54.84 to 100%.
The majority of healthcare professionals that filled in the questionnaire found the SMS service to simplify the workflow, reduce time, and help subjects to reach their target faster in a safe, hypoglycemia-free titration and that it is an effective start to insulin therapy with medium effort required (Supplementary Table 5).
Discussion
The assumption was that 40% of the subjects would reach their individual FBG target range in usual care as supported by Riddle et al. and Hu et al. 2018 [23, 24]. The fact that 72% of the subjects reached their FBG within 16 weeks shows an indication of superiority over the historical control. The results of this study are in line with similar management software services guiding once-daily long-acting insulin titration [25, 26]. Several (uncontrolled) trials showed a success rate between 45.9 and 76.8% [17, 25, 27–29]. Trials involving subjects who required retitration showed lower success rates compared to those that included both people new to insulin and those needing retitration. The real-world usage of management software services showed an even lower success rate with 34% reaching their goal [24, 27]. The duration until the FBG target was reached also varied from 25 days in the real-world study to 66 days in a clinical trial [17, 25]. SMS guidance implemented in the study here showed a median of 49 days to initially reach the individual range within the earlier mentioned range of other software. In addition to the finding of a median titration time of 49 days, HCPs agreed that the system was able to save them time and helped subjects reach their target values faster.
The subjects were, to a high degree, adherent to instructions of the SMS. This could have potentially contributed to the fact that subjects improved in almost all aspects. The HbA1c and FBG were both reduced to the same degree as other trials that started basal insulin people with type 2 diabetes [23, 24]. Where we observed a lowering of the HbA1c of 1.58%, other studies have shown a lowering of the HbA1c of 1.65% and 1.1% [23, 24]. There was no self-measured hypoglycemia in the majority of subjects while hypoglycemic events still did occur in three subjects. This amounted to 1.19 events per patient-year comparing quite favorably with the 4.0 events by Riddle et al. [23]. Patient-reported outcomes on distress, medication system, and general health all showed a positive impact, except for the sub-item of the DMSRQ on ”negative events”, potentially related to the hypoglycemia that still occurred due to the use of insulin. Finally, the use of SMS guidance did not yield any potentially related AEs.
In addition to the limited number of patients, there are several other limitations to consider in this study. Firstly, the absence of a control group and the selection of individuals who switched to insulin may have introduced bias into the results. Starting insulin therapy can be challenging for patients with diabetes, as they need to overcome significant hurdles such as injection pain, side effects, and the requirement for strict adherence to treatment [6]. Therefore, the fact that all study subjects were willing and able to make this transition may have influenced the outcomes.
Furthermore, the use of a historical control group instead of an actual control group warrants caution when drawing conclusions from the study. The positive results observed could potentially be confounded by other lifestyle changes that occurred over time.
Nonetheless, evidence-based titration schemes or treat-to-target approaches for insulin therapy have intrinsic benefits [30]. The SMS service has previously been evaluated in PWD that were already on basal insulin in need for a retritration of insulin. This showed favorable outcomes on HbA1c and patient-reported outcomes [31]. Similarly, this study suggests that the same service may offer benefits, but specifically to patients who are new to basal insulin and are being titrated on insulin for the first time.
Conclusions
The primary objective of this study was to determine the percentage of subjects with FBG stable in their individual target range after completion of SMS-supported basal insulin titration at the latest at visit 4 (week 16 [± 14 days]). Due to the limited number of subjects available for the study, the targeted number of 111 subjects could not be achieved. Accordingly, only 30 subjects were enrolled in the study. Although the target numbers were not achieved, the data do give an indication of the effect of the intervention. Specifically, it allows a unique look at the effect of an SMS service only in people who are insulin-naïve and not those already on insulin who might already have experience. It is important to assess the efficacy and safety of guided insulin titration in subjects new to insulin treatment and to evaluate the impact and benefits of starting insulin therapy supported by an SMS service.
Controlled trials with a bigger sample size would be needed to confirm the effect of SMS guidance during insulin titration. Despite not reaching the needed number of subjects, SMS guidance shows a first indication to perform better than usual care references and show success rates comparable to similar management software services.
Supplementary Information
Below is the link to the electronic supplementary material.
Table S1: Diabetes Distress Scale (DDS), Table S2: Hospital Anxiety and Depression Scale (HADS); Table S3: Diabetes Medication System Rating Questionnaire (DMSRQ); Table S4: Adherence to the SMS service; Table S5: SMS service healthcare professional (HCP) satisfaction questionnaire; Table S6: 12-Item Short-Form Health Survey (SF-12) (PDF 310 KB)
Acknowledgements
The authors of this study would like to thank the subjects for their hard work and dedication as well as the following investigators for their work in subject recruitment and study execution: Dr. med. Guido Freckmann from the Institut für Diabetes-Technologie Forschungs- und Entwick-lungsgesellschaft mbH an der Universität Ulm, Ulm, Germany; Dr. med. Georg Plaßmann from UHZ Klinische Forschung im Unterfrintroper Hausarztzentrum Gemeinschaftspraxis Dres. Dirk Weber, Ulrich Weber, Heiner Saueressig und Georg Plaßmann, Essen, Germany; Dr. med. Bern-hard Schmitt from Studiencentrum Mainz Mitte, Dres. B. Schmitt & S. Regner, Mainz, Germany; Dr. med. Dietrich Tews from Diabetes Zentrum Dr. Tews, Gelnhausen, Germany; Dr. med. Uta Stephan from MVZ am Bahnhof Spandau GbR, Berlin, Germany; Dr. med. Peter Witzel from Di-abetes Schwerpunktpraxis Wilhelmsburg, Hamburg, Germany; Dr. med. Astrid Schmidt-Reinwald from Praxis für Allgemeinmedizin, Dr. med. Astrid Schmidt-Reinwald, Trier, Germany; Dr. med. Jörg Simon from MVZ im Altstadt-Carree Fulda GmbH, Fulda, Germany; Dr. med. Christian Münch from gemeinschaftspraxis Dres. Münch, Immenhausen, Germany; Dr. med. Uwe Gerbaulet from Gemeinschaftspraxis Dr. Biesenbaum und Dr. Gerbaulet, Löhne, Germany.
Author Contributions
Conceptualization, Sven Reinhardt (SR) and Guido Freckmann (GF); methodology, S.R., Helena Koeing (HK), G.F.; formal analysis, H.K..; investigation, S.R. and G.F.; data curation, S.R., Tim Snel (TS) and H.K.; writing—original draft preparation, T.S.; writing—review and editing, T.S., S.R., G.F. and Stefan Pleus (SP); visualization, H.K.; supervision, S.R., T.S. and G.F.; project administration, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research, as well as the Rapid Service Fee, were funded by Roche Diabetes Care GmbH.
Data Availability Statement
The raw anonymized data supporting the conclusions of this article will be made available by the authors on request.
Declarations
Conflict of interest
Tim Snel, Helena Koenig. and Sven Reinhardt, have a paid position at Roche Diabetes Care, a company that markets tools related to diabetes self-management. Guido Freckmann is general manager and medical director of the Institute for Diabetes Technology (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies for example with medical devices for diabetes therapy on its own initiative and on behalf of various companies. G.F./IfDT have received research support, speakers’ honoraria, or consulting fees in the last 3 years from Abbott, Ascensia, Berlin Chemie, BOYDSense, Dexcom, Lilly, Novo Nordisk, Perfood, Pharmasens, Roche, Sinocare, Terumo, Ypsomed. Stefan Pleus is an employee of IDT.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Landesärztekammer Baden-Württemberg (protocol code DC000058, EU-DAMED-Nr.: CIV-20–01-031566) on 20-Apr-2020. Informed consent was obtained from all subjects involved in the study.
References
- 1.Weiss J. General practitioner shortage: with structured graduate education against the crisis. Dtsch Med Wochenschr (1946). 2010;135(28–29):26. 10.1055/s-0030-1247689. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen MAT, Thorsen T. Increasing shortage of general practitioners in social deprived Danish communities. Ugeskr Laeger. 2014;176(11):V08130497. [PubMed] [Google Scholar]
- 3.Majeed A. Shortage of general practitioners in the NHS. BMJ. 2017;358: j3191. 10.1136/bmj.j3191. [DOI] [PubMed] [Google Scholar]
- 4.Caley M, Sidhu K. Estimating the future healthcare costs of an aging population in the UK: expansion of morbidity and the need for preventative care. J Public Heal. 2011;33(1):117–22. 10.1093/pubmed/fdq044. [DOI] [PubMed] [Google Scholar]
- 5.Dall TM, Gallo PD, Chakrabarti R, West T, Semilla AP, Storm MV. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Heal Aff. 2017;32(11):2013–20. 10.1377/hlthaff.2013.0714. [DOI] [PubMed] [Google Scholar]
- 6.Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18(9):525–39. 10.1038/s41574-022-00690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MJ, Aroda VR, Collins BS, et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022;45(11):2753–86. 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–37. 10.1111/dom.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen Y, Wang F, Zhang X, et al. Effectiveness of internet-based interventions on glycemic control in patients with type 2 diabetes: meta-analysis of randomized controlled trials. J Méd Internet Res. 2018;20(5): e172. 10.2196/jmir.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Z, Tao H, Meng Q, Jing L. Management of endocrine disease: effects of telecare intervention on glycemic control in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Eur J Endocrinol. 2015;172(3):R93–101. 10.1530/eje-14-0441. [DOI] [PubMed] [Google Scholar]
- 11.Levy NK, Orzeck-Byrnes NA, Aidasani SR, et al. Transition of a text-based insulin titration program from a randomized controlled trial into real-world settings: implementation study. J Méd Internet Res. 2018;20(3): e93. 10.2196/jmir.9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy N, Moynihan V, Nilo A, et al. The mobile insulin titration intervention (MITI) for insulin adjustment in an urban, low-income population: randomized controlled trial. J Méd Internet Res. 2015;17(7): e180. 10.2196/jmir.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deerochanawong C, Bajpai S, Dwipayana IMP, et al. Optimizing glycemic control through titration of insulin glargine 100 U/mL: a review of current and future approaches with a focus on Asian populations. Diabetes Ther. 2017;8(6):1197–214. 10.1007/s13300-017-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang R, Deng X. Electronic messaging intervention for management of cardiovascular risk factors in type 2 diabetes mellitus: a randomised controlled trial. J Clin Nurs. 2018;27(3–4):612–20. 10.1111/jocn.13962. [DOI] [PubMed] [Google Scholar]
- 15.Peimani M, Rambod C, Omidvar M, et al. Effectiveness of short message service-based intervention (SMS) on self-care in type 2 diabetes: a feasibility study. Prim Care Diabetes. 2016;10(4):251–8. 10.1016/j.pcd.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med. 2011;28(4):455–63. 10.1111/j.1464-5491.2010.03180.x. [DOI] [PubMed] [Google Scholar]
- 17.Tamez-Pérez HE, Author D, Cantú-Santos OM, Gutierrez-González D, González-Facio R, Romero-Ibarguengoitia ME. Effect of digital-tool-supported basal insulin titration algorithm in reaching glycemic control in patients with type 2 diabetes in Mexico. J Diabetes Sci Technol. 2021. 10.1177/19322968211034533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortmann AL, Gallo LC, Garcia MI, et al. Dulce digital: an mHealth SMS-based intervention improves glycemic control in Hispanics with type 2 diabetes. Diabetes Care. 2017;40(10):1349–55. 10.2337/dc17-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osborn CY, Mulvaney SA. Development and feasibility of a text messaging and interactive voice response intervention for low-income, diverse adults with type 2 diabetes mellitus. J Diabetes Sci Technol. 2013;7(3):612–22. 10.1177/193229681300700305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer HH, Moore SL, Ginosar D, et al. Care by cell phone: text messaging for chronic disease management. Am J Manag Care. 2012;18(2):e42–7. [PubMed] [Google Scholar]
- 21.Arora S, Peters AL, Agy C, Menchine M. A mobile health intervention for inner city patients with poorly controlled diabetes: proof-of-concept of the TExT-MED Program. Diabetes Technol Ther. 2012;14(6):492–6. 10.1089/dia.2011.0252. [DOI] [PubMed] [Google Scholar]
- 22.Bajaj HS, Venn K, Ye C, Aronson R. Randomized trial of long-acting insulin glargine titration web tool (LTHome) versus enhanced usual therapy of glargine titration (INNOVATE Trial). Diabetes Technol Ther. 2016;18(10):610–5. 10.1089/dia.2016.0182. [DOI] [PubMed] [Google Scholar]
- 23.Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:308–3086. [DOI] [PubMed] [Google Scholar]
- 24.Hu J, Wang M, Zhao Y. SAR342434 - an insulin biosimilar for the treatment of type II diabetes. Expert Opin Biol Ther. 2018;18(11):1107–12. 10.1080/14712598.2018.1533548. [DOI] [PubMed] [Google Scholar]
- 25.Modi KD, Jha S, Panda M, et al. 138-LB: digital-tool-supported basal insulin (BI) titration: real-world effectiveness of my dose coach in India. Diabetes. 2019. 10.2337/db19-138-lb. [Google Scholar]
- 26.Hermanns N, Ehrmann D, Finke-Groene K, et al. Use of smartphone application versus written titration charts for basal insulin titration in adults with type 2 diabetes and suboptimal glycaemic control (My Dose Coach): multicentre, open-label, parallel, randomised controlled trial. Lancet Reg Health Eur. 2023;33: 100702. 10.1016/j.lanepe.2023.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies M, Bain S, Charpentier G, et al. A randomized controlled, treat-to-target study evaluating the efficacy and safety of insulin glargine 300 U/ml (Gla-300) administered using either device-supported or routine titration in people with type 2 diabetes. J Diabetes Sci Technol. 2019;13(5):881–9. 10.1177/1932296818821706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grdinovac K, Dc R, Ta L, Levin P, Sysko R. 122-LB: iSage: successful basal insulin titration managed by a prescription-only digital therapy for T2DM. Diabetes. 2019. 10.2337/db19-122-lb. [Google Scholar]
- 29.Philis-Tsimikas A, Al F, Bastian A, et al. 202-OR: basal insulin digital titration app vs. enhanced paper titration tool: a randomized control study. Diabetes. 2020. 10.2337/db20-202-or. [Google Scholar]
- 30.Khunti K, Giorgino F, Berard L, Mauricio D, Harris SB. The importance of the initial period of basal insulin titration in people with diabetes. Diabetes Obes Metab. 2020;22:722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortiz-Zúñiga Á, Simó-Servat O, Amigó J, Sánchez M, Morer C, Franch-Nadal J, Hernández C. Efficacy of insulin titration driven by SMS in improving glycemic control in people with type 2 diabetes. J Clin Med. 2023;12(19):6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Diabetes Distress Scale (DDS), Table S2: Hospital Anxiety and Depression Scale (HADS); Table S3: Diabetes Medication System Rating Questionnaire (DMSRQ); Table S4: Adherence to the SMS service; Table S5: SMS service healthcare professional (HCP) satisfaction questionnaire; Table S6: 12-Item Short-Form Health Survey (SF-12) (PDF 310 KB)
Data Availability Statement
The raw anonymized data supporting the conclusions of this article will be made available by the authors on request.