Abstract
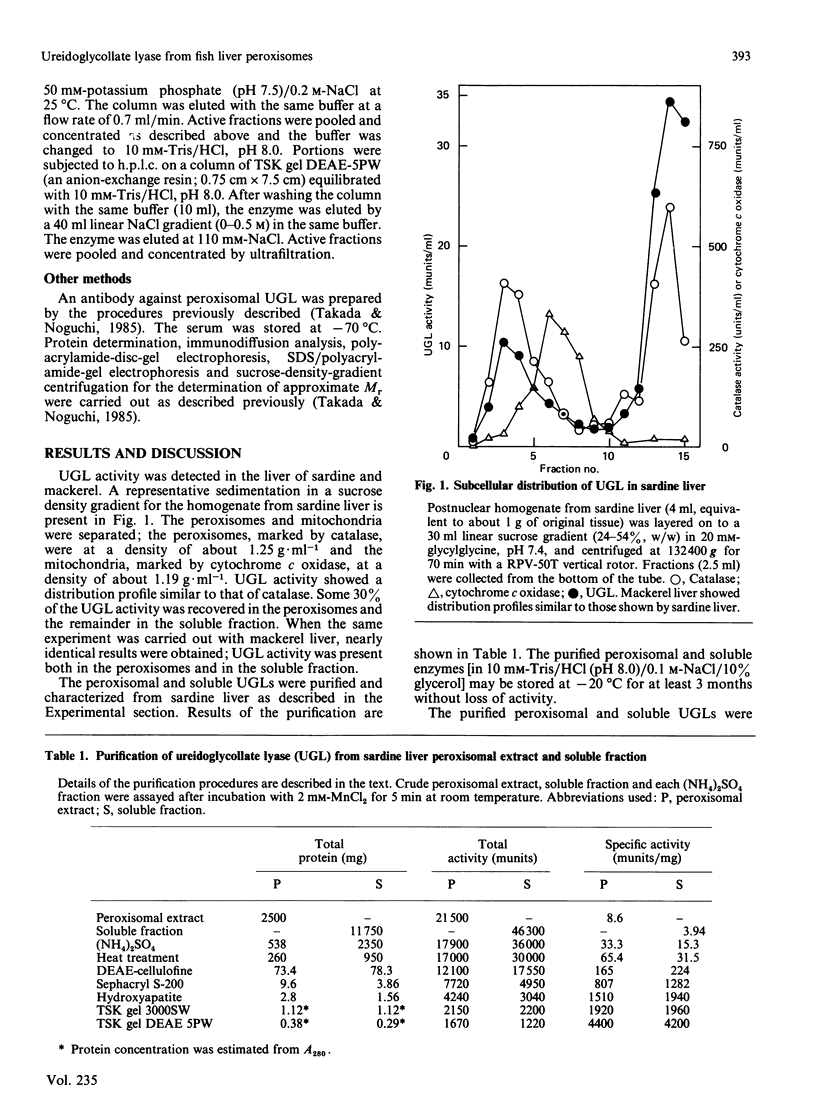
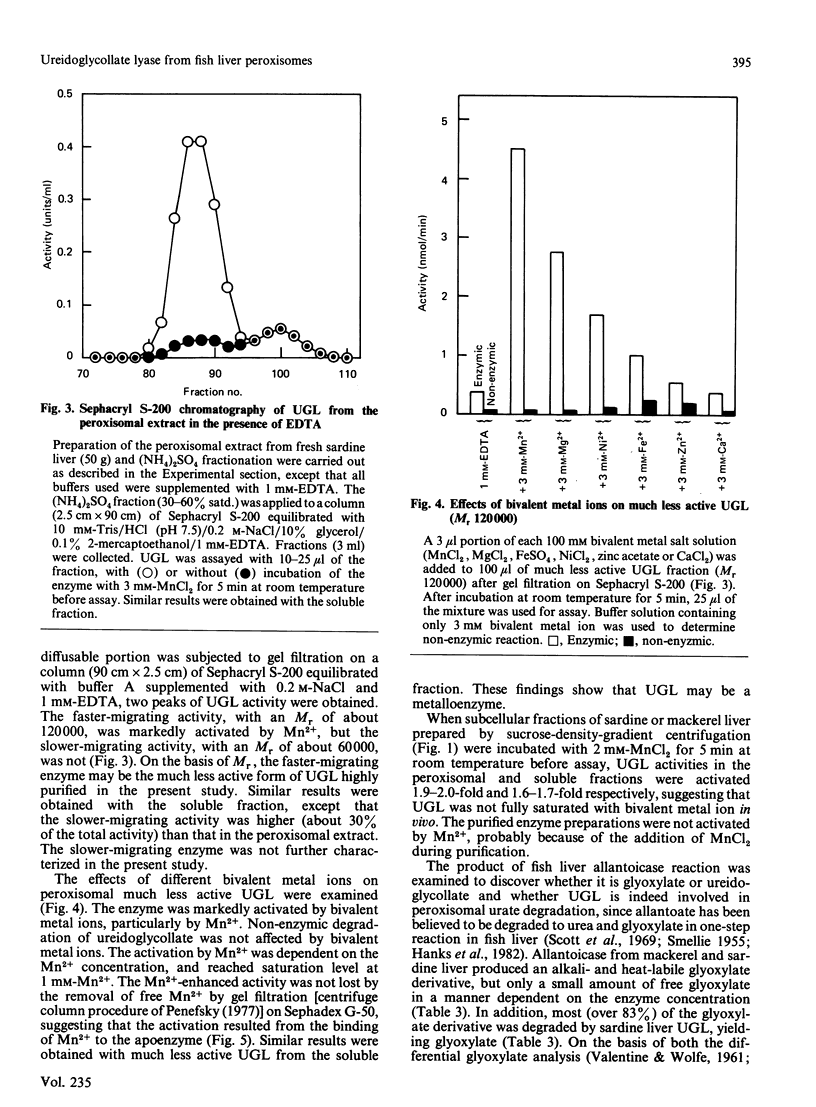
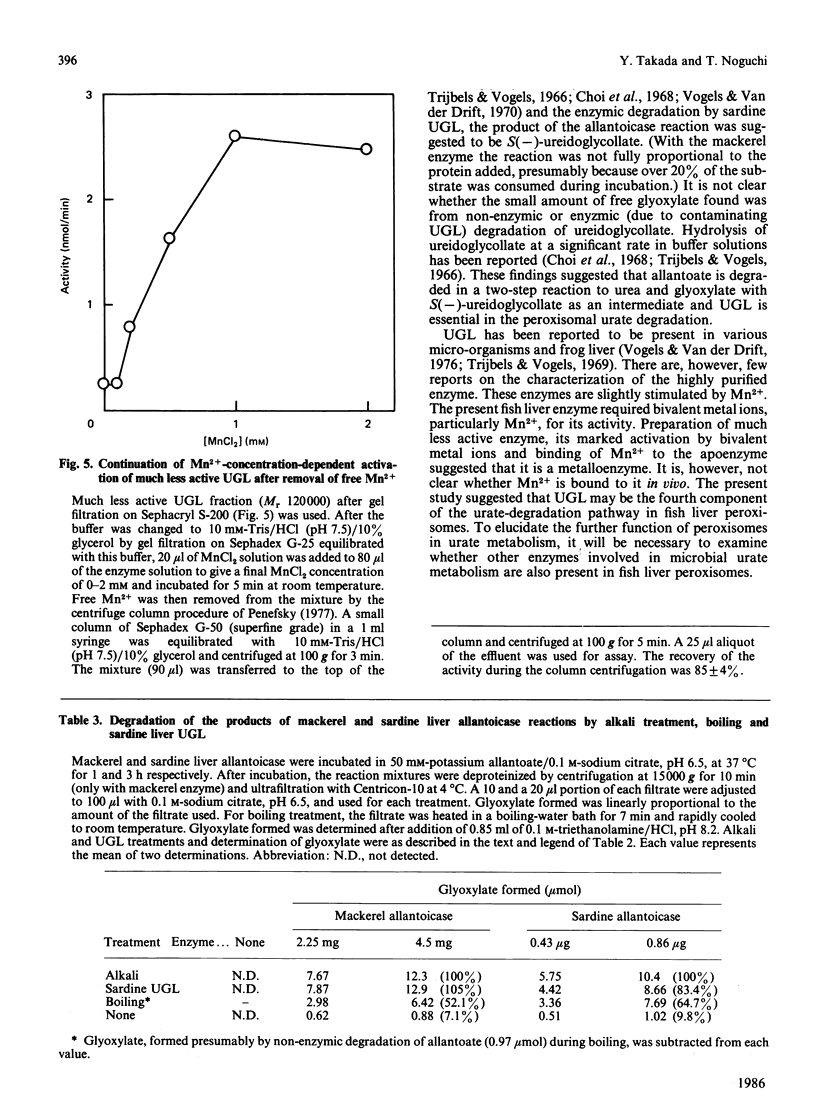
Ureidoglycollate lyase (UGL, EC 4.3.2.3), which catalyses the degradation of S(-)-ureidoglycollate to urea and glyoxylate, was found in the peroxisomes of marine fish (sardine and mackerel) liver. The enzyme highly purified from sardine liver had an Mr of about 121,000, with two identical subunits. When UGL was purified in the presence of 1 mM-EDTA, a much less active form was obtained. It was markedly activated by bivalent metal ions, particularly by Mn2+. The Mn2+-activated enzyme remained active when free Mn2+ was removed by gel filtration on Sephadex G-50, suggesting that UGL may be a metalloenzyme and the activation resulted from the binding of Mn2+ to the apoenzyme. UGL was found to be essential in peroxisomal urate degradation, since allantoate, the intermediate of urate catabolism, was found to be degraded to urea and glyoxylate in a two-step reaction catalysed by allantoicase (EC 3.5.1.5) and UGL via S(-)-ureidoglycollate as an intermediate in fish liver peroxisomes, but not in a one-step reaction as previously believed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choi K. S., Lee K. W., Hico S. C., Roush A. H. Assay, purification and properties of allantoicase from Candida utilis. Arch Biochem Biophys. 1968 Jul;126(1):261–268. doi: 10.1016/0003-9861(68)90582-1. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., de Duve C. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J Cell Biol. 1973 Nov;59(2 Pt 1):507–524. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton F., Coloma L., Koenig C. Structure, composition, physical properties, and turnover of proliferated peroxisomes. A study of the trophic effects of Su-13437 on rat liver. J Cell Biol. 1975 Nov;67(2PT1):281–309. doi: 10.1083/jcb.67.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Takada Y., Fujiwara S. Degradation of uric acid to urea and glyoxylate in peroxisomes. J Biol Chem. 1979 Jun 25;254(12):5272–5275. [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Scott P. J., Visentin L. P., Allen J. M. The enzymatic characteristics of peroxisomes of amphibian and avian liver and kidney. Ann N Y Acad Sci. 1969 Dec 19;168(2):244–264. doi: 10.1111/j.1749-6632.1969.tb43113.x. [DOI] [PubMed] [Google Scholar]
- Takada Y., Noguchi T. Characteristics of alanine: glyoxylate aminotransferase from Saccharomyces cerevisiae, a regulatory enzyme in the glyoxylate pathway of glycine and serine biosynthesis from tricarboxylic acid-cycle intermediates. Biochem J. 1985 Oct 1;231(1):157–163. doi: 10.1042/bj2310157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Noguchi T. The degradation of urate in liver peroxisomes. Association of allantoinase with allantoicase in amphibian liver but not in fish and invertebrate liver. J Biol Chem. 1983 Apr 25;258(8):4762–4764. [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Catabolism of allantoate and ureidoglycolate in Rana esculenta. Comp Biochem Physiol. 1969 Jul 15;30(2):359–365. doi: 10.1016/0010-406x(69)90817-2. [DOI] [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Degradation of allantoin by Pseudomonas acidovorans. Biochim Biophys Acta. 1966 Feb 14;113(2):292–301. doi: 10.1016/s0926-6593(66)80068-1. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., WOLFE R. S. Glyoxylurea. Biochem Biophys Res Commun. 1961 Jul 26;5:305–308. doi: 10.1016/0006-291x(61)90168-1. [DOI] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev. 1976 Jun;40(2):403–468. doi: 10.1128/br.40.2.403-468.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels G. D., Van der Drift C. Differential analyses of glyoxylate derivatives. Anal Biochem. 1970 Jan;33(1):143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]



