Abstract
Matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS), in combination with proteolytic protection assays, has been used to identify the functional epitope on human immunodeficiency virus envelope glycoprotein gp41 for the broadly neutralizing anti-gp41 human monoclonal antibody 2F5. In this protection assay-based procedure, a soluble gp140 protein with a stabilizing intermolecular disulfide bond between the gp120 and gp41 subunits (SOS gp140) was affinity bound to immobilized 2F5 under physiological conditions. A combination of proteolytic enzymatic cleavages was then performed to remove unprotected residues. Residues of SOS gp140 protected by their binding to 2F5 were then identified based on their molecular weights as determined by direct MALDI-MS of the immobilized antibody beads. The epitope, NEQELLELDKWASLWN, determined by this MALDI-MS protection assay approach consists of 16 amino acid residues near the C terminus of gp41. It is significantly longer than the ELDKWA core epitope previously determined for 2F5 by peptide enzyme-linked immunosorbent assay. This new knowledge of the structure of the 2F5 epitope may facilitate the design of vaccine antigens intended to induce antibodies with the breadth and potency of action of the 2F5 monoclonal antibody.
A vaccine to prevent human immunodeficiency virus type 1 (HIV-1) infection or to reduce disease progression in infected individuals is an urgent public health requirement (11, 26, 40). An effective vaccine is likely to include components able to induce both cellular and humoral immune responses (10, 29, 36, 37, 43, 49). Significant progress has been made in recent years on vaccines that induce cellular immunity, but no vaccine candidate has yet been designed that reproducibly stimulates broad and potent neutralizing antibody responses against primary HIV-1 isolates (1, 3–5, 9, 16, 21, 22, 37, 43, 53). That such responses are possible is demonstrated by the existence of a few human monoclonal antibodies (MAbs), isolated from HIV-1-infected individuals, that can neutralize most primary HIV-1 isolates in vitro (12, 23, 38, 43, 54, 55). Moreover, these antibodies, alone or in combination, can protect macaques from simian-HIV challenge when preadministered passively to the animals at a high enough concentration (2, 34, 35, 44). The epitopes for these MAbs, 2F5, 2G12, and immunoglobulin G1b12 (IgG1b12), are therefore of significant interest to vaccine designers (10, 11, 26, 40, 43). Thus, immunogens that present the epitopes for the above MAbs in a way that mimics their structure on the native HIV-1 envelope glycoproteins may be able to induce a polyclonal response that mimics the neutralization properties of one or more of the MAbs.
The 2F5 MAb (IAM-41-2F5) has strong neutralizing activity against a broad range of HIV-1 primary isolates (8, 17, 39, 46, 47, 54). Its epitope was previously determined by peptide reactivity as being a six-amino-acid sequence (ELDKWA) located near the C-terminal end of the gp41 ectodomain, close to the transmembrane domain (38). This segment of gp41 is one of the few regions of the envelope glycoprotein complex that is accessible to antibodies, as shown by experiments in which various MAbs were reacted with the surfaces of virus-infected cells, on which most of the envelope glycoproteins are present on budding virions (52). Also, the ELDKWA sequence is fairly well (although not absolutely) conserved among HIV-1 strains of different genetic subtypes, which is an important consideration in the development of a practical vaccine (17, 38, 39, 54).
The 2F5 MAb reacts strongly with peptides that contain the ELDKWA sequence, and the apparent simplicity of the 2F5 epitope has triggered multiple attempts to induce 2F5-like antibodies by presenting the ELDKWA sequence either as a peptide vaccine or after incorporation of the sequence into a more complex antigen (15, 18, 20, 30–32, 58–61). Invariably, these antigens have induced antibodies that react with the ELDKWA peptide or with the immunizing antigen but not with the native form of the HIV-1 envelope glycoprotein complex. In other words, none of these various immunization approaches have yielded antibodies that mimic 2F5 by being able to neutralize primary HIV-1 isolates.
The failure to induce antibodies with the same properties as 2F5 by presenting the ELDKWA epitope in various forms may be because the 2F5 epitope on the native, prefusion form of the gp41 glycoprotein has a complex structure. This idea is supported by the observation that 2F5 escape mutants, generated in vitro, did not contain mutations in the ELDKWA sequence (38, 46). Thus, the true 2F5 epitope might be discontinuous, perhaps involving sequences from a distal region of gp41, or even from the gp120 components of the native envelope glycoprotein complex. Alternatively, the epitope may be continuous but longer than the ELDKWA sequence (6).
Here, we have investigated the nature of the 2F5 epitope on the recombinant SOS gp140 (JR-FL) glycoprotein. This protein is posttranslationally cleaved in the cell, but the gp120 and gp41 ectodomain subunits are maintained in their association by a disulfide bond engineered between the subunits (7, 51). The SOS gp140 glycoprotein binds the 2F5 antibody strongly (7, 51). To define the 2F5 epitope, we have used a combination of proteolytic protection assays that involve digestion of the antigenic protein while it is bound in its native state to the MAb, followed by analysis of the peptide fragments using matrix-assisted laser desorption ionization (MALDI) mass spectrometry (MS) (27, 42).
Our results show that the 2F5 epitope on the SOS gp140 glycoprotein is NEQELLELDKWASLWN, with the end residues being partially protected. We suggest, therefore, that the 2F5 epitope on infectious virions is probably more complex than the simple ELDKWA sequence and that vaccine design should now reflect this additional complexity.
MATERIALS AND METHODS
Antibodies and reagents. (i) MAbs and recombinant SOS gp140.
Human HIV-1 gp41 monoclonal antibody 2F5 (IgG1 isotype, κ chain) was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. The anti-human Fc-specific IgG secondary antibody was obtained from Sigma Chemical Co. (St. Louis, Mo.).
(ii) The SOS gp140 (JR-FL) glycoprotein.
The gp140 protein was expressed from the HIV-1 JR-FL env gene, consists of gp120 disulfide linked to the gp41 ectodomain, and is designated SOS gp140 (51). The SOS gp140 (JR-FL) glycoprotein was expressed from CHO cells stably cotransfected with plasmids coding for SOS gp140 and furin. The secreted protein was purified under nondenaturing conditions from the cell culture supernatant by using lectin affinity, ion-exchange, and gel filtration chromatography (N. Schülke, R. Sanders, M. S. Vesanen, D. J. Anselma, A. R. Villa, K. A. Nagashima, S. I. Rosenfield, J. M. Binley, J. P. Moore, P. J. Maddon, and W. C. Olson, unpublished data).
(iii) Chemicals.
The cross-linking reagent bis(sulfosuccinimidyl) suberate (BS3) was obtained from Pierce Chemical Co. (Rockford, Ill.), and α-cyano-4-hydroxycinnamic acid was obtained from Aldrich Chemical Co. (Milwaukee, Wis.). Deionized water was prepared on a Hydro Service and Supplies, Inc. (Research Triangle Park, N.C.), RO40 water system.
Enzymes.
The enzymes used were obtained from the following sources: carboxypeptidase Y and aminopeptidase M, Boehringer Mannheim, Indianapolis, Ind.; endoproteinase LysC, Wako Chemical Co., Dallas, Tex.
Other materials.
CNBr-activated Sepharose 4B beads were from Pharmacia Biotech (Piscataway, N.J.). Compact reaction columns (CRCs) and 35-μm-pore-size filters were from USB Specialty Biochemicals, Cleveland, Ohio.
MS.
The MALDI mass spectrometer used to acquire the mass spectra was a Voyager-De STR (PerSeptive Biosystems, Framingham, Mass.). The instrument was equipped with a nitrogen laser (λ = 337 nm) to desorb and ionize the samples. The accelerating voltage used was 20 to 25 kV. External mass calibration was done by using two points that bracketed the mass range of interest.
A saturated solution of recrystallized α-cyano-4-hydroxycinnamic acid in 45:45:10 (vol/vol/vol) ethanol-water-concentrated formic acid was prepared fresh each day. A 0.5-μl aliquot of the sample (a liquid or an affinity bead slurry) was placed on the stainless steel MALDI target, followed by a 0.5-μl aliquot of the matrix solution, and the sample was allowed to dry at room temperature. For the experiments described here, the laser was aimed at or near the affinity beads on the target.
Preparation of CNBr-activated Sepharose-immobilized antibody columns.
CNBr-activated Sepharose beads were activated in accordance with the procedures described previously (45). Briefly, a 20-μl aliquot of washed beads was put into each of two CRCs. Twenty microliters (48 μg) of the secondary antibody, anti-human Fc-specific IgG, was added to each column and incubated for 1.5 h in 80 μl of 100 mM NaHCO3–150 mM NaCl, pH 8.2, with slow rotation.
The columns were rinsed, and a 50-μl (50 μg) aliquot of primary antibody 2F5 was added to one of the tubes while 50 μl of phosphate-buffered saline (PBS) was added to the other tube to serve as a control. The beads were incubated for 1 h at room temperature with slow rotation, drained, and washed three times with 0.5 ml of PBS.
The human 2F5 antibody was affinity captured from solution and cross-linked to the Fc-specific antibody with BS3 as previously described (45). A solution of 10 mM BS3 was prepared in pH 7.2 PBS. A 10-μl aliquot was added to the beads and incubated in the dark with rotation for 45 min. The beads were washed twice with 100 μl of 100 mM Tris, pH 8.0, and then resuspended in 50 μl of PBS.
The beads were washed three times with 0.4 ml of PBS. One-fourth of the beads were set aside as a control, while the remainder were used to couple the SOS gp140 protein. A 200-μl aliquot of a protein solution containing 50 μg of SOS gp140 was added to the CRC. Both CRCs were rotated at room temperature for 2 h. The beads were then drained and rinsed with PBS.
Proteolytic footprinting of SOS gp140 affinity bound to indirectly coupled 2F5 antibody. (i) Achromobacter protease (endoproteinase LysC)
A 0.1-μg/μl solution of Achromobacter protease (LysC) was prepared in 50 mM Tris HCl, pH 8.0. An aliquot containing 5 μg of LysC was added to CRCs containing SOS gp140 affinity bound to 2F5 beads or control 2F5 beads with no SOS gp140. The incubation was carried out in 100 μl of 50 mM Tris HCl, pH 8.0, for 2.5 h at 37°C with slow rotation. Each CRC was rinsed three times with 0.4 ml of PBS before MALDI analysis of the beads.
(ii) Carboxypeptidase Y.
On-column carboxypeptidase Y digestions of affinity-bound, LysC-digested SOS gp140 were carried out in PBS, pH 6.1. A 0.5-μg/μl concentration of carboxypeptidase Y in deionized water was added to each CRC. Incubations were carried out at 37°C with slow rotation. As described above, CRCs were rinsed with PBS, pH 6.1, before MALDI analysis. To continue the digestion, a fresh aliquot of carboxypeptidase was added to each CRC.
(iii) Aminopeptidase M.
A 1-μl aliquot of a 5-μg/μl solution of the enzyme in the original ammonium sulfate buffer solution was added to each compact reaction column. On-column digestions of affinity-bound fragments from SOS gp140 (after proteolysis with LysC and carboxypeptidase Y) were performed in PBS, pH 7.2, at 37°C with slow rotation. As described above, CRCs were rinsed with PBS, pH 6.1, before MALDI analysis.
RESULTS
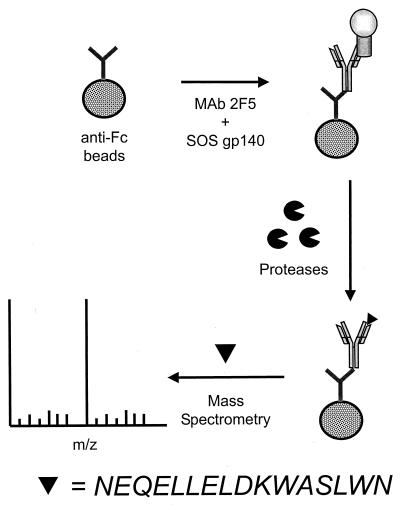
We used a purified, CHO cell-expressed SOS gp140 glycoprotein to map the 2F5 epitope. This glycoprotein binds the 2F5 MAb with high affinity, as does the same protein transiently expressed in 293T cells (7, 51; Schülke et al., unpublished data). To map the 2F5 epitope, a protection assay-based procedure was used (Fig. 1). The intact SOS gp140 glycoprotein was affinity bound to the immobilized 2F5 MAb under physiological conditions. A series of proteolytic enzymatic cleavages was then performed to remove gp140 residues that are unprotected by the MAb. The protected residues, i.e., the 2F5 epitope, were identified based on their molecular weights, as determined by MALDI-MS analysis of the peptides that were affinity bound to the immobilized antibody.
FIG. 1.
Schematic of the protection assay procedure used for MS analysis.
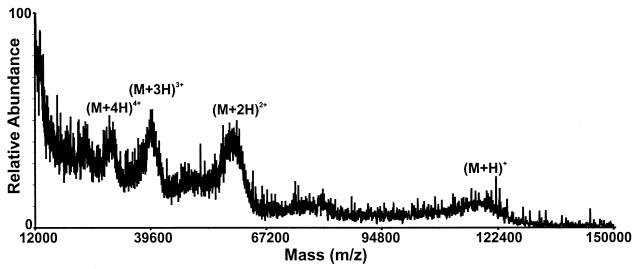
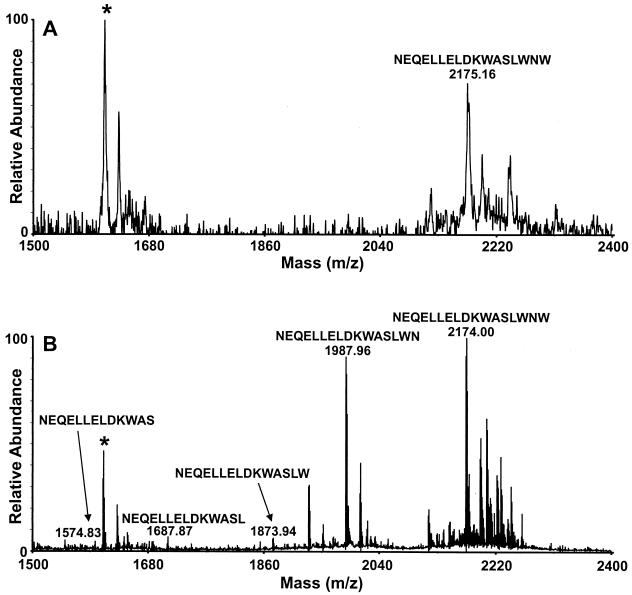
The MALDI-MS spectrum of the beads verified that the SOS gp140 glycoprotein was affinity bound to the beads (Fig. 2). The affinity-bound SOS gp140 was then digested for several hours with endoproteinase LysC, followed by shorter incubations with carboxypeptidase Y. The progress of the digestion was monitored by MALDI-MS of a small aliquot of the washed beads. Digestion of affinity-bound SOS gp140 by LysC (2.5 h) and a 1-min digestion with carboxypeptidase Y resulted in the observation of an abundant ion of m/z 2174.0 that corresponded in mass to the amino acid sequence NEQELLELDKWASLWNW from gp41 (Fig. 3A). Further digestion with carboxypeptidase Y (4 min) resulted in the observation of additional ions at m/z 1988.0 and m/z 1873.9 that corresponded in mass to the amino acid sequences NEQELLELDKWASLWN and NEQELLELDKWASLW. After a 5-min carboxypeptidase Y digestion, low-abundance ions that corresponded to subsequent loss of the C-terminal W and L residues were observed but the most abundant ions in the MALDI-MS spectrum still corresponded to the amino acid sequences NEQELLELDKWASLWN and NEQELLELDKWASLW (Fig. 3B). These ion abundances did not change, even after overnight digestion with carboxypeptidase Y.
FIG. 2.
MALDI-MS spectrum (average mass) of intact SOS gp140 affinity bound to 2F5 antibody beads.
FIG. 3.
MALDI-MS spectra obtained from a 0.5-μl aliquot of rinsed beads after digestion of affinity-bound SOS gp140 with LysC for 2.5 h, followed by digestion with carboxypeptidase Y for 1 min (average mass; panel A) and 5 min (exact mass; panel B). The ion indicated by the asterisk is a background ion.
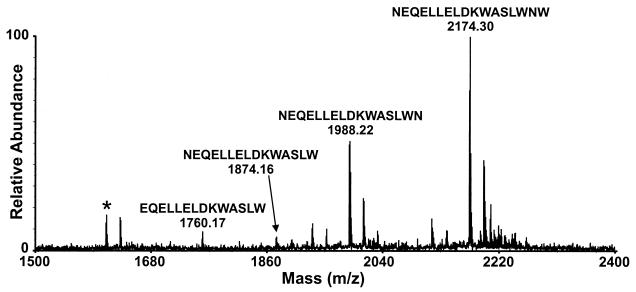
To determine the N terminus of the epitope, the affinity-bound peptide was again digested for 7 h with aminopeptidase M. A very weak ion at m/z 1760.2 was observed, indicating a small amount of cleavage of the N-terminal asparagine residue (Fig. 4). Because this ion is of low relative abundance, this asparagine residue appears to be protected and the large peaks in the spectrum still correspond to the NEQELLELDKWASLWN and NEQELLELDKWASLW peptides. Thus, both ends of the NEQELLELDKWASLWN sequence are protected in the 2F5-SOS gp140 complex.
FIG. 4.
MALDI-MS spectrum (exact mass) obtained from a 0.5-μl aliquot of rinsed beads after digestion of affinity-bound SOS gp140 with LysC for 2.5 h, carboxypeptidase Y for 6 min, and aminopeptidase M for 7 h. The ion indicated by the asterisk is a background ion.
DISCUSSION
The results of the protection assay show that the epitope on the SOS gp140 glycoprotein that is recognized by the 2F5 MAb, NEQELLELDKWASLWN, is continuous and that it consists of a longer stretch of amino acids than has been previously reported (38, 39). Whether the structure of the 2F5 epitope on infectious virions is similar remains to be determined; it is, for example, possible that the epitope is further modified by the trimerization of gp41 on the virion-associated envelope glycoprotein complex. The ELDKWA epitope that was previously determined for 2F5, based on peptide reactivity (38), probably identified the most tightly bound residues. Measurements of antibody-antigen affinities, however, have shown that the most tightly bound residues may only contribute 10% of the total binding energy (24). The additional, flanking amino acids that we have identified as being part of the 2F5 epitope may be required for the ELDKWA peptide to attain the optimal conformation for high-affinity binding of 2F5. Alternatively or additionally, they may contribute some binding energy to the antibody-antigen interaction.
The identification of NEQELLELDKWASLWN as a functional epitope recognized by a neutralizing antibody is also consistent with the known and predicted structures and functions of gp41 and MAb 2F5. Increasing the length of the ELDKWA sequence or inserting multiple copies of this sequence into the Escherichia coli MalE protein increased both the antigenicity and immunogenicity of the protein (15). This evidence suggests that additional interactions, and/or a conformational component, are involved in the 2F5 epitope. The importance of peptide conformation for binding affinity was also demonstrated by the observation that a cyclic peptide exhibited >1,000 times the binding affinity of the corresponding unconstrained peptide for anti-gp120 V3 MAb 58.2 (13). Additionally, it was recently predicted (48) that a functional immunogen for 2F5-like antibodies would have to include a portion of the adjacent C-terminal α-helix region of gp41 in order to hold the ELDKWA residues in the hairpin turn that X-ray crystallography has identified as existing in this region of the protein (41).
The mechanism of cell fusion is thought to involve the formation of a trimer of hairpins in which α-helices from the N- and C-terminal portions of gp41 form a structure that can embed itself in the cell membrane (14, 19, 33, 48, 56). A peptide (designated DP178) that consisted of 36 peptides from the C-terminal region of gp41 (YTSLIHSLIEESQNQQEKNEQELLELDKWASLWNWF) caused a 50% reduction in viral infectivity at a concentration of only 1.7 ng/ml in vitro (57). In a clinical trial, this peptide caused an approximately 100-fold decrease in plasma HIV-1 RNA when administered to infected individuals at a dose of 100 mg/day (28). The antiviral efficacy was attributed to inhibition by the peptide of the formation of the six-helix bundle structure, a process that drives membrane fusion and that is necessary for viral entry (19, 38, 57). The T20 peptide also contains the 16-residue 2F5 epitope. Similarly, it has been observed that 2F5 binds to a 43-residue-long peptide from the C-terminal helical region of gp41, terminating in ELDKW, that contains 11 residues of the 16-residue 2F5 epitope (25). The same report states that this binding is inhibited by the addition of a peptide from the N-terminal helical region, although whether such an event actually occurs within native gp41 during the fusion process is not known (25).
A small protein analog of the proposed helix bundle, designated 5-Helix, showed antiviral activity at nanomolar concentrations, presumably by trapping a C peptide of viral HIV-1 gp41 in a prefusion configuration (48). The C-terminal portion of gp41 contains a tryptophan-rich region that is present in different HIV strains, simian immunodeficiency virus, and visna virus and has been found to be critical for cell-cell fusion and viral infectivity (50). The 2F5 epitope contains three tryptophan residues from this region.
In summary, many different approaches all point to a critical role for the C-terminal region of HIV-1 gp41 in viral entry. The 2F5 antibody targets this region and presumably neutralizes infectivity by interfering with the complex structural changes in the envelope glycoprotein complex that are essential for fusion to occur. The 16-amino-acid epitope for 2F5, NEQELLELDKWASLWN, that is identified here should be a good candidate for vaccine studies intended to induce broadly neutralizing antibodies. It is important to learn how best to present this epitope to the immune system, as either a free or a constrained peptide or in the context of a more complex immunogen.
ACKNOWLEDGMENTS
C.E.P. and L.J.D. contributed equally to this work.
We thank Paul Maddon and Bill Olson for assistance with the expression and purification of the SOS gp140 glycoprotein.
Work on the SOS gp140 glycoproteins is supported by NIH grants RO1 AI39420 and RO1 AI45463 to J.P.M. and by grant UO1 AI49764 to P. J. Maddon. J.P.M. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation and a Stavros S. Niarchos Scholar. The Department of Microbiology and Immunology at the Weill Medical College gratefully acknowledges the support of the William Randolph Hearst Foundation.
REFERENCES
- 1.Amara R R, Villinger F, Altman J D, Lydy S L, O'Neil S P, Staprans S I, Montefiori D C, Xu Y, Herndon J G, Wyatt L S, Angelito Candido M, Kozyr N L, Earl P L, Smith J M, Ma H-L, Grimm B D, Hulsey M L, Miller J, McClure H M, McNicholl J M, Moss B, Robinson H L. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;292:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 2.Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 3.Barouch D H, Craiu A, Santra S, Egan M A, Schmitz J E, Kuroda M J, Fu T M, Nam J H, Wyatt L S, Lifton M A, Krivulka G R, Nickerson C E, Lord C I, Moss B, Lewis M G, Hirsch V M, Shiver J W, Letvin L N. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J Virol. 2001;75:2462–2467. doi: 10.1128/JVI.75.5.2462-2467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T-M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M-E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G, Emini E A, Shiver J W, Letvin N L. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 5.Belshe R B, Gorse G J, Mulligan M J, Evans T G, Keefer M C, Excler J-L, Duliege A M, Tartaglia J, Cox W I, McNamara J, Hwang K-L, Bradney A, Montefiori D C, Weinhold K J. Induction of immune responses to HIV-1 by canarypox virus (ALVAC) HIV-1 and gp120 SF-2 recombinant vaccines in uninfected volunteers. AIDS. 1998;12:2407–2415. doi: 10.1097/00002030-199818000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Binley J M, Ditzel H J, Barbas C F, Sullivan N, Sodroski J, Parren P W H I, Burton D R. Human antibody responses to HIV-1 glycoprotein 41 cloned in phage display libraries suggest three major epitopes are recognized and give evidence for conserved antibody motifs in antigen binding. AIDS Res Hum Retrovir. 1996;12:911–924. doi: 10.1089/aid.1996.12.911. [DOI] [PubMed] [Google Scholar]
- 7.Binley J M, Sanders R W, Clas B, Schülke N M, Master A, Guo Y, Kajumo F, Anselma D J, Maddon P J, Olson W C, Moore J P. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol. 2000;74:627–643. doi: 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A, Katinger H. Generation of human monoclonal antibodies against HIV-1 proteins: electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retrovir. 1994;10:359–369. doi: 10.1089/aid.1994.10.359. [DOI] [PubMed] [Google Scholar]
- 9.Burton D R. A vaccine for HIV type 1: the antibody perspective. Proc Natl Acad Sci USA. 1997;94:10018–10023. doi: 10.1073/pnas.94.19.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton D R, Montefiori D C. The antibody response in HIV-1 infection. AIDS. 1997;11(Suppl. A):587–598. [PubMed] [Google Scholar]
- 11.Burton D R, Moore J P. Why do we not have an HIV vaccine and how can we make one? Nat Med. 1998;4:495–498. doi: 10.1038/nm0598supp-495. [DOI] [PubMed] [Google Scholar]
- 12.Burton D R, Pyati J, Koduri R, Thornton G B, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Moore J P, Ho D D, Barbas C F., III Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 13.Cabezas E, Wang M, Parren P W H I, Stanfield R L, Satterthwait A C. A structure-based approach to a synthetic vaccine for HIV-1. Biochemistry. 2000;39:14377–14391. doi: 10.1021/bi0003691. [DOI] [PubMed] [Google Scholar]
- 14.Chan D C, Kim P S. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 15.Coeffier E, Clement J-M, Cussac V, Khodaei-Boorane N, Juahnno M, Rojas M, Dridi A, Latour M, El Habib R, Bare-Sinoussi F, Hofnung M, Leclerc C. Antigenicity and immunogenicity of the HIV-1 gp41 epitope ELDKWA inserted into permissive sites of the MalE protein. Vaccine. 2001;19:684–693. doi: 10.1016/s0264-410x(00)00267-x. [DOI] [PubMed] [Google Scholar]
- 16.Conley A J, Gorny M K, Kessler II J A, Boots L J, Ossorio-Castro M, Koenig S, Lineberger D W, Emini E A, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-v3 monoclonal-antibody, 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conley A J, Kessler II J A, Boots L J, Tung J S, Arnold B A, Keller P M, Shaw A R, Emini E A. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5: an anti-gp41 human monoclonal antibody. Proc Natl Acad Sci USA. 1994;91:3348–3352. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding J, Lu Y, Chen Y. Candidate multi-epitope vaccines in aluminium adjuvant induce high levels of antibodies with predefined multi-epitope specificity against HIV-1. FEMS Immunol Med Microbiol. 2000;29:123–127. doi: 10.1111/j.1574-695X.2000.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 19.Doms R W, Moore J P. HIV-1 membrane fusion: targets of opportunity. J Cell Biol. 2000;151:F9–F14. doi: 10.1083/jcb.151.2.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong X N, Xiao Y, Chen Y H. ELNKWA-epitope specific antibodies induced by epitope-vaccine recognize ELDKWA- and other two neutralizing-resistant mutated epitopes on HIV-1 gp41. Immunol Lett. 2001;75:149–152. doi: 10.1016/s0165-2478(00)00298-4. [DOI] [PubMed] [Google Scholar]
- 21.D'Souza M P, Livnat D, Bradac J A, Bridges S H. Evaluation of monoclonal antibodies to human immunodeficiency virus type 1 primary isolates by neutralization assays: performance criteria for selecting candidate antibodies for clinical trials. AIDS Clinical Trials Group Antibody Working Group. J Infect Dis. 1997;175:1056–1062. doi: 10.1086/516443. [DOI] [PubMed] [Google Scholar]
- 22.Engelmayer J, Larsson M, Lee A, Lee M, Cox W I, Steinman R M, Bhardwaj N. Mature dendritic cells infected with canarypox virus elicit strong anti-human immunodeficiency virus CD8+ and CD4+ T-cell responses from chronically infected individuals. J Virol. 2001;75:2142–2153. doi: 10.1128/JVI.75.5.2142-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fouts T R, Binley J M, Trkola A, Robinson J E, Moore J P. Neutralization of the human immunodeficiency virus type 1 primary isolate JR-FL by human monoclonal antibodies correlates with antibody binding to the oligomeric form of the envelope glycoprotein complex. J Virol. 1997;71:2779–2785. doi: 10.1128/jvi.71.4.2779-2785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorny M K, Conley A J, Karwowska S, Buchbinder A, Xu J-Y, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorny M K, Zolla-Pazner S. Recognition by human monoclonal antibodies of free and complexed peptides representing the prefusogenic and fusogenic forms of human immunodeficiency virus type 1 gp41. J Virol. 2000;74:6186–6192. doi: 10.1128/jvi.74.13.6186-6192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heilman C A, Baltimore D. HIV vaccines: where are we going? Nat Med. 1998;4:532–534. doi: 10.1038/nm0598supp-532. [DOI] [PubMed] [Google Scholar]
- 27.Hochleitner E O, Gorny M K, Zolla-Pazner S, Tomer K B. Mass spectrometric characterization of a discontinuous epitope of the human immunodeficiency virus (HIV) envelope protein HIV-gp120 recognized by the human monoclonal antibody 1331A. J Immunol. 2000;164:4156–4161. doi: 10.4049/jimmunol.164.8.4156. [DOI] [PubMed] [Google Scholar]
- 28.Kilby J M, Hopkins S S, Venetta T M, DiMassimo B, Cloud G A, Lee J Y, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson M R, Nowak M A, Shaw G M, Saag M S. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 29.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 30.Liao M, Lu Y, Xiao, Dierich, M. P. M P, Chen Y. Induction of high level of specific antibody response to the neutralizing epitope ELDKWA on HIV-1 gp41 by peptide-vaccine. Peptides. 2000;21:463–468. doi: 10.1016/s0196-9781(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 31.Lu Y, Xiao Y, Ding J, Dierich M, Chen Y H. Immunogenicity of neutralizing epitopes on multiple-epitope vaccines against HIV-1. Int Arch Allergy Immunol. 2000;121:80–84. doi: 10.1159/000024300. [DOI] [PubMed] [Google Scholar]
- 32.Lu Y, Xiao Y, Ding J, Dierich M P, Chen Y H. Multiepitope vaccines intensively increased levels of antibodies recognizing three neutralizing epitopes on human immunodeficiency virus-1 envelope protein. Scand J Immunol. 2000;51:497–501. doi: 10.1046/j.1365-3083.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- 33.Lu M, Blacklow S C, Kim P S. A trimeric structural domain of the HIV-1 transmembrane domain. Nat Struct Biol. 1995;12:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 34.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 35.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C R, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMichael A J, Rowland-Jones S L. Cellular immune responses to HIV. Nature. 2001;410:980–987. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 37.Montefiori D C, Evans T G. Toward an HIV type 1 vaccine that generates potent, broadly cross-reactive neutralizing antibodies. AIDS Res Hum Retrovir. 1999;15:689–698. doi: 10.1089/088922299310773. [DOI] [PubMed] [Google Scholar]
- 38.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nabel G J. Challenges and opportunities for development of an AIDS vaccine. Nature. 2001;410:1002–1007. doi: 10.1038/35073500. [DOI] [PubMed] [Google Scholar]
- 41.Pai, E. H., M. H. Klein, and P. Chong. 2000. World Intellectual Property Organization (www.wipo.org) patent WO-00/61618.
- 42.Parker C E, Tomer K B. Epitope mapping by a combination of epitope excision and MALDI-MS. Methods Mol Biol. 2000;146:185–201. doi: 10.1385/1-59259-045-4:185. [DOI] [PubMed] [Google Scholar]
- 43.Parren P W H I, Moore J P, Burton D R, Sattentau Q J. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS. 1999;13(Suppl. A):S137–S162. [PubMed] [Google Scholar]
- 44.Parren P W H I, Marx P, Hessell A J, Luckay A, Harouse J, Cheng-Mayer C, Moore J P, Burton D R. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peter J, Tomer K B. A general strategy for epitope mapping by direct MALDI-TOF mass spectrometry using secondary antibodies and crosslinking. Anal Chem. 2001;73:4012–4019. doi: 10.1021/ac010258n. [DOI] [PubMed] [Google Scholar]
- 46.Purtscher M, Trkola A, Grassauer A P M, Schulz, Klima A, Dopper S, Gruber G, Buchacher A, Muster T, Katinger H. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Purtscher M, Trkola A, Gruber G, Buchacher A, Predl R, Steindl F, Tauer, C. C, Berger R, Barrett N, Jungbauer A, Katinger H. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 1994;10:1651–1658. doi: 10.1089/aid.1994.10.1651. [DOI] [PubMed] [Google Scholar]
- 48.Root M J, Kay M S, Kim P S. Protein design of an HIV-1 entry inhibitor. Science. 2001;291:884–888. doi: 10.1126/science.1057453. [DOI] [PubMed] [Google Scholar]
- 49.Rowland-Jones S, Tan R, McMichael A. The role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:448–455. [PubMed] [Google Scholar]
- 50.Salzwedel K, West J T, Hunter E. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J Virol. 1999;73:2469–2480. doi: 10.1128/jvi.73.3.2469-2480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders R W, Schiffner L, Master A, Kajumo F, Guo Y, Dragic T, Moore J P, Binley J M. Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. J Virol. 2000;74:5091–5100. doi: 10.1128/jvi.74.11.5091-5100.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sattentau Q J, Zolla-Pazner S, Poignard P. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology. 1995;206:713–717. doi: 10.1016/s0042-6822(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 53.Seth A, Ourmanov I, Schmitz J E, Kuroda M J, Lifton M A, Nickerson C E, Wyatt L, Carroll M, Moss B, Venzon D, Letvin N L, Hirsch V M. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J Virol. 2000;74:2502–2509. doi: 10.1128/jvi.74.6.2502-2509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4 immunoglobulin G. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J P, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 57.Wild C, Greenwell T, Matthews T. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res Hum Retrovir. 1993;9:1051–1053. doi: 10.1089/aid.1993.9.1051. [DOI] [PubMed] [Google Scholar]
- 58.Xiao Y, Liao M, Lu Y, Dierich M P, Chen Y H. Epitope-vaccines: a new strategy to induce high levels of neutralizing antibodies against HIV-1. Immunobiology. 2000;201:323–331. doi: 10.1016/S0171-2985(00)80087-X. [DOI] [PubMed] [Google Scholar]
- 59.Xiao Y, Dong X, Chen Y. Induction of high levels of antibodies recognizing the neutralizing epitope ELDKWA and the D- or K-position-mutated epitopes by candidate epitope vaccines against HIV-1. Int Arch Allergy Immunol. 2000;122:287–292. doi: 10.1159/000024411. [DOI] [PubMed] [Google Scholar]
- 60.Xiao Y, Dong X N, Chen Y H. Induction of monoclonal antibody with predefined ELNKWA epitope specificity by epitope vaccine. Hybridoma. 2000;19:347–350. doi: 10.1089/027245700429918. [DOI] [PubMed] [Google Scholar]
- 61.Xiao Y, Zhao Y, Lu Y, Chen Y H. Epitope-vaccine induces high levels of ELDKWA-epitope-specific neutralizing antibody. Immunol Investig. 2000;29:41–50. doi: 10.3109/08820130009105143. [DOI] [PubMed] [Google Scholar]