Abstract
Background
Distinguishing postoperative fibrosis from isolated local recurrence (ILR) after resection of pancreatic ductal adenocarcinoma (PDAC) is challenging. A prognostic model that helps to identify patients at risk of ILR can assist clinicians when evaluating patients’ postoperative imaging. This nationwide study aimed to develop a clinically applicable prognostic model for ILR after PDAC resection.
Patients and Methods
An observational cohort study was performed, including all patients who underwent PDAC resection in the Netherlands (2014–2019; NCT04605237). On the basis of recurrence location (ILR, systemic, or both), multivariable cause-specific Cox-proportional hazard analysis was conducted to identify predictors for ILR and presented as hazard ratios (HRs) with 95% confidence intervals (CIs). A predictive model was developed using Akaike’s Information Criterion, and bootstrapped discrimination and calibration indices were assessed.
Results
Among 1194/1693 patients (71%) with recurrence, 252 patients (21%) developed ILR. Independent predictors for ILR were resectability status (borderline versus resectable, HR 1.42; 95% CI 1.03–1.96; P = 0.03, and locally advanced versus resectable, HR 1.11; 95% CI 0.68–1.82; P = 0.66), tumor location (head versus body/tail, HR 1.50; 95% CI 1.00–2.25; P = 0.05), vascular resection (HR 1.86; 95% CI 1.41–2.45; P < 0.001), perineural invasion (HR 1.47; 95% CI 1.01–2.13; P = 0.02), number of positive lymph nodes (HR 1.04; 95% CI 1.01–1.08; P = 0.02), and resection margin status (R1 < 1 mm versus R0 ≥ 1 mm, HR 1.64; 95% CI 1.25–2.14; P < 0.001). Moderate performance (concordance index 0.66) with adequate calibration (slope 0.99) was achieved.
Conclusions
This nationwide study identified factors predictive of ILR after PDAC resection. Our prognostic model, available through www.pancreascalculator.com, can be utilized to identify patients with a higher a priori risk of developing ILR, providing important information in patient evaluation and prognostication.
Supplementary Information
The online version contains supplementary material available at 10.1245/s10434-024-15664-4.
Pancreatic ductal adenocarcinoma (PDAC) is characterized by a poor prognosis and currently represents the fourth leading cause of cancer-related death.1 The best chances of survival are achieved through radical resection combined with (neo)adjuvant systemic therapy.2–4 Nevertheless, the majority of patients develop disease recurrence within 2 years after surgery, causing a disappointing 5-year survival rate of 17%.5,6
About a quarter of patients with disease recurrence after resection of PDAC develop isolated local recurrence (ILR).7 The prognosis of these patients is superior compared with patients with disease recurrence in the liver or at multiple sites, reflected by a median overall survival (OS) of 26 months versus 15 months and 18 months, respectively.7 As previously suggested, ILR might arise from residual microscopic tumor deposits in the pancreatic remnant or surrounding tissues, which lack the capability to survive at distant sites.7 This is considered to contribute to the relatively favorable prognosis observed in patients experiencing ILR.
The distinctive tumor biology of ILR, characterized by a less aggressive nature and a propensity for slower growth, might be more amenable to control through localized ablative treatment interventions (e.g., stereotactic radiotherapy). However, identification of ILR poses considerable challenges for healthcare professionals. Distinguishing postoperative fibrosis from disease recurrence in the pancreatic remnant or surgical bed is a difficult task, which often makes repetitive imaging necessary to confirm or negate the diagnosis of disease recurrence.8,9
Prognostic factors could be helpful to identify patients at risk of developing ILR after PDAC resection. However, studies investigating ILR-specific risk factors have reported conflicting results. For instance, a positive resection margin status (R1 < 1 mm, defined as presence of tumor cells within 1 mm of the resection margin) was associated with ILR in one study but not in two others, whilst one of those did show an association with R1 direct (tumor cells directly involved in the resection margin).5,10,11 Adjuvant chemoradiotherapy was associated with a reduced likelihood of ILR, but contradictory results were published regarding adjuvant chemotherapy.5,11 Moreover, nodal status (N1 and N2 compared with N0) was found to be associated with ILR.10 As reflected by these discrepancies, further clarification of factors predictive of ILR is desired. In addition, development of a clinically applicable prognostic model that incorporates the optimal combination of factors predictive of ILR might provide additional insights and has not been done before. Such a model has the potential to enable identification of patients with a higher a priori risk of developing ILR and might assist healthcare professionals when evaluating patients’ postoperative imaging.
Therefore, the aim of this study was to identify factors associated with ILR of PDAC and to develop a clinically applicable prognostic model with the best predictive ability.
Patients and Methods
Patient Selection
All Dutch centers performing pancreatic cancer surgery participated in this nationwide, observational cohort study (NCT04605237). The scientific committee of the Dutch Pancreatic Cancer Group (DPCG) has approved this study.12 In all participating centers, institutional board approval was obtained. Patients who underwent resection of PDAC between 2014 and 2019, as registered in the prospective, mandatory Dutch Pancreatic Cancer Audit (DPCA), were eligible.13 In case of complication-related mortality within 90 days after surgery, a macroscopically irradical resection (R2), or unknown resection margin status, patients were excluded. Patients with unknown recurrence status or location were also excluded. The Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD) and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed.14,15
Data Collection and Predictor Selection
Baseline and perioperative data were retrieved from the DPCA. Data regarding pathological features, follow-up, disease recurrence, and survival were additionally collected from the electronic patient records in each participating hospital.
Resectability status was defined according to DPCG criteria.16 (Neo)adjuvant therapy was considered completed if at least 80% of the planned number of cycles was received by the patient. During the study period, neoadjuvant chemo(radio)therapy was only given in the context of a clinical trial and the planned number of cycles therefore depended on the study protocol.17,18 Adjuvant strategies where unified in all participating institutions according to the Dutch national guidelines.19 Within the Netherlands, radiotherapy has no role in the adjuvant treatment of patients with pancreatic cancer. Postoperative complications for which surgical or radiological intervention or intensive care unit admittance were necessary or lead to single- or multi-organ failure or patient demise, were scored as major complications. Tumor (T) stage, lymph node (N) status, and tumor, node, metastasis (TNM)-status were defined according to the eighth edition of the American Joint Committee on Cancer TNM guidelines.20 Resection margin status was considered microscopically positive (R1 < 1 mm) if tumor cells were present within 1 mm of the closest resection margin, apart from the anterior surface.19
Potential predictors were selected on the basis of previously suggested associations with PDAC recurrence, including resectability status (resectable, borderline resectable, or locally advanced), completion of neoadjuvant therapy (yes or no), preoperative serum carbohydrate antigen 19-9 (CA 19-9) level (logarithmic in U/mL), location of the tumor (head or body/tail), vascular resection (yes or no), tumor size (continuous in mm), tumor differentiation (well/moderate or poor), perineural invasion (yes or no), lymphovascular invasion (yes or no), positive regional lymph nodes (continuous), resection margin status (R0 ≥ 1 mm or R1 < 1 mm), and completion of adjuvant chemotherapy (yes or no).
Outcomes and Definitions
The primary outcome was the presence of ILR. Diagnosis of disease recurrence was preferably based on histology, but if absent, consensus from a multidisciplinary team meeting based on results of imaging and serum tumor markers sufficed. In the Netherlands, national guidelines advise follow-up on the basis of clinical symptoms after resection of PDAC.19 If symptoms suspicious of disease recurrence arise, imaging can be performed (symptomatic strategy). However, in the case of study participation or patient preference, imaging could have been performed at set intervals (e.g., monthly, 3-monthly, yearly), which was defined as recurrence-focused follow-up.
Statistical Analysis
Patients were divided into three groups ono the basis of recurrence status at initial diagnosis of disease recurrence: ILR, systemic disease recurrence, or local and systemic disease recurrence. Descriptive statistics were used to present baseline characteristics. Multiple imputation with the iterative Markov chain Monte Carlo method (five imputations; ten iterations) was used for missing baseline data, which were considered missing at random.21 Categorical variables were shown as absolute numbers with corresponding percentages and compared via the Chi-Square test. Continuous variables were expressed as a mean ± standard deviation (SD) or median with interquartile range (IQR) and compared via an analysis of variance (ANOVA). Proportionality of predictors was examined by calculating Schoenfeld residuals, and variables were transformed in case of a nonnormal distribution. Multicollinearity between predictors was ruled out by determining variable inflation factors.22 Disease-free survival (DFS) and OS were determined by Kaplan–Meier survival curves and presented as median with 95% confidence intervals (95% CIs). Patients with missing survival data were excluded. DFS was defined as the time between the date of surgery and the date of recurrence diagnosis. OS was defined as the time between the date of surgery and the date of death from any cause. Patients without event were censored at the date of last follow-up.
Since development of ILR versus systemic or synchronous local and systemic recurrence were considered competing risks, multivariable cause-specific Cox proportional hazard analysis was performed to identify prognostic factors associated with ILR. Patients with disease recurrence in other locations than ILR were censored at date of recurrence diagnosis, and patients without disease recurrence were censored at the date of last follow-up. Results were presented as hazard ratios (HRs) with 95% CIs and probability values (P). HRs greater than 1 were associated with the development of ILR. The best predictive model was selected by Akaike’s information criterion and internally validated in 1000 bootstrap samples. The concordance index (C-index) was used to determine discriminative ability, in which perfect discrimination is reflected by a value of 1. Calibration plots with a calibration slope were constructed to assess calibration.
The HRs of predictors included in the final model were translated into risk scores. The sum of individual risk scores leads to a total score, which directly reflects the probability of an individual patient to develop ILR at a certain time point. The final model was made available as an online calculator on www.pancreascalculator.com.
R language environment was used to perform statistical analyses (version 3.3.0+, readxl, naniar, car, dplyr, tidyr, arsenal, mice, survival, survminer, rms, MASS packages; http://R-project.org). A two-sided P-value of less than 0.05 was considered statistically significant.
Results
In total, 1909 patients were identified. Of those, 216 patients (11%) were excluded (Supplementary Fig. 1). Consequently, 1693 patients (89%) were included, with a median follow-up of 45 months [interquartile range (IQR) 33–60 months] and median OS of 22 months (95% CI 20–23 months; Table 1).
Table 1.
Baseline characteristics and missing data of 1693 patients who underwent resection of pancreatic ductal adenocarcinoma
| Before imputation | Missing, n (%) | After imputation | |
|---|---|---|---|
| Age at diagnosis, mean (SD), years | 67 (9) | 0 (0) | 67 (9) |
| Male sex, n (%) | 899 (53) | 0 (0) | 899 (53) |
| BMI, mean (SD), kg/m2 | 25 (4) | 15 (1) | 25 (4) |
| CACI, mean (SD) | 3 (2) | 1 (0) | 3 (2) |
| ASA-score, n (%) | 18 (1) | ||
| I | 177 (10) | 180 (11) | |
| II | 1050 (62) | 1060 (63) | |
| III | 439 (26) | 444 (26) | |
| IV | 9 (1) | 9 (1) | |
| ECOG performance score at primary diagnosis, n (%) | 436 (26) | ||
| 0 | 621 (37) | 817 (48) | |
| 1 | 518 (31) | 707 (42) | |
| 2 | 95 (6) | 135 (8) | |
| 3 | 22 (1) | 31 (2) | |
| 4 | 1 (0) | 3 (0) | |
| Resectability, n (%) | 122 (7) | ||
| Resectable | 1209 (71) | 1305 (77) | |
| Borderline resectable | 242 (14) | 259 (15) | |
| Locally advanced | 120 (7) | 129 (8) | |
| Neoadjuvant therapy, n (%) | 13 (1) | ||
| None | 1442 (85) | 1442 (85) | |
| FOLFIRINOX chemotherapy | 156 (9) | 156 (9) | |
| Gemcitabine chemoradiotherapy | 76 (5) | 76 (5) | |
| Other chemotherapy | 6 (0) | 6 (0) | |
| Completed neoadjuvant therapya, n (%) | 228 (96) | 0 (0) | 228 (96) |
| Preoperative serum CA 19-9 level, median (IQR), U/mL | 129 (34−479) | 424 (25) | 130 (34–499) |
| Type of surgery, n (%) | 6 (0) | ||
| Open | 1436 (85) | 1441 (85) | |
| Laparoscopic | 123 (7) | 124 (7) | |
| Robot-assisted | 128 (8) | 128 (8) | |
| Surgical procedure, n (%) | 0 (0) | ||
| Pancreatoduodenectomy | 1343 (79) | 1343 (79) | |
| Distal pancreatectomy | 262 (16) | 262 (16) | |
| Total pancreatectomy | 57 (3) | 57 (3) | |
| Other | 31 (2) | 31 (2) | |
| Tumor location, n (%) | 39 (2) | ||
| Head | 1356 (80) | 1388 (82) | |
| Body/tail | 298 (18) | 305 (18) | |
| Vascular resection, n (%) | 479 (28) | 3 (0) | 479 (28) |
| Tumor differentiation, n (%) | 278 (16) | ||
| Well/moderate | 1027 (61) | 1230 (73) | |
| Poor | 388 (23) | 463 (27) | |
| Microscopic lymphovascular invasion, n (%) | 896 (53) | 238 (14) | 1034 (61) |
| Microscopic perineural invasion, n (%) | 1275 (75) | 143 (8) | 1384 (82) |
| Tumor stage 8th AJCC edition, n (%) | 32 (2) | ||
| T1 | 227 (13) | 232 (14) | |
| T2 | 996 (59) | 1012 (60) | |
| T3 | 417 (25) | 428 (25) | |
| T4 | 21 (1) | 21 (1) | |
| Lymph node stage eighth AJCC edition, n (%) | 5 (0) | ||
| N0 | 513 (30) | 515 (30) | |
| N1 | 638 (38) | 640 (38) | |
| N2 | 537 (32) | 538 (32) | |
| Total resected lymph nodes, median (IQR) | 15 (11–21) | 15 (1) | 15 (11–21) |
| Lymph node ratio, n (%) | 18 (1) | ||
| ≤ 0.2 | 1072 (63) | 1086 (64) | |
| > 0.2 | 603 (36) | 607 (36) | |
| TNM stage eighth AJCC edition, n (%) | 35 (2) | ||
| ≤ 2A | 498 (29) | 511 (30) | |
| ≥ 2B | 1160 (69) | 1182 (70) | |
| Major postoperative complicationsb, n (%) | 580 (34) | 0 (0) | 580 (34) |
| Resection margin statusc, n (%) | 0 (0) | ||
| R0 ≥ 1 mm | 882 (52) | 882 (52) | |
| R1 < 1 mm | 811 (48) | 811 (48) | |
| Adjuvant chemotherapy, n (%) | 1050 (62) | 27 (2) | 1064 (62) |
| Completed adjuvant chemotherapya, n (%) | 664 (63) | 0 (0) | 664 (62) |
| Type adjuvant chemotherapy, n (%) | 31 (3) | ||
| Gemcitabine monotherapy | 720 (69) | 720 (68) | |
| FOLFIRINOX | 155 (15) | 155 (15) | |
| Gemcitabine combination therapy | 133 (13) | 133 (13) | |
| Other | 11 (1) | 11 (1) |
Percentages may not add up to 100 because of rounding
a(Neo)adjuvant therapy was considered completed in cases where 80% of the planned number of cycles was received by the patient
bMajor postoperative complications were defined as complications requiring surgical or radiologic intervention, intensive care unit admittance, single- or multi-organ failure, of the patients’ demise
cResection margin status was considered microscopically positive (R1 < 1 mm) if tumor cells were present within 1 mm of the closest resection margin, apart from the anterior surface
AJCC American Joint Committee on Cancer, ASA American Society of Anesthesiologists, BMI body mass index, CA 19-9 carbohydrate antigen 19-9, CACI Charlson age-adjusted comorbidity index, ECOG Eastern Cooperative Oncology Group, FOLFIRINOX fluorouracil, leucovorin, irinotecan, oxaliplatin, IQR interquartile range, SD standard deviation
A total of 1194 patients (71%) developed disease recurrence with a median DFS of 11 months (95% CI 11–12 months). Of those, 252 patients (21%) developed ILR. Systemic disease recurrence without local recurrence occurred in 473 patients (40%), of whom 182 patients (38%) had liver only recurrence, 86 patients (18%) had lung only recurrence, 182 patients (38%) had multiple site recurrence, and 23 patients (5%) had isolated recurrence at another distant site. Synchronous local and systemic recurrence was present in 469 patients (39%; Table 2). When comparing patients with ILR to those with synchronous local and systemic recurrence, patients with ILR more often underwent a vascular resection (39% versus 31%; P = 0.03), had a favorable tumor stage (P < 0.01), and had tumors that were more often well/moderately differentiated (76% versus 69%; P = 0.02) with less lymphovascular invasion (55% versus 71%; P < 0.001). In addition, they more often received and completed adjuvant chemotherapy (67% versus 56%; P < 0.01 and 71% versus 63%; P < 0.01, respectively). In patients with ILR, standardized follow-up imaging to detect disease recurrence was more frequently applied (22% versus 14%; P = 0.02; Supplementary Table 1).
Table 2.
Descriptive statistics comparing patients with isolated local pancreatic ductal adenocarcinoma recurrence with patients with local and systemic recurrence
| ILR (n = 252) |
Systemic (n = 473) |
Local and systemic (n = 469) |
P | |
|---|---|---|---|---|
| Age at diagnosis, mean (SD), years | 66 (10) | 67 (9) | 67 (10) | 0.76 |
| Male sex, n (%) | 136 (54) | 260 (55) | 244 (52) | 0.66 |
| BMI, mean (SD), kg/m2 | 25 (4) | 25 (4) | 25 (4) | 0.58 |
| CACI, mean (SD) | 3 (2) | 3 (2) | 3 (2) | 0.94 |
| Resectability, n (%) | 0.04 | |||
| Resectable | 175 (70) | 374 (79) | 355 (76) | |
| Borderline resectable | 56 (22) | 62 (13) | 81 (17) | |
| Locally advanced | 21 (8) | 36 (8) | 33 (7) | |
| Neoadjuvant therapy, n (%) | 42 (17) | 79 (17) | 66 (14) | 0.47 |
| Completed neoadjuvant therapya, n (%) | 37 (71) | 72 (91) | 61 (92) | 0.60 |
| Preoperative serum CA 19-9 level, median (IQR), U/mL | 157 (34–514) | 154 (47–499) | 179 (48–593) | 0.13 |
| Tumor location, n (%) | < 0.001 | |||
| Head | 226 (90) | 370 (78) | 397 (85) | |
| Body/tail | 26 (11) | 103 (22) | 72 (15) | |
| Vascular resection, n (%) | 99 (39) | 132 (28) | 145 (31) | < 0.01 |
| Tumor stage 8th AJCC edition, n (%) | < 0.01 | |||
| T1 | 22 (9) | 56 (12) | 52 (11) | |
| T2 | 176 (70) | 272 (57) | 264 (56) | |
| T3 | 49 (20) | 140 (29) | 147 (31) | |
| T4 | 5 (2) | 5 (1) | 6 (1) | |
| Tumor differentiation, n (%) | 0.03 | |||
| Well/moderate | 192 (76) | 320 (68) | 323 (69) | |
| Poor | 60 (24) | 153 (32) | 146 (31) | |
| Perineural invasion, n (%) | 217 (86) | 393 (83) | 405 (86) | 0.30 |
| Lymphovascular invasion, n (%) | 139 (55) | 316 (67) | 334 (71) | < 0.001 |
| Lymph node status eighth AJCC edition, n (%) | 0.30 | |||
| N0 | 69 (27) | 119 (25) | 116 (25) | |
| N1 | 103 (41) | 189 (40) | 168 (36) | |
| N2 | 80 (32) | 165 (35) | 185 (39) | |
| Resection margin statusb, n (%) | < 0.01 | |||
| R0 ≥ 1 mm | 103 (41) | 262 (55) | 210 (45) | |
| R1 < 1 mm | 149 (59) | 211 (45) | 259 (55) | |
| Adjuvant chemotherapy, n (%) | 170 (67) | 296 (63) | 264 (56) | 0.01 |
| Completed adjuvant chemotherapya, n (%) | 120 (71) | 199 (74) | 166 (63) | < 0.01 |
| Use of imaging procedures during follow-upc, n (%) | 0.11 | |||
| None/nonstandardized | 195 (78) | 376 (82) | 390 (83) | |
| Standardized | 54 (22) | 84 (18) | 68 (14) |
Percentages may not add up to 100 because of rounding and missing data
a(Neo)adjuvant therapy was considered completed in cases where 80% of the planned number of cycles was received by the patient
bResection margin status was considered microscopically positive (R1 < 1 mm) if tumor cells were present within 1 mm of the closest resection margin, apart from the anterior surface
cPostoperative imaging could have been performed in a standardized fashion at set intervals, or when indicated by clinical symptoms
AJCC American Joint Committee on Cancer, BMI body mass index, CA 19-9 carbohydrate antigen 19-9, CACI Charlson age-adjusted comorbidity index, IQR interquartile range, SD standard deviation
Survival Estimates
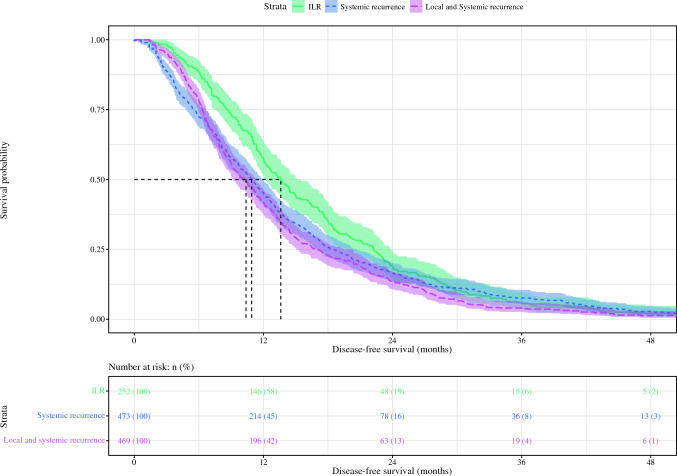
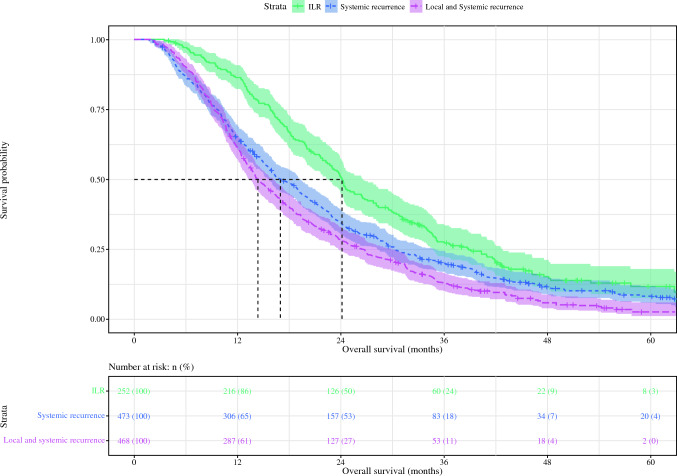
Patients with ILR had a DFS of 14 months (95% C 12–15 months) compared with 11 months (95% CI 10–12 months) in patients who developed systemic recurrence (P = 0.04) and 10 months (95% CI 9–11 months) in patients with synchronous local and systemic disease recurrence (P < 0.001; Figure 1). Patients who developed ILR had a higher OS of 24 months (95% CI 23–27 months) compared to 17 months (95% CI 16–19 months) in patients who developed systemic disease recurrence (P < 0.001) and 14 months (95% CI 13–16 months) in patients with synchronous local and systemic disease recurrence (P < 0.001; Fig. 2).
Fig. 1.
Kaplan–Meier comparing disease-free survival of patients with ILR with patients with (local and) systemic disease recurrence after resection of pancreatic ductal adenocarcinoma
Fig. 2.
Kaplan–Meier comparing overall survival of patients with ILRwith patients with (local and) systemic disease recurrence after resection of pancreatic ductal adenocarcinoma
Factors Associated with ILR
Since the proportionality assumption did not hold for adjuvant chemotherapy, this variable was included as a time-varying covariate. The best performing model included six covariates: resectability status (borderline resectable versus resectable, HR 1.42; 95% CI 1.03–1.96; P = 0.03; and locally advanced versus resectable, HR 1.11; 95% CI 0.68–1.82; P = 0.66), tumor location (head versus body/tail, HR 1.50; 95% CI 1.00–2.25; P = 0.05), vascular resection (yes versus no, HR 1.86; 95% CI 1.41–2.45; P < 0.001), perineural invasion (yes versus no, HR 1.47; 95% CI 1.01–2.13; P = 0.02), number of positive lymph nodes (continuous, HR 1.04; 95% CI 1.01–1.08; P = 0.02), and resection margin status (R1 < 1 mm versus R0 ≥ 1 mm, HR 1.64; 95% CI 1.25–2.14; P < 0.001; Table 3).
Table 3.
Multivariable cause-specific Cox proportional hazard analysis to identify independent predictors of isolated local disease recurrence after resection of pancreatic ductal adenocarcinoma
| HR | 95% CI | P | |
|---|---|---|---|
| Resectability status | |||
| Resectable | Ref | Ref | Ref |
| Borderline resectable | 1.42 | 1.03–1.96 | 0.03 |
| Locally advanced | 1.11 | 0.68–1.82 | 0.66 |
| Tumor location (head versus body/tail) | 1.50 | 1.00–2.25 | 0.05 |
| Vascular resection (yes versus no) | 1.86 | 1.41–2.45 | < 0.001 |
| Perineural invasion (yes versus no) | 1.47 | 1.02–2.13 | 0.04 |
| Number of positive regional lymph nodes (continuous) | 1.04 | 1.01–1.08 | 0.02 |
| Resection margin statusa | |||
| R0 ≥ 1 mm | Ref | Ref | Ref |
| R1 < 1 mm | 1.64 | 1.25–2.14 | < 0.001 |
aResection margin status was considered microscopically positive (R1 < 1 mm) if tumor cells were present within 1 mm of the closest resection margin, apart from the anterior surface
95% CI 95% confidence interval, HR Hazard ratio
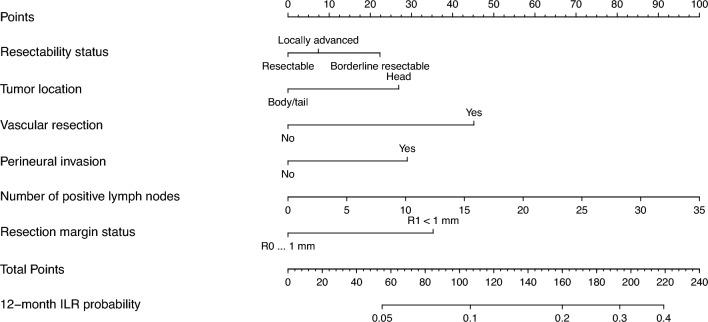
Prognostic Model
Risk scores were assigned to each predictor, with a total maximum score of 258 (Supplementary Table 2). The best predictive model had a C-index of 0.66 and calibration slope of 0.99 (Fig. 3, Supplementary Fig. 2).
Fig. 3.
Nomogram to predict the probability of ILR 12 months after resection of pancreatic ductal adenocarcinoma
Discussion
This large, nationwide cohort study identified several predictive factors for ILR among patients after resection of PDAC. The online model based on the best performing combination of these factors included resectability status, location of the tumor, vascular resection, perineural invasion, number of positive regional lymph nodes, and resection margin status.
Literature on predictive factors for ILR is relatively scarce. Although R1 < 1 mm seems to be a prognostic factor based on the study by Groot et al. and our study, Jones et al. did not find a significant association with ILR.5,10 Interestingly, the anterior margin was not included in the determination of resection margin in the study by Groot et al. and our study, while Jones et al. did include this margin in the assessment.5,10 This resulted in a higher number of R1 resections in the latter study and suggests that a positive resection margin is only predictive of ILR when the anterior surface is disregarded.10 Moreover, the inclusion of factors, such as resection margin status, resectability status, the need for vascular resection, presence of perineural invasion, and a higher number of positive locoregional lymph nodes in the final model, indicates that ILR mainly occurs from tumors that are locally more advanced. In addition, ILR seems to occur from biologically more favorable tumors compared with tumors that result in synchronous local and systemic recurrence. This suggests that tumors that develop into local recurrences have a different tumor biology than tumors that tend to spread systemically. Future studies should aim at revealing the underlying biological differences that contribute to distinct patterns of recurrence.
Adjuvant chemotherapy was found to be associated with reduced occurrence of ILR in the meta-analysis by Tanaka et al. (n = 894), whilst this association was not seen in the study by Groot et al. (n = 692) and our study.5,11 However, the five studies included in the meta-analysis by Tanaka et al. to determine the prognostic value of adjuvant chemotherapy exhibit considerable heterogeneity. Both retrospective cohort studies and prospective randomized controlled trials (RCTs) were analyzed.23–27 In the two retrospective studies included, the effect of adjuvant chemotherapy on ILR was calculated on the basis of analyses, which were not designed to determine this outcome.23,24 In one of the three RCTs, adjuvant chemotherapy was compared with adjuvant chemoradiotherapy, which impeded evaluation of the true effect of adjuvant chemotherapy.25 Additionally, the remaining two RCTs did not find a significant correlation between adjuvant chemotherapy and ILR, which aligns with the results of Groot et al. and our study.5,26,27 Therefore, the prognostic value of adjuvant chemotherapy in light of ILR seems questionable at least. Regarding the type of adjuvant chemotherapy, Jones et al. found that patients who received adjuvant gemcitabine/capecitabine showed a reduced likelihood of developing ILR compared with receiving gemcitabine alone.10 In addition, the 5-year results of the PRODIGE 24/Canadian Cancer Trials Group PA6 demonstrated a similar proportion of patients with ILR after modified 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin combination (mFOLFIRINOX) as after Gemcitabine monotherapy.28 Therefore, whether or not adjuvant chemotherapy is related to the development of ILR and whether it depends on the specific regimen received remains unclear and requires further investigation. However, Groot et al. did demonstrate that adjuvant chemoradiotherapy reduces the chance of developing ILR.5 This finding supports the hypothesis that ILR originates from residual microscopic tumor deposits, warranting further improvement of local therapies. Lastly, pathologic response to chemotherapy was not collected in this study but would be an interesting factor to investigate in future studies.
Patients with ILR after resection of PDAC demonstrate superior OS compared with patients with disease recurrence in the liver or at multiple sites and might specifically benefit from local ablative treatment.7 Over the past years, image-guided stereotactic body radiation therapy (SBRT) gained interest as potential local treatment for ILR, since it allows higher dose administration to the intended target area while sparing the surrounding organs.29–33 Additionally, magnetic resonance guided RT (MRgRT) with daily online adaptive treatment planning and tumor gating using continuous cine MR-images significantly enhances the visibility of both tumor and organs at risk.34–37 These advancements have made it possible to deliver higher biologically equivalent radiation doses to pancreatic lesions, potentially increasing treatment effiveness.38,39 Currently, the value of additional SBRT in patients with ILR is investigated in the nationwide randomized controlled ARCADE trial (NCT04881487).40 Besides the combination of SBRT and chemotherapy, SBRT and immunotherapy might be promising as well for the treatment of ILR. Radiotherapy might trigger tumor-associated antigens and upregulation of immune checkpoints, which are targeted by immunotherapy.41,42 Zhu et al. combined CT-guided SBRT with pembrolizumab and trametinib and compared it with CT-guided SBRT and gemcitabine.43 They have observed improved survival in patients with local recurrence after resection of PDAC that received pembrolizumab and trametinib, although this coincided with increased serious adverse events. The optimal combination of local and systemic treatment for ILR, however, is yet to be determined.
Before disease dissemination might occur, depriving patients’ opportunity to receive local ablative treatment, early identification of ILR through recurrence-focused follow-up with imaging at certain intervals seems important. Within the Netherlands, it is uncommon to conduct recurrence-focused follow-up imaging after PDAC resection, following recommendations in Dutch and European guidelines.19,44,45 Nevertheless, if routine postoperative imaging enables early detection of disease recurrence, this could potentially enhance timely treatment, which is anticipated to have a positive impact on survival. This hypothesis is currently being investigated in the RADAR-PANC trial (NCT04875325).
The detection of ILR on follow-up imaging often poses a challenging task. Distinguishing recurrent tumor tissue from postoperative fibrosis on postoperative imaging can be difficult. As a result, repetitive imaging is frequently required to assess whether the lesion grows over time, indicating tumor recurrence.8,9 Promising developments in computer science might provide a solution for this challenge, as artificial intelligence (AI) techniques have shown to be particularly helpful for imaging evaluation. For example, AI finds great utility in highlighting suspicious regions in imaging and classifying abnormalities as benign or malignant.46 The IMPACT consortium (NCT06055010) focuses on the development of an AI algorithm that can help clinicians to differentiate between postoperative fibrosis and pancreatic cancer recurrence, aiming to enhance an early and more accurate diagnosis of ILR.47 Until diagnostic accuracy is approved, however, the prognostic model developed in this study can be used to identify patients with a higher risk of developing ILR, which might be informative when evaluating patients’ follow-up imaging.
Main strengths of this study include the largest study population so far to investigate ILR specific risk factors, with subsequent integration of selected factors into a comprehensive online available prognostic model. However, the results of this study should be interpreted with acknowledgement of several limitations. First, baseline and perioperative data were collected in a prospective manner, while data on follow-up and recurrence were obtained retrospectively from the patients’ records. Second, the proportion of patients with ILR of 21% found in this study might be an underestimation as imaging is generally only performed in patients with symptoms of disease recurrence according to the Dutch national guidelines.19 This might have caused patients who suffered from initial ILR but developed systemic metastases before the diagnosis of disease recurrence to be misclassified into the group with local and systemic disease recurrence. Third, histological evidence of disease recurrence was only obtained in a minority of patients. When histological evidence was absent, presence of recurrence and corresponding recurrence location was based on consensus of a multidisciplinary meeting based on imaging and CA 19-9 levels. In case of ILR, this might mean that a small number of patients with postoperative fibrosis have wrongfully been classified as ILR. Lastly, borderline resectable disease is awarded more points than locally advanced disease in the nomogram, which seems unusual. However, the subgroup of patients with locally advanced disease was relatively small (only 7%), which could impede robustness of findings. Additionally, only patients with locally advanced disease who underwent pancreatic cancer resection were included. These patients reflect the best patients amongst the total group with locally advanced disease, as they responded well to neoadjuvant systemic therapy, which downsized their tumor, making them eligible for tumor resection. In contrast, most patients with borderline resectable disease in this study underwent upfront resection, as neoadjuvant therapy was only administered as part of a clinical trial during the study period.17,18 This is also reflected by their higher R1 resection margin rate (66% versus 44% in patients with locally advanced disease). As microscopically irradical resections (R1 < 1 mm) were associated with a higher probability of developing ILR, the higher number of points awarded to borderline disease might be explained by a higher probability of developing ILR based on the higher rate of microscopically irradical resections related to the difference in neoadjuvant treatment and patient selection.5
To conclude, multiple factors associated with ILR of PDAC have been identified in this nationwide, observational cohort study. The developed prognostic model, available at www.pancreascalculator.com, can be helpful to identify patients with a higher risk of developing ILR. This can be informative to healthcare professionals when evaluating patients’ postoperative imaging and for patient counseling.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgement
This work was supported by the Dutch Cancer Foundation (KWF) under Grant Agreement no. 12568.
Disclosure
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. P. W. Intven and L. A. Daamen has shared senior authorship.
Contributor Information
I. W. J. M. van Goor, Email: i.w.j.vangoor-5@umcutrecht.nl.
L. A. Daamen, Email: l.a.daamen-3@umcutrecht.nl.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer [published correction appears in N Engl J Med. 2004 Aug 12;351(7):726]. N Engl J Med. 2004;350(12):1200–10. 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473–81. 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395–406. 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 5.Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936–45. 10.1097/SLA.0000000000002234. [DOI] [PubMed] [Google Scholar]
- 6.Latenstein AEJ, van der Geest LGM, Bonsing BA, et al. Nationwide trends in incidence, treatment and survival of pancreatic ductal adenocarcinoma. Eur J Cancer. 2020;125:83–93. 10.1016/j.ejca.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Groot VP, Gemenetzis G, Blair AB, et al. Implications of the pattern of disease recurrence on survival following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2018;25(8):2475–83. 10.1245/s10434-018-6558-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balaj C, Ayav A, Oliver A, et al. CT imaging of early local recurrence of pancreatic adenocarcinoma following pancreaticoduodenectomy. Abdom Radiol (NY). 2016;41(2):273–82. 10.1007/s00261-015-0564-z. [DOI] [PubMed] [Google Scholar]
- 9.Jung W, Jang JY, Kang MJ, et al. The clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) in follow-up of curatively resected pancreatic cancer patients. HPB (Oxford). 2016;18(1):57–64. 10.1016/j.hpb.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones RP, Psarelli EE, Jackson R, et al. Patterns of recurrence after resection of pancreatic ductal adenocarcinoma: a secondary analysis of the ESPAC-4 randomized adjuvant chemotherapy trial. JAMA Surg. 2019;154(11):1038–48. 10.1001/jamasurg.2019.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M, Mihaljevic AL, Probst P, et al. Meta-analysis of recurrence pattern after resection for pancreatic cancer. Br J Surg. 2019;106(12):1590–601. 10.1002/bjs.11295. [DOI] [PubMed] [Google Scholar]
- 12.Strijker M, Mackay TM, Bonsing BA, et al. Establishing and coordinating a nationwide multidisciplinary study group: lessons learned by the Dutch Pancreatic Cancer Group. Ann Surg. 2020;271(4):e102–4. 10.1097/SLA.0000000000003779. [DOI] [PubMed] [Google Scholar]
- 13.Dutch Institute for Clinical Auditing. Dutch Pancreatic Cancer Audit (DPCA). Accessed January 9, 2024. http://www.dica.nl/dpca
- 14.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med. 2015;13:1. 10.1186/s12916-014-0241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 16.Dutch Pancreatic Cancer Group. Defining resectability in pancreatic cancer. Accessed May 11, 2023. https://dpcg.nl/richtlijnen/#:~:text=DPCG%2Ddefinities%20resectabiliteit%20pancreascarcinoom%20(PREOPANC%20trial%2C%20DPCG%202012)
- 17.Versteijne E, van Eijck CH, Punt CJ, et al. 2016 Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials. 2016;17(1):127. 10.1186/s13063-016-1262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen QP, van Dam JL, Bonsing BA, et al. Total neoadjuvant FOLFIRINOX versus neoadjuvant gemcitabine-based chemoradiotherapy and adjuvant gemcitabine for resectable and borderline resectable pancreatic cancer (PREOPANC-2 trial): study protocol for a nationwide multicenter randomized controlled trial. BMC Cancer. 2021;21(1):300. 10.1186/s12885-021-08031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Federatie Medische Specialisten. Richtlijn Pancreascarcinoom 2019. Accessed January 9, 2024. https://richtlijnendatabase.nl/richtlijn/pancreascarcinoom/startpagina.html
- 20.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):9399. 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 21.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59(10):1087–91. 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Dodge Y. The concise encyclopedia of statistics. Berlin: Springer; 2008. [Google Scholar]
- 23.Watanabe Y, Nishihara K, Niina Y, et al. Patients with lung recurrence after curative resection for pancreatic ductal adenocarcinoma have a better prognosis than those with recurrence at other sites. J Pancreas. 2017;18:54–61. [Google Scholar]
- 24.Labori KJ, Katz MH, Tzeng CW, et al. Impact of early disease progression and surgical complications on adjuvant chemotherapy completion rates and survival in patients undergoing the surgery first approach for resectable pancreatic ductal adenocarcinoma - A population-based cohort study. Acta Oncol. 2016;55(3):265–77. 10.3109/0284186X.2015.1068445. [DOI] [PubMed] [Google Scholar]
- 25.Van Laethem JL, Hammel P, Mornex F, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol. 2010;28(29):4450–6. 10.1200/JCO.2010.30.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101(6):908–15. 10.1038/sj.bjc.6605256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–77. 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 28.Conroy T, Castan F, Lopez A, et al. Five-year outcomes of FOLFIRINOX vs gemcitabine as adjuvant therapy for pancreatic cancer: a randomized clinical trial [published correction appears in JAMA Oncol. 2023 Jan 1;9(1):151]. JAMA Oncol. 2022;8(11):1571–8. 10.1001/jamaoncol.2022.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghaly M, Gogineni E, Saif MW. The evolving field of stereotactic body radiation therapy in pancreatic cancer. Pancreas (Fairfax). 2019;3(1):9–14. 10.17140/POJ-3-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crane CH. Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer. J Radiat Res. 2016;57(11):i53–7. 10.1093/jrr/rrw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong J, Patel K, Switchenko J, et al. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123(18):3486–93. 10.1002/cncr.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JJ, Hajj C, Reyngold M, et al. Stereotactic body radiation vs. intensity-modulated radiation for unresectable pancreatic cancer. Acta Oncol. 2017;56(12):1746–53. 10.1080/0284186X.2017.1342863. [DOI] [PubMed] [Google Scholar]
- 33.Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol. 2019;14(1):95. 10.1186/s13014-019-1309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudra S, Jiang N, Rosenberg SA, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8(5):2123–32. 10.1002/cam4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassanzadeh C, Rudra S, Bommireddy A, et al. Ablative five-fraction stereotactic body radiation therapy for inoperable pancreatic cancer using online MR-guided adaptation. Adv Radiat Oncol. 2020;6(1):100506. 10.1016/j.adro.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raaymakers BW, Lagendijk JJ, Overweg J, et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009;54(12):N229–37. 10.1088/0031-9155/54/12/N01. [DOI] [PubMed] [Google Scholar]
- 37.Lagendijk JJ, Raaymakers BW, van Vulpen M. The magnetic resonance imaging-linac system. Semin Radiat Oncol. 2014;24(3):207–9. 10.1016/j.semradonc.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Kontaxis C, Bol GH, Lagendijk JJ, Raaymakers BW. A new methodology for inter- and intrafraction plan adaptation for the MR-linac. Phys Med Biol. 2015;60(19):7485–97. 10.1088/0031-9155/60/19/7485. [DOI] [PubMed] [Google Scholar]
- 39.Henke L, Kashani R, Robinson C, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126(3):519–26. 10.1016/j.radonc.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 40.van Goor IWJM, Daamen LA, Besselink MG, et al. A nationwide randomized controlled trial on additional treatment for isolated local pancreatic cancer recurrence using stereotactic body radiation therapy (ARCADE) [published correction appears in Trials. 2023 Jan 24;24(1):55]. Trials. 2022;23(1):913. 10.1186/s13063-022-06829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hörner-Rieber J, Klüter S, Debus J, Adema G, Ansems M, Verheij M. MR-guided radiotherapy: the perfect partner for immunotherapy? Front Oncol. 2021;10:615697. 10.3389/fonc.2020.615697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gajiwala S, Torgeson A, Garrido-Laguna I, Kinsey C, Lloyd S. Combination immunotherapy and radiation therapy strategies for pancreatic cancer-targeting multiple steps in the cancer immunity cycle. J Gastrointest Oncol. 2018;9(6):1014–26. 10.21037/jgo.2018.05.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X, Cao Y, Liu W, et al. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: an open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2021;22(8):1093–102. 10.1016/S1470-2045(21)00286-2. [DOI] [PubMed] [Google Scholar]
- 44.Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up [published correction appears in Ann Oncol. 2017 Jul 1;28(suppl_4):iv167-iv168]. Ann Oncol. 2015;26(Suppl 5):v56–68. 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 45.Takaori K, Bassi C, Biankin A, et al. International Association of Pancreatology (IAP)/European Pancreatic Club (EPC) consensus review of guidelines for the treatment of pancreatic cancer. Pancreatology. 2016;16(1):14–27. 10.1016/j.pan.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Liu KL, Wu T, Chen PT, et al. Deep learning to distinguish pancreatic cancer tissue from non-cancerous pancreatic tissue: a retrospective study with cross-racial external validation. Lancet Digit Health. 2020;2(6):e303–13. 10.1016/S2589-7500(20)30078-9. [DOI] [PubMed] [Google Scholar]
- 47.Impact consortium. Accessed January 9, 2024. Available via https://impact-consortium.github.io/IMPACT/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.