Abstract
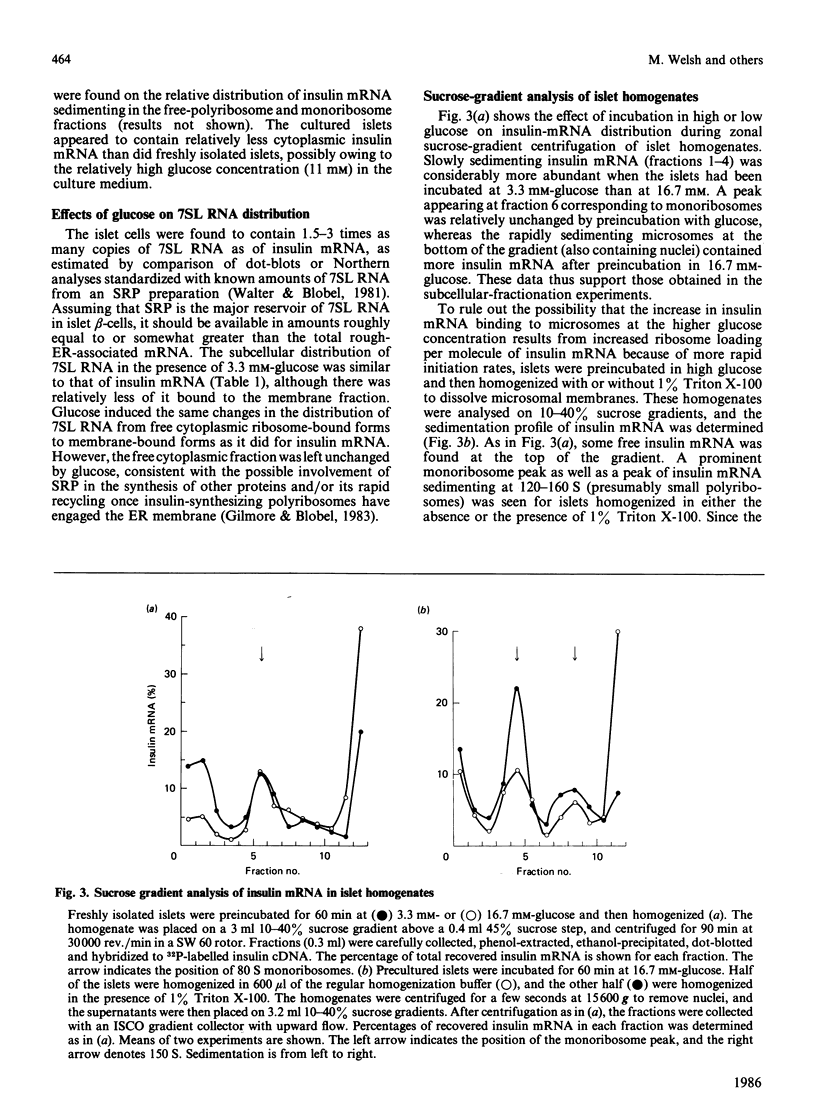
The biosynthesis of insulin in the islets of Langerhans is strongly controlled at the translational level by glucose. We have used a variety of experimental approaches in efforts to dissect the mechanisms underlying the stimulatory effect of glucose. To assess its effects on rates of peptide-chain elongation, isolated rat islets were labelled with [3H]leucine at different glucose concentrations in the presence or absence of low concentrations of cycloheximide. Under these conditions, at glucose concentrations up to 5.6 mM, endogenous insulin mRNA did not become rate-limiting for the synthesis of insulin, whereas stimulation of non-insulin protein synthesis was abolished by cycloheximide at all glucose concentrations, indicating either that insulin synthesis is selectively regulated at the level of elongation at glucose concentrations up to 5.6 mM, or that at these concentrations inactive insulin mRNA is transferred to an actively translating pool. Glucose-induced changes in the intracellular distribution of insulin mRNA in cultured islets were assessed by subcellular fractionation and blot-hybridization using insulin cDNA probes. At glucose concentrations above 3.3 mM, cytoplasmic insulin mRNA was increasingly transferred to fractions co-sedimenting with ribosomes, and relatively more of the ribosome-associated insulin mRNA became membrane-associated, consistent with effects of glucose above 3.3 mM on both the initiation of insulin mRNA and SRP (signal recognition particle)-mediated transfer of cytosolic nascent preproinsulin to the endoplasmic reticulum. When freshly isolated islets were homogenized and incubated with 125I-Tyr-tRNA, run-off incorporation of 125I into preproinsulin was increased by prior incubation of the islets at 16.7 mM-glucose. The addition of purified SRP receptor increased the run-off incorporation of [125I]iodotyrosine into preproinsulin, especially when the islets had been preincubated at 16.7 mM-glucose. These findings taken together suggest that glucose may stimulate elongation rates of nascent preproinsulin at concentrations up to 5.6 mM, stimulates initiation of protein synthesis involving both insulin and non-insulin mRNA at concentrations above 3.3 mM, and increases the transfer of initiated insulin mRNA molecules from the cytoplasm to microsomal membranes by an SRP-mediated mechanism that involves the modification of interactions between SRP and its receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Mostov K. E., Blobel G. Mechanisms of integration of de novo-synthesized polypeptides into membranes: signal-recognition particle is required for integration into microsomal membranes of calcium ATPase and of lens MP26 but not of cytochrome b5. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7249–7253. doi: 10.1073/pnas.80.23.7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978 Jun;14(6):397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- Anjaneyulu K., Anjaneyulu R., Sener A., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. Thiol: disulfide balance in pancreatic islets. Biochimie. 1982 Jan;64(1):29–36. doi: 10.1016/s0300-9084(82)80606-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Bunce J., Lowry M., Hansen S. E., Hedeskov C. J. The effect of sugars on (pro)insulin biosynthesis. Biochem J. 1978 Aug 15;174(2):517–526. doi: 10.1042/bj1740517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Bassett J. M., Randle P. J. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972 Feb;126(3):525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft S. J., Weerasinghe L. C., Randle P. J. Interrelationship of islet metabolism, adenosine triphosphate content and insulin release. Biochem J. 1973 Feb;132(2):223–231. doi: 10.1042/bj1320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag J., Sarkar S. Cytoplasmic nonpolysomal messenger ribonucleoprotein containing actin messenger RNA in chicken embryonic muscles. Biochemistry. 1975 Aug 26;14(17):3800–3807. doi: 10.1021/bi00688a012. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A. H., Walter P., Blobel G. Translocation of a lysosomal enzyme across the microsomal membrane requires signal recognition particle. Biochem Biophys Res Commun. 1983 Aug 30;115(1):275–280. doi: 10.1016/0006-291x(83)91000-8. [DOI] [PubMed] [Google Scholar]
- Eskridge E. M., Shields D. Cell-free processing and segregation of insulin precursors. J Biol Chem. 1983 Oct 10;258(19):11487–11491. [PubMed] [Google Scholar]
- Gilmore R., Blobel G. Transient involvement of signal recognition particle and its receptor in the microsomal membrane prior to protein translocation. Cell. 1983 Dec;35(3 Pt 2):677–685. doi: 10.1016/0092-8674(83)90100-9. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Danielsson A. Adenosine triphosphate levels of mammalian pancreatic B cells after stimulation with glucose and hypoglycemic sulfonylureas. Diabetes. 1969 Aug;18(8):509–516. doi: 10.2337/diab.18.8.509. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Taylor K. W. Effects of glucose concentration on incorporation of [3H]leucine into insulin using isolated mammalian islets of Langerhans. Biochim Biophys Acta. 1966 Dec 28;130(2):519–521. doi: 10.1016/0304-4165(66)90250-9. [DOI] [PubMed] [Google Scholar]
- Itoh N., Okamoto H. Translational control of proinsulin synthesis by glucose. Nature. 1980 Jan 3;283(5742):100–102. doi: 10.1038/283100a0. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Herbert P., Campbell E. A., Hunt T. The roles of sugar phosphates and thiol-reducing systems in the control of reticulocyte protein synthesis. Eur J Biochem. 1983 Mar 15;131(2):313–324. doi: 10.1111/j.1432-1033.1983.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Jagus R., Safer B. Activity of eukaryotic initiation factor 2 is modified by processes distinct from phosphorylation. I. Activities of eukaryotic initiation factor 2 and eukaryotic initiation factor 2 alpha kinase in lysate gel filtered under different conditions. J Biol Chem. 1981 Feb 10;256(3):1317–1323. [PubMed] [Google Scholar]
- Konieczny A., Safer B. Purification of the eukaryotic initiation factor 2-eukaryotic initiation factor 2B complex and characterization of its guanine nucleotide exchange activity during protein synthesis initiation. J Biol Chem. 1983 Mar 10;258(5):3402–3408. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lernmark A., Nathans A., Steiner D. F. Preparation and characterization of plasma membrane-enriched fractions from rat pancreatic islets. J Cell Biol. 1976 Nov;71(2):606–623. doi: 10.1083/jcb.71.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Cell-free modulation of proinsulin synthesis. Science. 1977 Nov 11;198(4317):620–622. doi: 10.1126/science.918657. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Hutton J. C., Kawazu S., Herchuelz A., Valverde I., Sener A. The stimulus-secretion coupling of glucose-induced insulin release. XXXV. The links between metabolic and cationic events. Diabetologia. 1979 May;16(5):331–341. doi: 10.1007/BF01223623. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis. I. On the mechanism of glucose stimulation. J Biol Chem. 1972 Feb 25;247(4):1194–1199. [PubMed] [Google Scholar]
- Permutt M. A., Kipnis D. M. Insulin biosynthesis: studies of Islet polyribosomes (nascent peptides-sucrose gradient analysis-gel filtration). Proc Natl Acad Sci U S A. 1972 Feb;69(2):505–509. doi: 10.1073/pnas.69.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. A procedure for the quantitative recovery of homogeneous populations of undegraded free and bound polysomes from rat liver. Biochemistry. 1976 Apr 20;15(8):1704–1712. doi: 10.1021/bi00653a018. [DOI] [PubMed] [Google Scholar]
- Scherberg N. H., Seo H., Hynes R. Incorporation of radioiodotyroisines into proteins formed during cell-free translation. J Biol Chem. 1978 Mar 25;253(6):1773–1779. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle C. A., Mankin H. J., Avruch J., Treadwell B. V. Insulin promoted decrease in the phosphorylation of protein synthesis initiation factor eIF-2. Biochem Biophys Res Commun. 1984 May 31;121(1):134–140. doi: 10.1016/0006-291x(84)90697-1. [DOI] [PubMed] [Google Scholar]
- Ullu E., Murphy S., Melli M. Human 7SL RNA consists of a 140 nucleotide middle-repetitive sequence inserted in an alu sequence. Cell. 1982 May;29(1):195–202. doi: 10.1016/0092-8674(82)90103-9. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Subcellular distribution of signal recognition particle and 7SL-RNA determined with polypeptide-specific antibodies and complementary DNA probe. J Cell Biol. 1983 Dec;97(6):1693–1699. doi: 10.1083/jcb.97.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]