Abstract
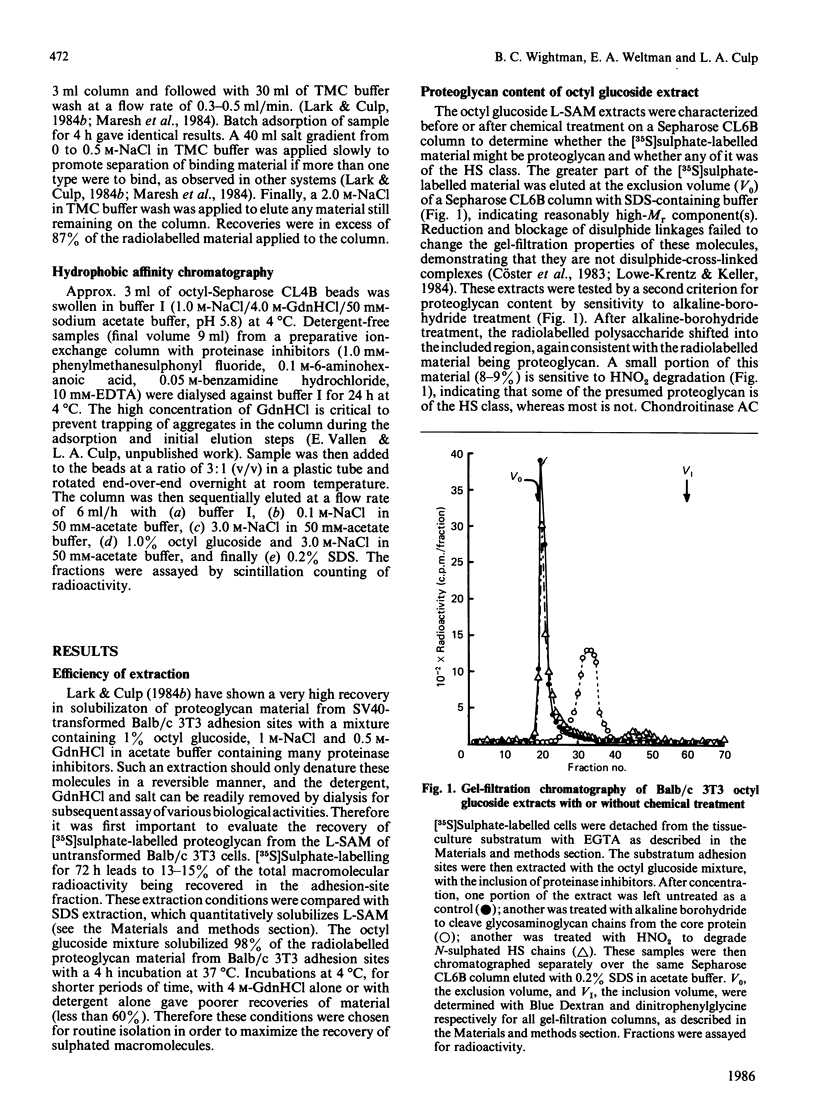
Proteoglycans on the cell surface play critical roles in the adhesion of fibroblasts to a fibronectin-containing extracellular matrix, including the model mouse cell line Balb/c 3T3. In order to evaluate the biochemistry of these processes, long-term [35S]sulphate-labelled proteoglycans were extracted quantitatively from the adhesion sites of 3T3 cells, after their EGTA-mediated detachment from the substratum, by using an extractant containing 1% octyl glucoside, 1 M-NaCl and 0.5 M-guanidinium chloride (GdnHCl) in buffer with many proteinase inhibitors. Greater than 90% of the material was identified as a large chondroitin sulphate proteoglycan (Kav. = 0.4 on a Sepharose CL2B column), and the remainder was identified as a smaller heparan sulphate proteoglycan; only small amounts of free chains of glycosaminoglycan were observed in these sites. These extracts were fractionated on DEAE-Sepharose columns under two different sets of elution conditions: with acetate buffer (termed DEAE-I) or with acetate buffer supplemented with 8 M-urea (termed DEAE-II). Under DEAE-I conditions about one-half of the material was eluted as a single peak and the remainder required 4 M-GdnHCl in order to recover it from the column; in contrast, greater than 90% of the material was eluted as a single peak from DEAE-II columns. Comparison of the elution of [35S]sulphate-labelled proteoglycan with that of 3H-labelled proteins from these two columns, as well as mixing experiments, indicated that the GdnHCl-sensitive proteoglycans were trapped at the top of columns, partially as a consequence of their association with proteins in these adhesion-site extracts. Affinity chromatography of these proteoglycans on columns of either immobilized platelet factor 4 or immobilized plasma fibronectin revealed that most of the chondroitin sulphate proteoglycan and the heparan sulphate proteoglycan bound to platelet factor 4 but that only the heparan sulphate proteoglycan bound to fibronectin, providing a ready means of separating the two proteoglycan classes. Affinity chromatography on octyl-Sepharose columns to test for hydrophobic domains in their core proteins demonstrated that a high proportion of the heparan sulphate proteoglycan but none of the chondroitin sulphate proteoglycan bound to the hydrophobic matrix. These results are discussed in light of the possible functional importance of the chondroitin sulphate proteoglycan in the detachment of cells from extracellular matrix and in light of previous affinity fractionations of proteoglycans from the substratum-adhesion sites of simian-virus-40-transformed 3T3 cells.
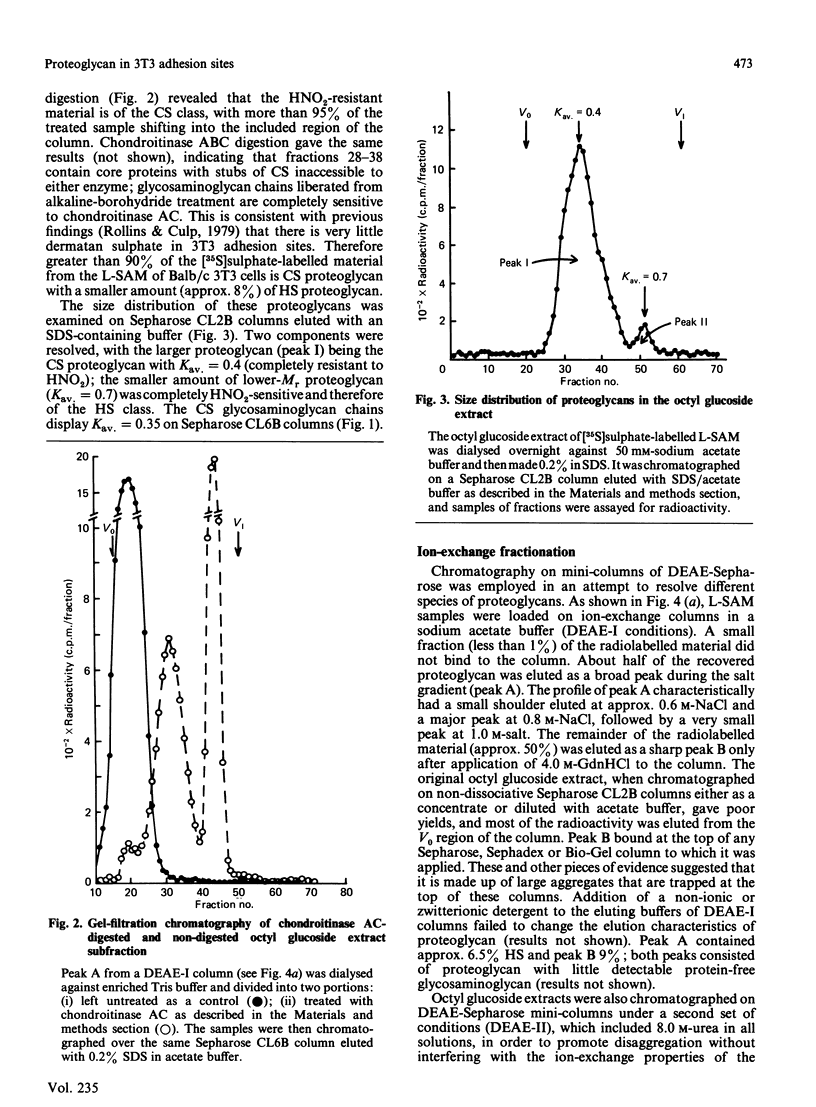
Full text
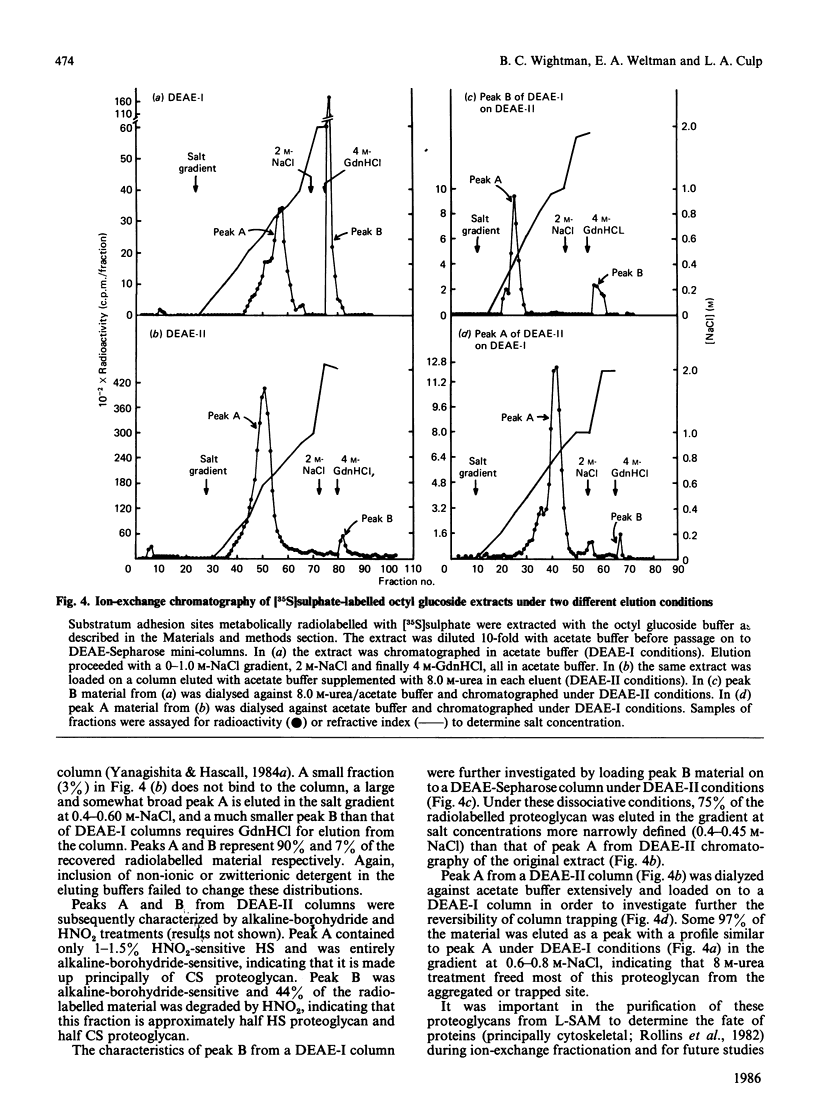
PDF


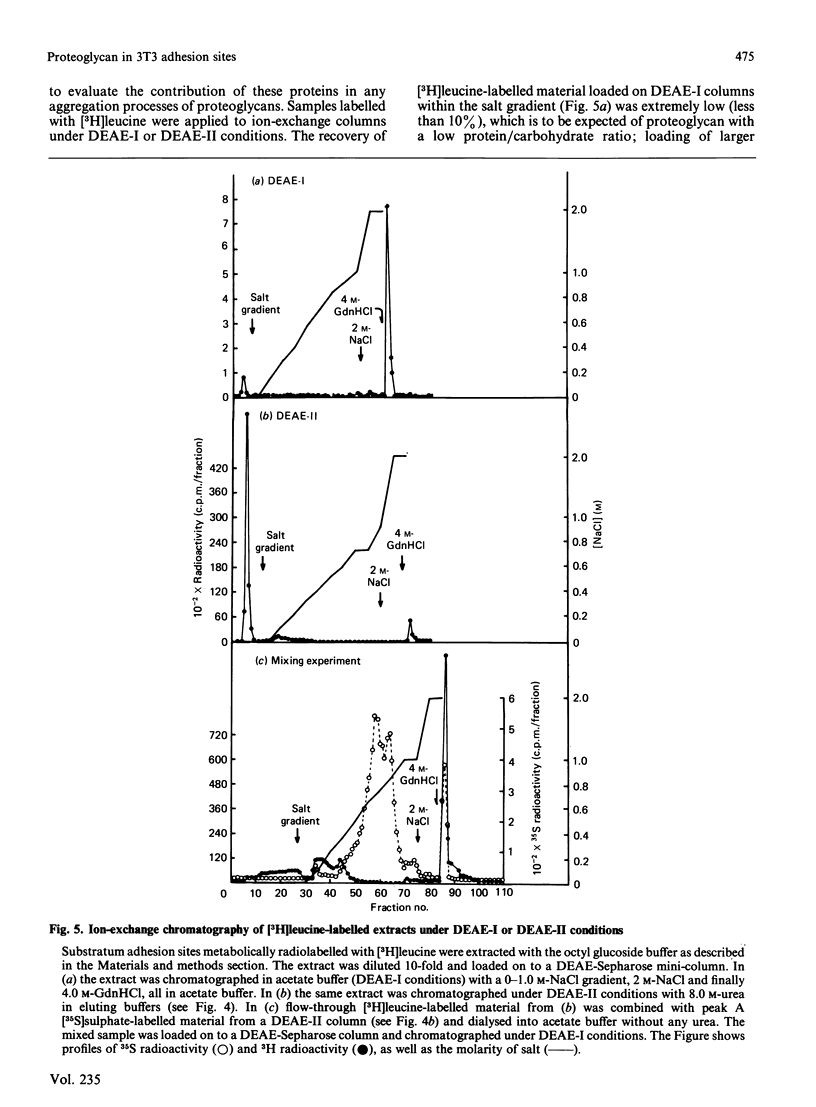


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aplin J. D., Hughes R. C., Jaffe C. L., Sharon N. Reversible cross-linking of cellular components of adherent fibroblasts to fibronectin and lectin-coated substrata. Exp Cell Res. 1981 Aug;134(2):488–494. doi: 10.1016/0014-4827(81)90453-5. [DOI] [PubMed] [Google Scholar]
- Barber A. J., Käser-Glanzmann R., Jakábová M., Lüscher E. F. Characterization of a chondroitin 4 -sulfate proteoglycan carrier for heparin neutralizing activity (platelet factor 4 ) released from human blood platelets. Biochim Biophys Acta. 1972 Dec 29;286(2):312–329. [PubMed] [Google Scholar]
- Beyth R. J., Culp L. A. Complementary adhesive responses of human skin fibroblasts to the cell-binding domain of fibronectin and the heparan sulfate-binding protein, platelet factor-4. Exp Cell Res. 1984 Dec;155(2):537–548. doi: 10.1016/0014-4827(84)90213-1. [DOI] [PubMed] [Google Scholar]
- Bienkowski M. J., Conrad H. E. Kinetics of proteoheparan sulfate synthesis, secretion, endocytosis, and catabolism by a hepatocyte cell line. J Biol Chem. 1984 Nov 10;259(21):12989–12996. [PubMed] [Google Scholar]
- Brennan M. J., Oldberg A., Hayman E. G., Ruoslahti E. Effect of a proteoglycan produced by rat tumor cells on their adhesion to fibronectin-collagen substrata. Cancer Res. 1983 Sep;43(9):4302–4307. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Carrino D. A., Caplan A. I. Isolation and preliminary characterization of proteoglycans synthesized by skeletal muscle. J Biol Chem. 1982 Dec 10;257(23):14145–14154. [PubMed] [Google Scholar]
- Cathcart M. K., Culp L. A. Initial studies of the molecular organization of the cell-substrate adhesion site. Biochim Biophys Acta. 1979 Sep 21;556(2):331–343. doi: 10.1016/0005-2736(79)90052-x. [DOI] [PubMed] [Google Scholar]
- Cöster L., Malström A., Carlstedt I., Fransson L. A. The core protein of fibroblast proteoheparan sulphate consists of disulphide-bonded subunits. Biochem J. 1983 Nov 1;215(2):417–419. doi: 10.1042/bj2150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Tarone G., Knudsen K., Damsky C., Comoglio P. M. Cleavage of a 135 kD cell surface glycoprotein correlates with loss of fibroblast adhesion to fibronectin. Exp Cell Res. 1985 Jan;156(1):182–190. doi: 10.1016/0014-4827(85)90272-1. [DOI] [PubMed] [Google Scholar]
- Hascall V. C. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. doi: 10.1002/jss.400070110. [DOI] [PubMed] [Google Scholar]
- Huang S. S., Huang J. S., Deuel T. F. Proteoglycan carrier of human platelet factor 4. Isolation and characterization. J Biol Chem. 1982 Oct 10;257(19):11546–11550. [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976 Jun;21(1):129–159. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflexion study. J Cell Sci. 1980 Apr;42:81–116. doi: 10.1242/jcs.42.1.81. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Pettersson I., Hök M. Cell-surface heparan sulfate: an intercalated membrane proteoglycan. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5371–5375. doi: 10.1073/pnas.78.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox P., Wells P. Cell adhesion and proteoglycans. I. The effect of exogenous proteoglycans on the attachment of chick embryo fibroblasts to tissue culture plastic and collagen. J Cell Sci. 1979 Dec;40:77–88. doi: 10.1242/jcs.40.1.77. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Vogel K. G., Nicolson G. L. Solubilization and degradation of subendothelial matrix glycoproteins and proteoglycans by metastatic tumor cells. J Biol Chem. 1982 Mar 10;257(5):2678–2686. [PubMed] [Google Scholar]
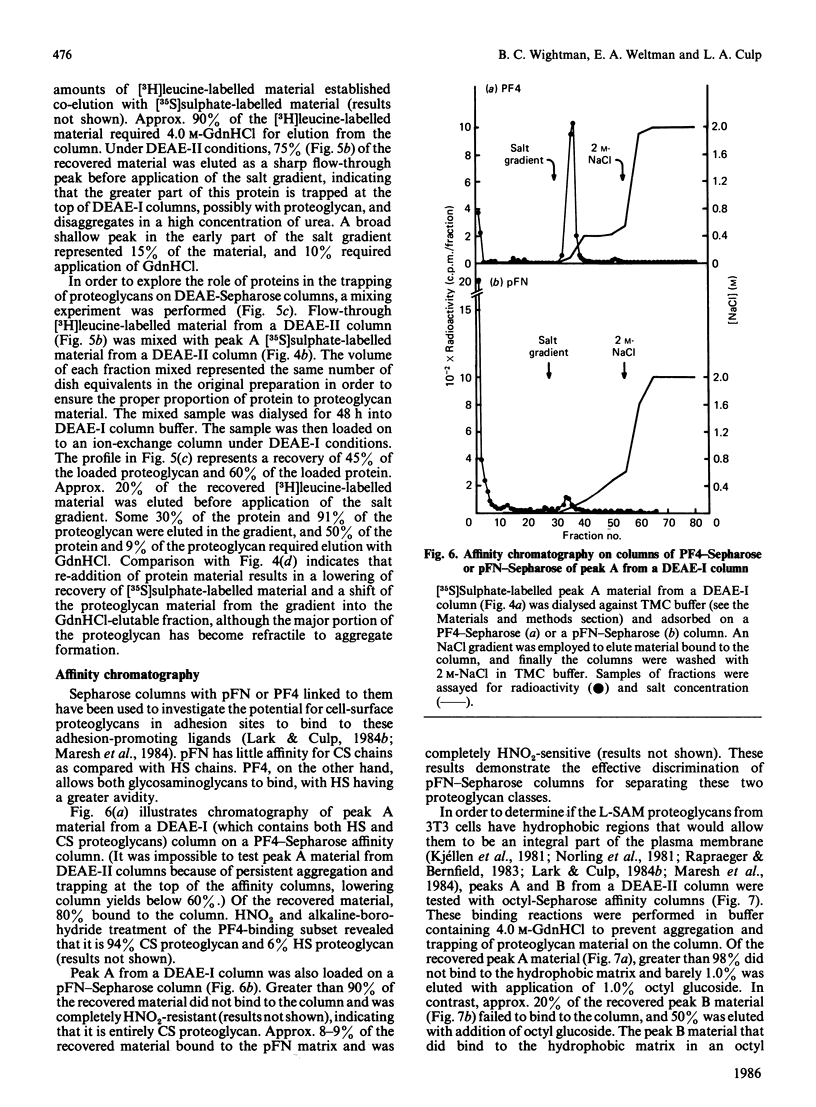
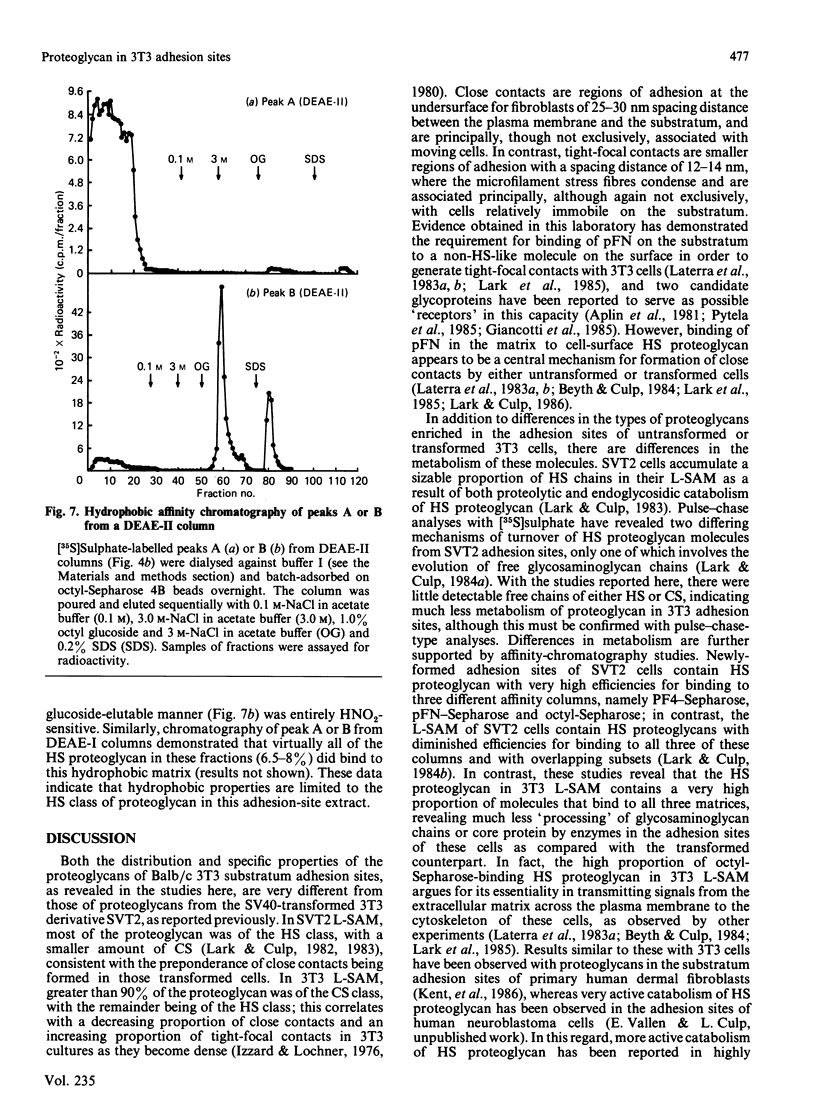
- Lark M. W., Culp L. A. Modification of proteoglycans during maturation of fibroblast substratum adhesion sites. Biochemistry. 1983 Apr 26;22(9):2289–2296. doi: 10.1021/bi00278a036. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Culp L. A. Multiple classes of heparan sulfate proteoglycans from fibroblast substratum adhesion sites. Affinity fractionation on columns of platelet factor 4, plasma fibronectin, and octyl-sepharose. J Biol Chem. 1984 Jun 10;259(11):6773–6782. [PubMed] [Google Scholar]
- Lark M. W., Culp L. A. Selective solubilization of hyaluronic acid from fibroblast substratum adhesion sites. J Biol Chem. 1982 Dec 10;257(23):14073–14080. [PubMed] [Google Scholar]
- Lark M. W., Culp L. A. Turnover of heparan sulfate proteoglycans from substratum adhesion sites of murine fibroblasts. J Biol Chem. 1984 Jan 10;259(1):212–217. [PubMed] [Google Scholar]
- Lark M. W., Laterra J., Culp L. A. Close and focal contact adhesions of fibroblasts to a fibronectin-containing matrix. Fed Proc. 1985 Feb;44(2):394–403. [PubMed] [Google Scholar]
- Laterra J., Norton E. K., Izzard C. S., Culp L. A. Contact formation by fibroblasts adhering to heparan sulfate-binding substrata (fibronectin or platelet factor 4). Exp Cell Res. 1983 Jun;146(1):15–27. doi: 10.1016/0014-4827(83)90320-8. [DOI] [PubMed] [Google Scholar]
- Laterra J., Silbert J. E., Culp L. A. Cell surface heparan sulfate mediates some adhesive responses to glycosaminoglycan-binding matrices, including fibronectin. J Cell Biol. 1983 Jan;96(1):112–123. doi: 10.1083/jcb.96.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Lowe-Krentz L. J., Keller J. M. Disulfide-bonded aggregates of heparan sulfate proteoglycans. Biochemistry. 1984 Jun 5;23(12):2621–2627. doi: 10.1021/bi00307a013. [DOI] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Maresh G. A., Chernoff E. A., Culp L. A. Heparan sulfate proteoglycans of human neuroblastoma cells: affinity fractionation on columns of platelet factor-4+. Arch Biochem Biophys. 1984 Sep;233(2):428–437. doi: 10.1016/0003-9861(84)90464-8. [DOI] [PubMed] [Google Scholar]
- Norling B., Glimelius B., Wasteson A. Heparan sulfate proteoglycan of cultured cells: demonstration of a lipid- and a matrix-associated form. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1265–1272. doi: 10.1016/0006-291x(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Hayman E. G., Ruoslahti E. Isolation of a chondroitin sulfate proteoglycan from a rat yolk sac tumor and immunochemical demonstration of its cell surface localization. J Biol Chem. 1981 Nov 10;256(21):10847–10852. [PubMed] [Google Scholar]
- Oldberg A., Ruoslahti E. Interactions between chondroitin sulfate proteoglycan, fibronectin, and collagen. J Biol Chem. 1982 May 10;257(9):4859–4863. [PubMed] [Google Scholar]
- Oohira A., Wight T. N., Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983 Feb 10;258(3):2014–2021. [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. A putative membrane proteoglycan associates quantitatively with lipid vesicles. J Biol Chem. 1983 Mar 25;258(6):3632–3636. [PubMed] [Google Scholar]
- Rich A. M., Pearlstein E., Weissmann G., Hoffstein S. T. Cartilage proteoglycans inhibit fibronectin-mediated adhesion. Nature. 1981 Sep 17;293(5829):224–226. doi: 10.1038/293224a0. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Culp L. A. Glycosaminoglycans in the substrate adhesion sites of normal and virus-transformed murine cells. Biochemistry. 1979 Jan 9;18(1):141–148. doi: 10.1021/bi00568a022. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Metabolism of proteoglycans in rat ovarian granulosa cell culture. Multiple intracellular degradative pathways and the effect of chloroquine. J Biol Chem. 1984 Aug 25;259(16):10270–10283. [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Proteoglycans synthesized by rat ovarian granulosa cells in culture. Isolation, fractionation, and characterization of proteoglycans associated with the cell layer. J Biol Chem. 1984 Aug 25;259(16):10260–10269. [PubMed] [Google Scholar]


