Abstract
Vitiligo is a dermatological disease characterized by loss of melanocytes, causing non-scaly white macules on the skin. Zinc, copper, and selenium are important micronutrients that play a role in the normal functioning of the body and have been found to potentially aid in vitiligo treatment, although the relationship between their serum levels and vitiligo is not yet fully understood. This is a systematic review aimed at assessing the levels of serum zinc, copper, and selenium and their association with vitiligo. This review was performed following the Preferred Reporting Items of the systematic Review and Meta-Analysis (PRISMA) checklist and Cochrane guidelines. A comprehensive literature search was conducted on PubMed, Google Scholar and 41 studies published between 1970 and 2022 including 3353 vitiligo cases and 10,638 controls were included in the meta-analysis conducted from August 2022 till September 2023. The quality of the studies was assessed using the National Heart Lung and Blood Institute Study Quality Assessment tool, and the risk of bias was represented using the RobVis tool. The statistical analysis was performed using Review Manager (RevMan) Version 5.4. This meta-analysis indicate a significant decline in serum zinc levels (Z = 4.97; P < 0.0001; SMD = − 0.86; 95% CI − 1.19 to − 0.52) in vitiligo group with high statistical heterogeneity (Tau2 = 0.74; Chi2 = 513.95, d.f. = 26 [P < 0.00001]; I2 = 95%). Similarly for serum copper levels there was decline (Z = 2.43; P < 0.0001; SMD = − 0.50; 95% confidence interval [CI] − 0.91 to − 0.10) in vitiligo group and high statistical heterogeneity (Tau2 = 0.92; Chi2 = 475.10, d.f. = 22 [P < 0.00001]; I2 = 95%). On the other hand, there was a increase of serum selenium levels in the vitiligo group (Z = 0.56; P < 0.0001; SMD = 0.23; 95% confidence interval [CI], 0.58 to 1.04) and the results reveals high statistical heterogeneity among studies (Tau2 = 1.93; Chi2 = 406.44, d.f. = 11 [P < 0.00001]; I2 = 97%) in vitiligo patients compared to healthy controls. Publication bias was not found for the studies analysed. This study analyses the association of serum micronutrient levels and vitiligo among patients and controls from published research along with sub-group analysis specific to Asian populations using a meta-analysis. Low serum levels of Zinc and copper and high selenium levels are associated with Vitiligo.
Keywords: [Mesh], Vitiligo, Copper, Selenium, Zinc, Oxidative stress, Genetic predisposition to disease
Subject terms: Biochemistry, Diseases, Medical research
Introduction
Vitiligo is an acquired dermatological disease characterised by selective and progressive loss of melanocytes that lead to milky white, non-scaly macules on the skin, hair and mucous membranes1. Vitiligo is reported in people of all ethnic groups irrespective of their race, gender & skin types2. Though the condition has been known for centuries, vitiligo remains a target for stigmatization and causes quality of life impairment in a large population of patients. It affects up to 1% of the world population making it the most common depigmentation disorder. Vitiligo occursinall races but is clearly more visible in dark-skinned people because of strong contrast. Emotional stress, sunburn, a major illness or surgical procedure, any physical trauma are some of the major triggers for vitiligo3. More than 50% of the patients develop the disease before 20 years of age. Onset of disease at later stage is unusual and also raises concerns about other autoimmune associated disorders such as thyroid dysfunction, rheumatoid arthritis, diabetes mellitus, and alopecia areata as co-morbidity with vitiligo.
Zinc (Zn), Copper (Cu) and Selenium are crucial micronutrients found in trace quantities in the human body and have major functions in homeostatic mechanisms of the body, such as specific immunity, inflammation and oxidative stress (OS)4,5. Zinc acts as a co-factor for over 300 metalloenzymes and over 2000 transcription factors and is responsible for the cells, tissues and human body to function normally. For healthy adults, normal zinc levels in serum ranges from 70 to 180 µg per 100 ml with RDA of 8 mg per day for women and 11 mg per day for men. Zinc performs numerous functions in our body such as in the synthesis of melanin, in immune system and in reproductive health. It is also observed that supplementation of zinc is valuable adjuvant treatment in vitiligo patients because of its cell reinforcement and against apoptotic properties5,6. It is seen that use of zinc and other trace elements not only regresses the size of lesions but also increase melanogenesis7. During melanogenesis, copper and zinc serve as metalloenzymes which therefore catalyzes 5,6-dihydroxy indole-2 carboxylic acid (DHICA) leading to eu-melanin development by causing a rearrangement in dopachrome8. Also due to antioxidant property of zinc, copper and other trace elements also prevents the toxic effect of free radicals9. It is still not known that how levels of zinc and copper are associated with sera of vitiligo patients. Many studies have reported reduction in serum Zinc10–15 and Copper levels16–21 in patients suffering from vitiligo, while on the other hand, Zaki et al. 2020, Salem et al. 2018, and several other studies states that high serum zinc levels are associated with vitiligo risk7,18,19,22–27. Similarly Wang et al. 2012, Yao et al. 2011 and several similar studies showed higher levels of serum copper to be associated with vitiligo risk23,25,28–32. The available evidence is not uniform and contradictory conclusions have been reported.
Selenium is an important mineral for normal homeostasis in human because it is present in more than 30 seleno-proteins and can influence in the immune system. Selenium plays a vital role in function of Glutathione peroxidase enzyme which plays pivotal role in redox regulation and is implicated in vitiligo pathogenesis. Selenium deficiency predisposes to several dermatological disorders and low selenium levels are observed in severe psoriasis. Selenium supplementation has been found to be useful in vitiligo therapy. Several reports concluded that the selenium level in vitiligo were found to be slightly elevated11,33,34, while others reported reduced levels20,35,36 or no difference37,38Therefore, such controversial reports encouraged us to conduct a meta analysis and systematic review to summarize the studies on level of serum zinc, copper and selenium levels in vitiligo patients, so as to analyse their association with vitiligo and to understand its role in pathogenesis and treatment outcome.
Materials and method
The meta-analysis was conducted to assess the serum levels of zinc, copper and selenium in individuals with Vitiligo and analyse it association with Vitiligo. This review has been performed in concordance with the Preferred Reporting Items of the systematic Review and Meta-Analysis (PRISMA) checklist and also each step of the review has been performed as per the Cochrane guidelines.
Review registration
This Systematic review and Metanalysis’ protocol was analysed and registered with PROSPERO (International Prospective Register of Systematic Reviews) database vide registration number CRD42022367699after confirming that no similar study assessing the effect of three metal ions, other influencing factors and vitiligo was being performed.
Search strategy
A computer based comprehensive literature search was conducted on PubMed, Google Scholar using the search terms “vitiligo”, “vitiligo micronutrients”, “zinc”, “copper”, “selenium”, “serum zinc levels vitiligo”, “serum copper levels vitiligo” and “serum selenium levels vitiligo” studies from 3rdAugust 2022 till 10th of September 2023 and 1501 articles were found. Further, a secondary search was performed by the expert reviewer and 82 articles were found. Additionally, a manual search from the bibliography of selected studies was used to add articles if they met the standards of the selection criteria for the study and 10 articles were found.
Selection criteria
The selection criteria were defined based on the PICO construct, where studies that included:
-
i.
Patients with physicians’ diagnosed vitiligo with overt symptoms including localised, generalized, or universal vitiligo and stable or active vitiligo and control groups involving healthy individuals;
-
ii.
The study participants who were subjected to biochemical analysis of their blood to reveal amounts of specific mineral micronutrients selenium, zinc and copper.
-
iii.
All observational studies (Cohorts, Case Controls) studies with the search terms where comparators exist were included.
-
iv.
Published, Pre-Published, Pre-Prints available as full texts online in English language published globally were included.
The selected articles were further filtered as per the pre-decided exclusion criteria if they were:
-
i.
Case reports, Case series, Review articles of any sort, editorial or expert comments, conference presentations, authors’ response to publications or texts, incomplete papers or papers with only abstracts available and incomplete clinical trials.
-
ii.
Animal Studies, AI models, in-vitro studies and other non-human clinical studies
-
iii.
Studies whose full-text cannot be found online
-
iv.
Studies with unclear, incomplete or overlapping data and evidences that cannot be reliably extracted for analysis.
Data search
After completion of the search, articles from PubMed, Google Scholar, the collected articles (n = 1583) were screened and irrelevant studies were excluded, and remaining studies were exported onto a Microsoft Excel Spreadsheet for further screening (n = 127). Duplicates were removed (n = 80) through the Data cleanup tool using the ‘Title-Author-Date of publication’ criteria and insufficient data articles were removed, the remaining 41 articles were selected for full-text analysis (AS and AnK). The following data were collected from each study such as first author, year of publication, country, number of cases and controls, mean values and standard deviation values. General characteristics of included studies are presented in Table 1 (by AnK) and further reviewed (by AsK and AS).
Table 1.
General characteristics of 41 included studies in meta- analysis.
| S. No. | Author | Year | Ions analysed | Country | Total participants | Vitiligo cases | Healthy control |
|---|---|---|---|---|---|---|---|
| 1 | Archana | 2021 | Copper | India | 120 | 60 | 60 |
| 2 | Muawia | 2020 | Copper | Sudan | 100 | 50 | 50 |
| 3 | Zaki | 2020 | Zinc | Egypt | 100 | 50 | 50 |
| 4 | Saniee | 2019 | Zinc | Iran | 196 | 98 | 98 |
| 5 | Muhammad | 2019 | Zinc | Sudan | 100 | 50 | 50 |
| 6 | Narang | 2018 | Zinc, Copper | India | 160 | 100 | 60 |
| 7 | Salem | 2018 | Zinc, Copper | Egypt | 100 | 50 | 50 |
| 8 | Mirnezami | 2018 | Zinc | Iran | 206 | 103 | 103 |
| 9 | Wacewicz | 2017 | Zinc, Copper, Selenium | Poland | 108 | 50 | 58 |
| 10 | Song | 2017 | Selenium | China | 124 | 62 | 62 |
| 11 | Mogaddam | 2017 | Zinc | Iran | 200 | 100 | 100 |
| 12 | Mirza | 2016 | Zinc | Bangladesh | 110 | 60 | 50 |
| 13 | Basha | 2014 | Zinc | Egypt | 120 | 60 | 60 |
| 14 | Wang | 2012 | Zinc, Copper | China | 240 | 120 | 120 |
| 15 | Yao | 2011 | Zinc, Copper, Selenium | China | 140 | 90 | 50 |
| 16 | Wang | 2011 | Zinc, Copper | China | 58 | 28 | 30 |
| 17 | Barikbin | 2011 | Selenium | Iran | 105 | 60 | 45 |
| 18 | Zhao | 2011 | Selenium | China | 52 | 36 | 16 |
| 19 | Wu | 2010 | Zinc, Copper | China | 140 | 70 | 70 |
| 20 | Haider | 2010 | Zinc | Bangladesh | 60 | 30 | 30 |
| 21 | Ali | 2010 | Zinc | Bangladesh | 120 | 60 | 60 |
| 22 | Ozturk | 2008 | Selenium | Turkey | 60 | 30 | 30 |
| 23 | Wang | 2007 | Zinc, Copper | China | 88 | 68 | 20 |
| 24 | Ines | 2006 | Selenium | Tunisia | 76 | 36 | 40 |
| 25 | Gu | 2005 | Copper | China | 66 | 30 | 36 |
| 26 | Kang | 2002 | Zinc, Copper, Selenium | China | 64 | 30 | 34 |
| 27 | Li | 2001 | Copper | China | 126 | 96 | 30 |
| 28 | Beazley | 1999 | Selenium | UK | 122 | 61 | 61 |
| 29 | Tu | 1998 | Selenium | China | 66 | 29 | 37 |
| 30 | Tan | 1997 | Zinc. Copper | China | 787 | 37 | 750 |
| 31 | Zhou | 1996 | Zinc, Copper | China | 46 | 26 | 20 |
| 32 | Wang | 1996 | Zinc, Copper | China | 189 | 48 | 141 |
| 33 | Wang | 1993 | Zinc, Copper, Selenium | China | 64 | 30 | 34 |
| 34 | Shi | 1993 | Zinc, Copper | China | 270 | 120 | 150 |
| 35 | Tu | 1991 | Zinc, Copper | China | 63 | 27 | 36 |
| 36 | Chen | 1989 | Zinc, Copper | China | 90 | 60 | 30 |
| 37 | Li | 1988 | Zinc, Copper | China | 142 | 7 | 135 |
| 38 | Wang | 1986 | Selenium | China | 200 | 100 | 100 |
| 39 | Teherani | 1986 | Selenium | Austria | 13 | 5 | 8 |
| 40 | Genov | 1972 | Copper | Bulgaria | 104 | 84 | 20 |
| 41 | Lal | 1970 | Copper | India | 50 | 30 | 20 |
Data extraction
Eligible studies
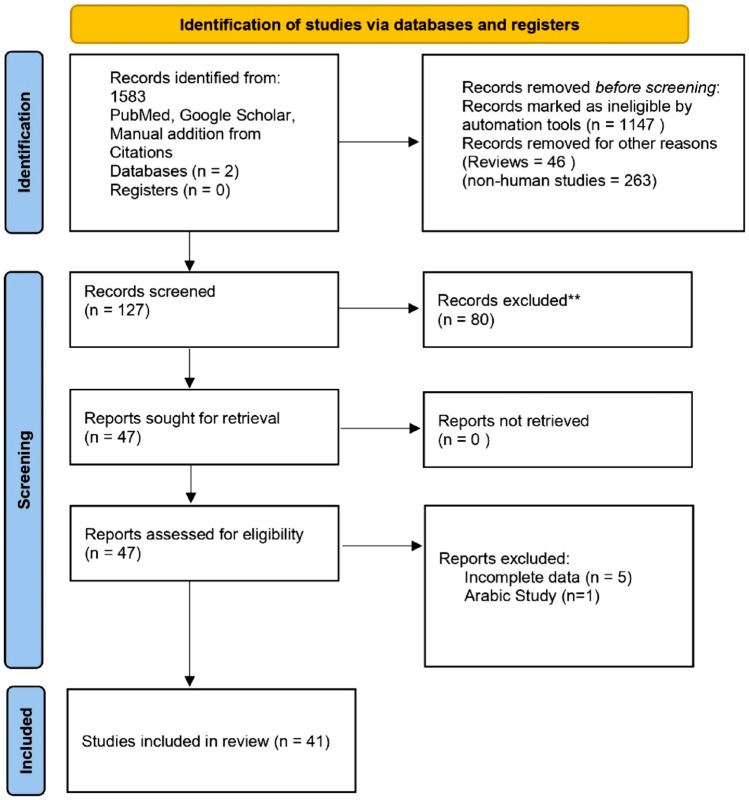
After a thorough literature search on PubMedand reference matching on Google scholar, 1583 articles were found. 1456 articles were excluded as 1147 were irrelevant to vitiligo and/or serum Zn, Cu and Se levels, 263 articles were not case- control studies and 46 were review articles. 127 articles were screened from which 80 articles were duplicate, 5 articles had insufficient data and 1 was Arabic study. 41 studies including 3353 vitiligo cases and 10,638 controls were included in the meta- analysis. The PRISMA flow diagram of the included studies is shown in Fig. 1.
Figure 1.
PRISMA flow diagram for included studies.
Data outcomes
The main outcome was to understand the association in change in levels of Metal Ions of copper and/or zinc and/or selenium and vitiligo. Other factors in the cascade of impact analysed were ethnicity, genetic predisposition via genetic analysis, trauma preceding vitiligo, medications and diet patterns and known oxidative stressors.
Quality assessment
Two authors independently (AnK and AS) assessed the methodological quality of the studies included in the review by using the National Heart Lung and Blood Institute (NHLBI) Study Quality Assessment tool for case–control studies (12 questions), systematic review and meta-analysis (8 Questions); observational cohort and cross-sectional studies (14 questions)39.Each of the domains contained multiple questions, each of the questions could be marked as Yes, No, and Cannot Determine (CD) or Not Applicable (NA) or Not Reported (NR) by the reporters. The reported domains measured confounding, measurement of exposure, selection of participants, post exposure interventions, missing data, measurement of outcome and reporting of results.
The risk of bias was further represented using the RobVis tool for schematically representing the risk of bias from the domains of various studies as a traffic signal plot (Fig. 2).
Figure 2.
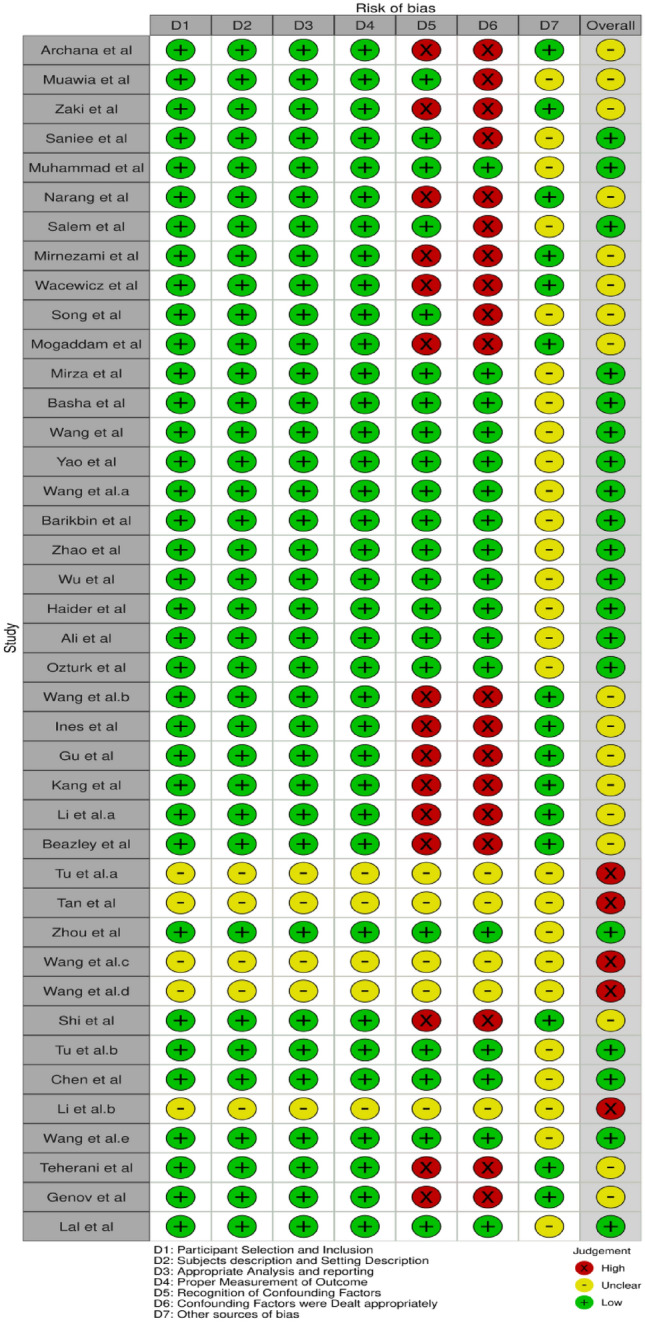
Traffic signal plot for included studies.
Statistical analysis
The presence of any association or correlation between change in serum levels of copper, selenium and Zinc and vitiligo was summarized as means and standard deviations which were further compared by odds ratios (OR) in 95% confidence intervals (CI). Review Manager (RevMan) Version 5.4 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was used for generation of the weighted forest plot. Heterogeneity was assessed statistically using I2 and Cochrane Q statistic.For the assessment of publication bias, Begg’s and Egger’s test was performed using MedCalc Version 20.210 . A P-value of ≤ 0.05 or 5% was considered statistically significant. In the absence of a publication bias and statistical heterogeneity (I2 < 50%), estimation of a pooled effect by fixed-effect model using the Mantel–Haenszel method was carried out.
Results
Study characteristics
This Meta analysis includes studies published between 1970 and 2022.
It was found that twenty-seven studies showed serum zinc levels in vitiligo patients. Geographically, 14 studies were from China, 4 from Egypt, 3 from Iran and Bangladesh and 1 each from India, Poland and Sudan (Table 2).
Table 2.
Characteristics of studies showing serum zinc levels in patients with vitiligo and healthy individuals included in Meta analysis.
| N | Author (References) | Year | Country | Zinc | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||||
| M* | SD** | T*** | M* | SD** | T*** | ||||
| 1 | Zaki7 | 2020 | Egypt | 50.93 | 11.02 | 50 | 77.09 | 12.16 | 50 |
| 2 | Saniee14 | 2019 | Iran | 90.94 | 20.88 | 98 | 84.68 | 21.08 | 98 |
| 3 | Muhammad13 | 2019 | Sudan | 104.0 | 20.1 | 60 | 93.2 | 19.5 | 60 |
| 4 | Narang18 | 2018 | India | 24.4 | 10.8 | 100 | 159.4 | 70.8 | 60 |
| 5 | Salem19 | 2018 | Egypt | 37.16 | 9.16 | 50 | 50.49 | 11.02 | 50 |
| 6 | Mirnezami9 | 2018 | Iran | 81.3 | 12.7 | 63 | 103 | 91.8 | 103 |
| 7 | Mogaddam40 | 2017 | Iran | 80.11 | 17.10 | 100 | 96.10 | 16.16 | 100 |
| 8 | Wacewicz20 | 2017 | Poland | 0.848 | 0.120 | 50 | 0.997 | 0.292 | 58 |
| 9 | Mirza41 | 2016 | Bangladesh | 74.17 | 24.70 | 60 | 95.50 | 27.30 | 50 |
| 10 | Basha42 | 2014 | Egypt | 104.0 | 20.1 | 60 | 93.2 | 19.5 | 60 |
| 11 | Wang31 | 2012 | China | 12.79 | 2.31 | 120 | 13.02 | 3.53 | 120 |
| 12 | Yao32 | 2011 | China | 0.88 | 0.26 | 90 | 1.07 | 0.31 | 50 |
| 13 | Wang26 | 2011 | China | 9.9 | 0.51 | 28 | 15.62 | 2.94 | 30 |
| 14 | Wu43 | 2010 | China | 6.416 | 1.758 | 70 | 7.194 | 1.412 | 70 |
| 15 | Haider12 | 2010 | Bangladesh | 1.08 | 0.07 | 30 | 0.95 | 0.35 | 30 |
| 16 | Ali44 | 2010 | Bangladesh | 30.27 | 11.09 | 60 | 32.27 | 11.08 | 60 |
| 17 | Wang25 | 2007 | China | 0.61 | 0.05 | 68 | 1.31 | 0.33 | 20 |
| 18 | Kang35 | 2002 | China | 0.9 | 0.51 | 30 | 1.06 | 2.25 | 34 |
| 19 | Beazley11 | 1999 | UK | 104 | 20.1 | 60 | 93.2 | 19.5 | 60 |
| 20 | Tan23 | 1997 | China | 17.45 | 3.56 | 37 | 22.85 | 3.09 | 750 |
| 21 | Zhou27 | 1996 | China | 60.08 | 31.33 | 26 | 97.17 | 22.54 | 20 |
| 22 | Wang24 | 1996 | China | 74.23 | 18.99 | 48 | 97.4 | 13.8 | 141 |
| 23 | Wang30 | 1993 | China | 0.9 | 0.51 | 30 | 1.06 | 2.25 | 34 |
| 24 | Shi45 | 1993 | China | 15 | 3.81 | 120 | 17.21 | 5.51 | 150 |
| 25 | Tu46 | 1991 | China | 11.5 | 3.15 | 22 | 13.54 | 2.34 | 36 |
| 26 | Chen47 | 1989 | China | 118.7 | 30.9 | 60 | 151.9 | 46.9 | 30 |
| 27 | Li29 | 1988 | China | 0.88 | 0.25 | 7 | 1.2 | 0.45 | 135 |
*Mean **Standard Deviation ***Total.
Twenty-three studies showed serum copper levels in vitiligo patients. Geographically, 16 studies were from China, 3 from India, and 1 each from Egypt, Bulgaria, Poland and Sudan (Table 3).
Table 3.
Characteristics of studies showing serum copper levels in patients with vitiligo and healthy individuals included in Meta analysis.
| S. No | Author (References) | Year | Country | Copper | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||||
| Mean | SD** | T*** | Mean | SD** | T*** | ||||
| 1 | Archana66 | 2021 | India | 59.8 | 68.4 | 60 | 55.4 | 37.7 | 60 |
| 2 | Muawia17 | 2020 | Sudan | 21.69 | 5.17 | 50 | 18.05 | 3.51 | 50 |
| 3 | Narang18 | 2018 | India | 32.5 | 10.3 | 100 | 24.2 | 6.7 | 60 |
| 4 | Salem19 | 2018 | Egypt | 141.62 | 32.56 | 50 | 128.38 | 29.03 | 50 |
| 5 | Wacewicz20 | 2017 | Poland | 1.099 | 0.273 | 50 | 1.038 | 0.336 | 58 |
| 6 | Wang31 | 2012 | China | 13.1 | 2.56 | 120 | 14.78 | 2.4 | 120 |
| 7 | Yao32 | 2011 | China | 0.69 | 0.15 | 90 | 1.12 | 0.22 | 50 |
| 8 | Wang26 | 2011 | China | 18.95 | 0.39 | 28 | 19.35 | 4.32 | 30 |
| 9 | Wu43 | 2010 | China | 1.46 | 0.471 | 70 | 1.536 | 0.345 | 70 |
| 10 | Wang31 | 2007 | China | 0.81 | 0.12 | 68 | 1.06 | 0.38 | 20 |
| 11 | Gu28 | 2005 | China | 0.6107 | 0.0917 | 30 | 1.0156 | 0.4367 | 36 |
| 12 | Kang35 | 2002 | China | 0.88 | 0.71 | 30 | 1.13 | 0.21 | 34 |
| 13 | Li48 | 2001 | China | 0.807 | 0.143 | 96 | 1.091 | 0.181 | 30 |
| 14 | Tan23 | 1997 | China | 16.23 | 1.76 | 37 | 22.1 | 2.19 | 750 |
| 15 | Zhou27 | 1996 | China | 103.74 | 24.29 | 26 | 122.9 | 39.21 | 20 |
| 16 | Wang24 | 1996 | China | 107.6 | 24.29 | 26 | 122.9 | 39.21 | 20 |
| 17 | Wang30 | 1993 | China | 0.88 | 0.17 | 34 | 1.13 | 0.21 | 30 |
| 18 | Shi45 | 1993 | China | 12.21 | 4.36 | 120 | 12.17 | 4.13 | 150 |
| 19 | Tu46 | 1991 | China | 13.05 | 2.74 | 27 | 14.68 | 2.33 | 36 |
| 20 | Chen47 | 1989 | China | 88.2 | 25.47 | 60 | 99.66 | 15.49 | 30 |
| 21 | Li29 | 1988 | China | 0.86 | 0.13 | 7 | 0.95 | 0.19 | 135 |
| 22 | Genov16 | 1972 | Bulgaria | 129 | 33 | 84 | 99 | 19 | 20 |
| 23 | Lal49 | 1970 | India | 121.70 | 29.24 | 30 | 126.75 | 27.07 | 20 |
**Standard Deviation ***Total participants.
Twelve studies which showed serum selenium levels in vitiligo patients. Geographically, 6 studies were from China, 1 each study were from Austria, Iran, Poland, Turkey, Tunisia, and UK (Table 4).
Table 4.
Characteristics of studies showing serum Selenium levels in patients with vitiligo and healthy individuals included in Meta analysis.
| S. No | Author (References) | Year | Country | Selenium | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | ||||||||
| M* | SD** | T*** | M* | SD** | T*** | ||||
| 1 | Song36 | 2017 | China | 0.11 | 0.02 | 62 | 0.16 | 0.05 | 62 |
| 2 | Wacewicz20 | 2017 | Poland | 51.30 | 13.99 | 50 | 79.42 | 18.97 | 58 |
| 3 | Barikbin34 | 2011 | Iran | 1.021 | 0.31 | 60 | 0.909 | 0.07 | 45 |
| 4 | Zhao50 | 2011 | China | 121.9 | 46.16 | 36 | 129.27 | 23.67 | 16 |
| 5 | Yao32 | 2011 | China | 0.09 | 0.03 | 90 | 0.14 | 0.07 | 50 |
| 6 | Ozturk38 | 2008 | Turkey | 122.33 | 30.17 | 30 | 120.77 | 21.8 | 30 |
| 7 | Ines51 | 2006 | Tunisia | 1.52 | 0.14 | 18 | 0.93 | 0.07 | 40 |
| 8 | Kang35 | 2002 | China | 0.10 | 0.02 | 30 | 0.13 | 0.04 | 34 |
| 9 | Beazley11 | 1999 | UK | 1.27 | 0.32 | 61 | 0.93 | 0.20 | 5932 |
| 10 | Tu37 | 1998 | China | 99.41 | 14.93 | 29 | 101.13 | 12.87 | 37 |
| 11 | Wang30 | 1993 | China | 0.10 | 0.02 | 34 | 0.13 | 0.14 | 28 |
| 12 | Teherani52 | 1986 | Austria | 0.388 | 0.044 | 5 | 0.304 | 0.087 | 8 |
*Mean **Standard Deviation ***Total participants.
Overall findings of meta-analysis
Levels of serum zinc and its association with vitiligo
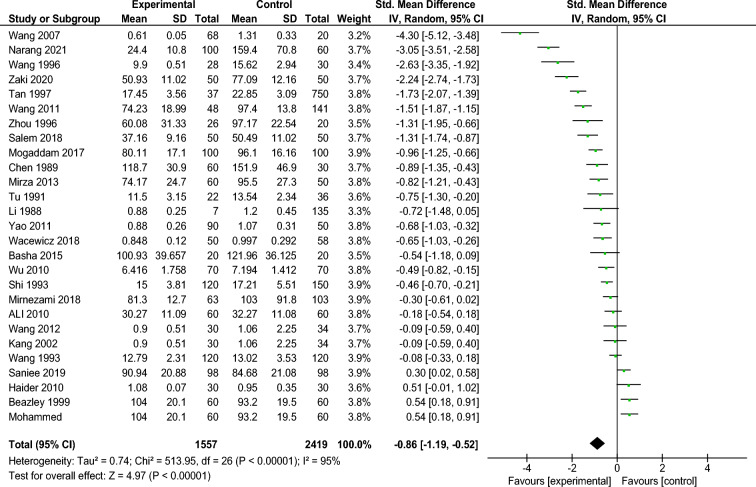
Out of the 27 investigations reported, the serum Zn levels, eleven studies announced no significant Zn level change between the vitiligo group and the healthy control group. The others introduced critical abatement of Zn level in the vitiligo group. Heterogeneity among the 27 articles was examined first. The results revealed a high statistical heterogeneity among studies (Tau2 = 0.74; Chi2 = 513.95, d.f. = 26 [P < 0.00001]; I2 = 95%). Then, a random effect model was utilized for meta-analysis. In the collective examination, there was a critical decline of Zn level in the vitiligo group (Z = 4.97; P < 0.0001; SMD = − 0.86; 95% CI − 1.19 to − 0.52; Fig. 3).
Figure 3.
Meta analysis of the serum Zn level between the two groups Vitiligo patients (Experimental) and controls. Compared with the control, there was a significant decrease of Zn level in the vitiligo group (Z = 4.97, P < 0.00001; standardized mean difference, − 0.86; 95% confidence interval [CI] − 1.19 to − 0.52). SD, standard deviation.
Levels of serum copper and its association with vitiligo
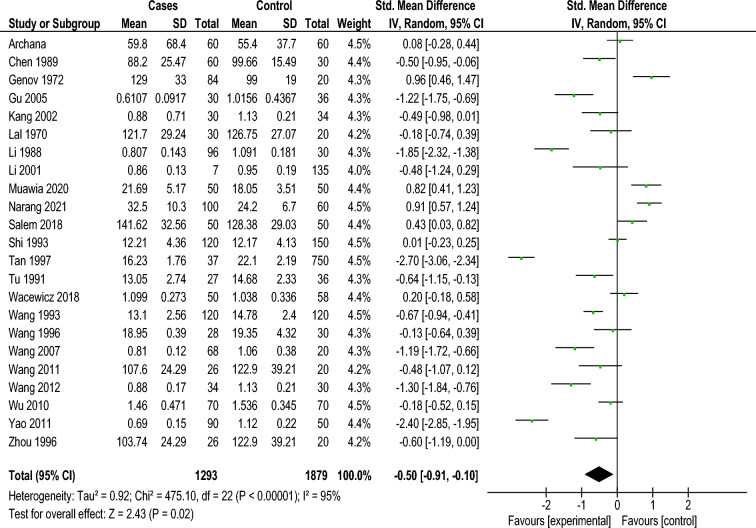
Of the 23 investigation that distinguished the serum Cu level, thirteen investigations reported no statistically significant Cu level change between the vitiligo group and the healthy control group. The others introduced huge abatement of Cu level in the vitiligo group. Heterogeneity among the 23 articles was examined first. The results revealed a high statistical heterogeneity among studies (Tau2 = 0.92; Chi2 = 475.10, d.f. = 22 [P < 0.00001]; I2 = 95%). Then, a random effect model was used for the meta-analysis and in the combined examination, there was again a critical decline of Cu levels in the vitiligo group (Z = 2.43; P < 0.0001; SMD = -0.50; 95% confidence interval [CI] − 0.91 to − 0.10) (Fig. 4).
Figure 4.
Meta analysis of the serum Cu level between the two groups Vitiligo patients (Experimental) and controls. Compared with the control, there was a significant decrease of Cu level in the vitiligo group (Z = 2.43; P < 0.0001; SMD = − 0.50; 95% confidence interval [CI] − 0.91 to − 0.10). SD, standard deviation.
Levels of serum selenium and its association with vitiligo
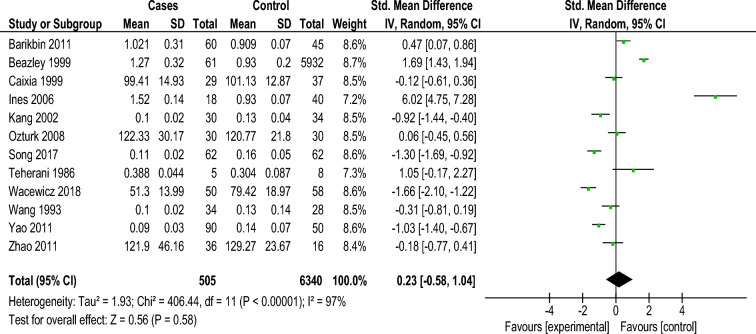
Among the investigations that reported the serum Se levels, five out of twelve investigations announced no statistically significant Se level change between the vitiligo group and the healthy control group. Two investigations showed slight elevation in sera Se levels, one study reported significant high levels, and four investigations showed decreased levels of selenium in vitiligo patients. Heterogeneity among the 12 articles was examined and the results reveals statistical heterogeneity among studies (Tau2 = 1.93; Chi2 = 406.44, d.f. = 11 [P < 0.00001]; I2 = 97%). Then, a random effect model was utilized for meta-analysis and the pooled examination, there was an increment of Se levels in the vitiligo group (Z = 0.56; P < 0.0001; SMD = 0.23; 95% confidence interval [CI], 0.58 to 1.04) (Fig. 5).
Figure 5.
Meta analysis of the serum Se level between the two groups Vitiligo patients (Experimental) and Healthy Individuals (Controls). Compared with the control, there was a significant increase of Se level in the vitiligo group (Z = 0.56; P < 0.0001; SMD = 0.23; 95% confidence interval [CI], 0.58 to 1.04).SD, standard deviation.
Sensitivity analysis and publication bias
The results from 27 studies in serum zinc levels showed Test for Heterogeneity Q = 757.2414, DF = 26, Significance level at P < 0.00001; I2 = 96.57% and CI at 95% for I2 is 95.77 to 97.21.
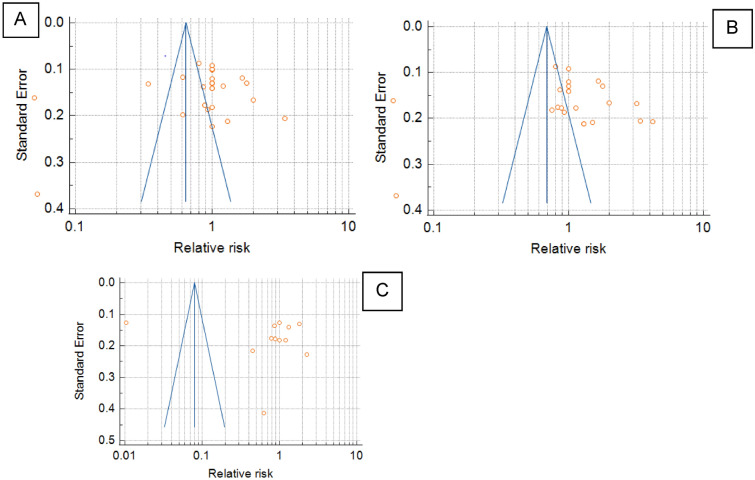
For publication bias Egger’s Test showed Intercept at − 2.5534, 95% CI = − 9.5070 to 4.4003 and Significance level P = 0.4566; Begg’s Test showed Kendall’s tau = -0.1850 at significance level P = 0.1759 [Fig. 6A].
Figure 6.
Publication bias for included studies: (A) in serum Zinc for Vitiligopatients versus healthy controls; (B) in serum copper for Vitiligo patients versus healthy controls; (C) in serum selenium for Vitiligo patients versus healthy controls.
The results from 23 studies on serum copper levels showed Test for Heterogeneity Q = 792.0325, DF = 22, Significance level at P < 0.00001; I2 = 97.22% and CI at 95% for I2 is 96.57 to 97.75.
For publication bias Egger’s Test showed Intercept at − 0.1169, 95% CI = − 8.1912 to 7.9574 and Significance level P = 0.9763; Begg’s Test showed Kendall’s tau = − 0.1198 at significance level P = 0.4236 [Fig. 6B].
The results from 12 studies on serum selenium levels showed Test for Heterogeneity Q = 3010.2988, DF = 11, Significance level at P < 0.00001; I2 = 99.63% and CI at 95% for I2 is 99.58 to 99.69.
For publication bias Egger’s Test showed Intercept at 8.0349, 95% CI = − 18.6917 to 34.7615 and Significance level P = 0.5181; Begg’s Test showed Kendall’s tau = − 0. 1818 at significance level P = 0.4106 (Fig. 6C).
Discussion
Vitiligo is an autoimmune disorder that causes depigmentation of the skin, hair, and mucous membranes. The condition has been described in ancient medical texts, indicating that humans have been aware of vitiligo for thousands of years. Despite this long history, the precise mechanisms underlying the development and progression of vitiligo are still not fully understood, and there exists significant amount ongoing research enabling better comprehension of the condition. Even though the exact cause has not been understood, but individual studies have shown that oxidative stress and trace elements such as zinc, copper, and selenium play a significant role in its pathogenesis. This meta-analysis has multiple exclusive advantages, where by far this is the largest meta-analysis performed on the association of zinc, copper and selenium serum levels and vitiligo. This study does not derail into other antioxidants and provides a specific perspective of metallo-micronutrients and the various possible pathogenetic mechanisms of vitiligo.
These specific micronutrients were chosen due to extensive existing but contradictory evidences present among them, where most studies reflect only on their anti-oxidant or free radical stress pathways of these micronutrients. This study also analyses a subgroup analysis for Asian and Caucasian populations separately, as previous meta-analyses by Chen et al. focuses only on Chinese populations, with high pre-existing risk of skin infections as reported by Lv et al.8,53.
Association between oxidative stress and zinc/copper ratio
Oxidative stress is known to damage cells by generating free radicals that can cause DNA damage, protein oxidation, and lipid peroxidation. A study has reported an association between oxidative stress and the ratio of zinc and copper (Zn/Cu) in vitiligo patients. The study showed that patients with vitiligo had a higher Cu/Zn ratio compared to healthy controls20. Zinc and copper are known to be involved in the defence mechanisms against free radicals by comprising Cu/Zn superoxide dismutase, which catalyzes the dismutation of reactive oxygen species to O2 or H2O2. Bagherani et al.6 and Yaghoobi et al.54 highlighted the association between among vitiligo and Zinc, due to its anti-apoptotic activity, has been suggested to be useful in the control of vitiligo55,56.Zinc deficiency can also impact the innate and adaptive immune system by affecting the survival, proliferation, and maturation of monocytes, polymorphonuclear, natural killer T and B-cells. Zinc also possesses antioxidant properties that can counter the toxic effects of free radicals9. Another study suggest that higher levels of serum zinc levels denote lower levels of Glutathione S-transferase in females57,58. Deficiency of zinc causes the suppression of non-specific cell mediated immunity, so zinc may cause the stimulation of cell mediated immunity against the infective and other probable factors which contributes to the development of vitiligo. α-melanocytes hormone is responsible for the melanogenesis and zinc plays a key role in the synthesis and release of α-melanocytes hormone. Regulation in normal and malignant melanocytes production is by ZAG (zinc-α2-glycoprotein), and zinc precipitates the ZAG at the vitiligo patch site and can be effective in the treatment of vitiligo59,60.
Role of copper and zinc in melanogenesis
Both copper and zinc are integral parts of many metalloenzymes necessary in the process of melanogenesis20. Melanin protein, responsible for imparting pigment to the skin, needs the amino acid tyrosine and the enzyme, tyrosinase for its synthesis61. The synthesis of melanin is initiated for the conversion of tyrosine to dihydroxyphenylalanine (DOPA), a key regulatory enzyme called tyrosinase. Copper is indispensable for the action of this enzyme, hence playing an essential role in melanogenesis 7. Zinc is also necessary for the proper functioning of tyrosinase and melanogenesis, making it crucial for the maintenance of normal skin pigmentation62.
Selenium, glutathione and vitiligo
Selenium is a chemical element that occurs naturally and has been extensively studied in medicine and biology due to its nutritional effects. The liver produces a protein called selenoprotein P (SELENOP), which is the primary component of circulating selenium. SELENOP provides essential tissues, such as endocrine glands and cells expressing insulin receptors, with the necessary amount of selenium. In tissues and hair, selenium is found in various forms, including selenocysteine, selenomethionine, selenoproteins, and other selenium-containing proteins and enzymes. Selenium plays a crucial role in the synthesis of selenoproteins, which have a wide range of pleiotropic effects, including antioxidant and anti-inflammatory effects. Several selenoproteins possess antioxidant properties that can help protect the human body against diseases caused by free radical damage, including malignant, infectious, and cardiovascular diseases63. The association between serum levels of copper, zinc, and vitiligo is still unclear due to conflicting results reported in various studies. Glutathione peroxidase is a selenium-containing enzyme that helps protect cells against oxidative damage. Together, selenium and glutathione peroxidase play a critical role in maintaining cellular health and preventing a range of diseases associated with oxidative stress. Previous meta-analyses have investigated the importance of selenium levels and glutathione peroxidase (GPX) levels in vitiligo11,34,38.
Our pooled analysis showed that the overall selenium level was similar between vitiligo patients and healthy controls, but a subgroup analysis unveiled that selenium levels were slightly higher in vitiligo patients than in healthy controls in the Asian population. This suggests that high selenium levels may contribute to the pathogenesis of vitiligo in the Asian population. Race could also be the reason for high selenium levels in the Asian population, as it is a structural component of the GPX enzyme. This falls in similar conclusions as observed by the systematic review by Huo et al. indicated that vitiligo patients observed a higher serum concentration of vitiligo in line with the existing biological evidence in mice models64,65. In contrast,systematic review by Lv et al.53 shows low serum selenium levels to be associated with vitiligo. This lack of congruence on selenium levels and associated risks needs to be further evaluated by studies for viable treatment options. This study bears some limitations; the study involves a large pooling of data on a controversial yet significant medical pathology. Vitiligo treatment modalities have been largely experimental as the evidence has not been concordant so far. This study draws the associations of serum micronutrient levels and vitiligo among patients and controls over large populations along with sub-group analysis specific to Asian population to enable treatment personalization and further incentive to perform high quality research. The study is limited by the limited number of databases it had access to, and may have missed out on studies that may have tilted the final outcome. This study also acknowledges the limited statistical conclusivity that exists due to the discordant nature of studies’ results.
Conclusion
The pathogenesis of vitiligo is complex and involves oxidative stress, trace elements such as zinc, copper, and selenium, and melanogenesis. There exists a significant decline in serum micronutrient levels in patients of vitiligo as compared to individuals who were taken as healthy controls. A more significant association exists as per sub-group analysis among the Asian population with serum selenium levels (reduction) as compared to overall reductions. Although the association between these trace elements and vitiligo is not yet fully understood, this article highlights the current understanding of their role in the disease. Further research is needed to elucidate the mechanisms underlying the association between these trace elements and vitiligo and to develop effective therapies for this debilitating condition.
Author contributions
A.K. and A.S. wrote main manuscript, R.M. prepared figures and helped in tables. The manuscript was reviewed by D.A. and A.K.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nath, S. K., Majumder, P. P. & Nordlund, J. J. Genetic epidemiology of vitiligo: Multilocusrecessivity cross-validated. Am. J. Hum. Genet.55(5), 981–990 (1994). [PMC free article] [PubMed] [Google Scholar]
- 2.Frisoli, M. L., Essien, K. & Harris, J. E. Vitiligo: Mechanisms of pathogenesis and treatment. Annu. Rev. Immunol.26(38), 621–648 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Stojanovich, L. & Marisavljevich, D. Stress as a trigger of autoimmune disease. Autoimmun. Rev.7(3), 209–213. 10.1016/j.autrev.2007.11.007 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Praneeth, N. G. et al. Study on trace elements levels in vitiligo and normal subjects. IP Indian J. Clin. Exp. Dermatol.5, 295–298 (2019). [Google Scholar]
- 5.Sanad, E. M., El-Fallah, A. A., Al-Doori, A. R. & Salem, R. M. Serum zinc and inflammatory cytokines in vitiligo. J. Clin. Aesthet. Dermatol.13(12 Suppl 1), S29-33 (2020). [PMC free article] [PubMed] [Google Scholar]
- 6.Bagherani, N., Yaghoobi, R. & Omidian, M. Hypothesis: zinc can be effective in treatment of vitiligo. Indian J. Dermatol.56(5), 480–484 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaki, A. M. et al. Therapeutic implications of assessment of serum zinc levels in patients with vitiligo: A patient controlled prospective study. Dermatol. Ther.33(6), e13998 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Zeng, Q. et al. Decreased copper and zinc in sera of Chinese vitiligo patients: A meta-analysis. J. Dermatol.41(3), 245–251 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Mirnezami, M. & Rahimi, H. Serum zinc level in vitiligo: A case-control study. Indian J. Dermatol.63(3), 227–230 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashighi, M. & Harris, J. E. Vitiligo pathogenesis and emerging treatments. Dermatol. Clin.35(2), 257–265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beazley, W. D., Gaze, D., Panske, A., Panzig, E. & Schallreuter, K. U. Serum selenium levels and blood glutathione peroxidase activities in vitiligo. Br. J. Dermatol.141(2), 301–303 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Haider, N. et al. Oxidative stress and antioxidant status in vitiligo patients. Dhaka Univ. J. Pharmaceut. Sci.9(2), 103–108 (2010). [Google Scholar]
- 13.Mohammed, M. S., Ahmed, S. A., Hamid, S. H. & Modawe, G. A. Assessment of serum zinc levels among Sudanese patients with vitiligo. JUMJ1(6), 1 (2019). [Google Scholar]
- 14.Saniee, S., Ghareaghaji Zare, A. & Radmehr, A. Zinc, vitamin D, and TSH levels in patients with vitiligo. J. Clin. Pract. Res.41(2), 148 (2019). [Google Scholar]
- 15.Shameer, P., Prasad, P. V. & Kaviarasan, P. K. Serum zinc level in vitiligo: A case control study. Indian J. Dermatol. Venereal. Leprol.71(3), 206–207 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Genov, D., Bozhkov, B. & Zlatkov, N. B. Copper pathochemistry in vitiligo. Clin. Int. J. Clin. Chem.37, 207–211 (1972). [DOI] [PubMed] [Google Scholar]
- 17.Muawia, M., Suad, H. H. & Modawe, G. A. Assessment of serum copper level among Sudanese patients with vitiligo. Sudan J. Med. Sci.15(1), 73–84 (2020). [Google Scholar]
- 18.Narang, I., Barman, K. D., Sahoo, B. & Lali, P. Evaluation of serum levels of zinc and copper in vitiligo. Pigment Int.8(1), 42–47 (2021). [Google Scholar]
- 19.Salem, M. A., El-Raheem, T. A. A. & Aboraia, N. M. Serum copper and zinc levels in vitiligo patients. Egypt. J. Hosp. Med.70(2), 273–281 (2018). [Google Scholar]
- 20.Wacewicz, M. et al. Selenium, zinc, copper, Cu/Zn ratio and total antioxidant status in the serum of vitiligo patients treated by narrow-band ultraviolet-B phototherapy. J. Dermatol. Treat.29(2), 190–195 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Zlatkov, N. B., Petkov, I., Genov, D. & Boshkov, B. Kupferstoffwechsel bei Kranken mit Vitiligo nach Heliotherapie [Copper metabolism in vitiligo patients after heliotherapy]. Dermatologica143(2), 115–120 (1971). [PubMed] [Google Scholar]
- 22.Arora, P. N., Dhillon, K. S., Rajan, S. R., Sayal, S. K. & Das, A. L. Serum zinc levels in cutaneous disorders. Med. J. Armed Forces India58(4), 304–306 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan, Q. M., Wang, C. F. & Gan, Z. J. Detecting of copper and zinc levels in serum and skin lesions, and free radical eliminated system in serum in vitiligo patients. J. Clin. Dermat.3, 171–173 (1997). [Google Scholar]
- 24.Wang, X. H. & Chen, X. D. The zinc and copper levels in serum of 48 vitiligo patients. J. Nantong Med. Coll16, 277 (1996). [Google Scholar]
- 25.Wang, S. H., Zhang, J. & Xin, M. The clinical study of treatment with Yuliaoling particles in vitiligo patients. JN Chin. Med39, 54–56 (2007). [Google Scholar]
- 26.Wang, X. M. A correlative study on SOD and serum zinc copper iron in patients with vitiligo. World Elem. Med.18, 31–32 (2011). [Google Scholar]
- 27.Zhou, Y. X., Yang, L. & Ou, Y. H. The influences of Zitongxiaobai prescription on the serum levels of zinc and copper in vitiligo patients. Hunan Guid.2, 32–33 (1996). [Google Scholar]
- 28.Gu, C. L., Zhou, A. H. & Wang, J. P. (2005). Serum level of copper in vitiligo patients. In Conference proceedings of the 9th academic meeting of laboratory medicine in the five northwestern provinces. Hospital of Ningxia Medical College. Ningxia. https://docslide.com. br› Documents.
- 29.Li, Y. G., Zhou, J. G. & Shao, Z. H. Determination of the levels of copper and zinc in plasma of serum of some patients. Journal of Tianjin Univ. Commer.4, 24–30 (1988). [Google Scholar]
- 30.Wang, F. & Xu, H. Q. Changes of some enzymes and trace elements in serum and skin lesions in vitiligo patients. Chin J. Derm. Venereol7, 142–143 (1993). [Google Scholar]
- 31.Wang, Y. D., Liu, X. H. & Lv, X. H. Analysis of serum trace elements of vitiligo patients in DaQing district. J. Qiqihar Univ. Med.33, 39–40 (2012). [Google Scholar]
- 32.Yao, A. P. The clinical analysis of trace elements in vitiligo patients. China. Higher Med. Educ7, 145–146 (2011). [Google Scholar]
- 33.Whitton, M. E., Ashcroft, D. M. & González, U. Therapeutic interventions for vitiligo. J. Am. Acad. Dermatol.59(4), 713–717 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Barikbin, B., Kavand, S., Yousefi, M., Hedayati, M. & Saeedi, M. No differences in serum selenium levels and blood glutathione peroxidase activities in patients with vitiligo compared with healthy control subjects. J. Am. Acad. Dermatol.64(2), 444–445 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Kang, A. J., Su, B. S. & Xu, H. Q. Research on the melanocytes apoptosis in vitiligo caused by oxygen free radicals and microelement. J. Chin. Clin. Med.3, 4–7 (2002). [Google Scholar]
- 36.Song, J., Zhou, P. & Yang, L. Observation on levels of trace elements and cytokines in patients with leukoderma. Chongqing Medicine, 1191–1192 (2017).
- 37.Caixia, T., Hongwen, F. & Xiran, L. Levels of soluble interleukin-2 receptor in the sera and skin tissue fluids of patients with vitiligo. J. Dermatol. Sci.21(1), 59–62 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Ozturk, I. C., Batcioglu, K., Karatas, F., Hazneci, E. & Genc, M. Comparison of plasma malondialdehyde, glutathione, glutathione peroxidase, hydroxyproline and selenium levels in patients with vitiligo and healthy controls. Indian J. Dermatol.53(3), 106–110 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sass, D. et al. Blood-based biomarkers of frailty in solid tumors: A systematic review. Front. Public Health11, 1171243. 10.3389/fpubh.2023.1171243 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mogaddam, M. R., Ardabili, N. S., Maleki, N., Chinifroush, M. M. & Fard, E. M. Evaluation of the serum zinc level in patients with vitiligo. Adv. Dermatol. Allergol.2, 116–119. 10.5114/ada.2017.67073 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wrzzt, S. et al. Implication of serum zinc level with duration and clinical type of vitiligo. BSMMU J.6(2), 99–103 (2013). [Google Scholar]
- 42.Basha, M. A., Amin, O. A., Azmy, R. M., El-Khalik, A. & Seham, R. Study of serum zinc in vitiligo. Menoufia Med. J.28(2), 377–381 (2014). [Google Scholar]
- 43.Wu, Y., He, N., Li, J. & Liang, L. The zinc and copper levels in serum of 70 vitiligo patients from Guangxi province. Chin. J. Dermatovenereol.24(8), 722–723 (2010). [Google Scholar]
- 44.Ali, R., Akhtar, N., Ahsan, M. S., Hassan, A. & Asifuzzaman, M. Serum Zinc level in patients with vitiligo in a tertiary hospital in Bangladesh. Assoc. Phys. Bangladesh21, 43 (2010). [Google Scholar]
- 45.Shi, D. R., Pu, X. M. & Ha, L. S. A correlative study on serum copper and zinc in patients with vitiligo. J. Clin. Dermatol.5, 241–243 (1993). [Google Scholar]
- 46.Tu, C. X., Lin, X. R. & Yin, F. Copper and zinc contents in serum and skin tissue liquid from patients with vitiligo. Chin. J. Derm. Venereol1, 20–25 (1991). [Google Scholar]
- 47.Chen, X. Q. & Luo, H. C. Copper and zinc contents in hair and serum of vitiligo patients. Chin. J. Dermatol.22, 21–23 (1989). [Google Scholar]
- 48.Li, Z. P. & Zhu, M. R. The serum level of copper in vitiligo patients. Shanghai J Prev. Med13, 239 (2001). [Google Scholar]
- 49.Lal, S. & Rajagopal, G. Serum Copper Levels in Vitiligo. Indian J. Dermatol. Venereol.36(1), 12–14 (1970). [PubMed] [Google Scholar]
- 50.Zhao, J., Li, W. & Li, S. Y. Serum selenium levels in vitiligo patients. Chin. J. Lepr. Skin Dis.17(1), 28–29 (2011). [Google Scholar]
- 51.Ines, D. et al. A comparative study of oxidant-antioxidant status in stable and active vitiligo patients. Arch. Dermatol. Res.298(4), 147–152 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Teherani, D. & Nagy-Vezekényi, K. Neutron activation analysis of some trace elements/selenium, chromium, cobalt and nickel/in the blood of vitiligo patients. J. Radioanal. Nucl. Chem.104(1), 53–58 (1986). [Google Scholar]
- 53.Lv, J. et al. Selenium levels and skin diseases: Systematic review and meta-analysis. J. Trace Elem. Med. Biol.62, 126548. 10.1016/j.jtemb.2020.126548 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Yaghoobi, R., Omidian, M. & Bagherani, N. Original article title: “Comparison of therapeutic efficacy of topical corticosteroid and oral zinc sulfate-topical corticosteroid combination in the treatment of vitiligo patients: A clinical trial”. BMC Dermatol.11(1), 7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonaventura, P., Benedetti, G., Albarède, F. & Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev.14(4), 277–285 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Prasad, A. S. Zinc in human health: Effect of zinc on immune cells. Mol. Med.14(5–6), 353–357 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roestijawati, N., Ernawati, D. A. & Krisnansari, D. High serum iron and zinc decrease glutathione S-transferase among women with breast cancer. UniversaMedicina.37(1), 25–30 (2018). [Google Scholar]
- 58.Kasperczyk, A., Dobrakowski, M., Horak, S., Zalejska-Fiolka, J. & Birkner, E. The influence of macro and trace elements on sperm quality. J. Trace Elem. Med. Biol.30, 153–159 (2015). [DOI] [PubMed] [Google Scholar]
- 59.El-Rifaie, A. A. A. S., Gohary, Y. M., Abd-El Aziz, G. M. & Owies, F. O. Zinc-α2-glycoprotein (ZAG): A new deficiency in vitiligo patients. Skinmed17(4), 248–253 (2019). [PubMed] [Google Scholar]
- 60.Bagherani, N. The newest hypothesis about vitiligo: most of the suggested pathogeneses of vitiligo can be attributed to lack of one factor, zinc-α2-glycoprotein. ISRN Dermatol.2012, 405268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molokhia, M. M. & Portnoy, B. Neutron activation analysis of trace elements in skin: VII. Copper and zincinvitiligo, moles and seborrhoeic warts. Br. J. Dermatol.88(4), 347–353 (1973). [PubMed] [Google Scholar]
- 62.Abdel-Malek, Z. A. et al. The enigma and challenges of vitiligo pathophysiology and treatment. Pigment Cell Melanoma Res.33(6), 778–787 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Rayman, M. P. Selenium and human health. Lancet (London, England)379(9822), 1256–1268. 10.1016/S0140-6736(11)61452-9 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Huo, J., Liu, T., Huan, Y., Li, F. & Wang, R. Serum level of antioxidant vitamins and minerals in patients with vitiligo, a systematic review and meta-analysis. J. Trace Elem. Med. Biol.62, 126570. 10.1016/j.jtemb.2020.126570 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Jalel, A., Soumaya, G. S. & Hamdaoui, M. H. Vitiligo treatment with vitamins, minerals and polyphenol supplementation. Indian J. Dermatol.54(4), 357–360. 10.4103/0019-5154.57613 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Archana, S. A., Vinutha Rangappa, T. G., Savitha, B., Jayadev, P. A. & Kushalappa, P. K. A. Does copper has a role in vitiligo? Analysis of tissue and serum copper in vitiligo. Pigment Int.8(1), 30. 10.4103/Pigmentinternational.Pigmentinternational_5_20 (2021). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.