Abstract
Objectives
Natural language processing (NLP) and large language models (LLMs) have emerged as powerful tools in healthcare, offering advanced methods for analysing unstructured clinical texts. This systematic review aims to evaluate the current applications of NLP and LLMs in rheumatology, focusing on their potential to improve disease detection, diagnosis and patient management.
Methods
We screened seven databases. We included original research articles that evaluated the performance of NLP models in rheumatology. Data extraction and risk of bias assessment were performed independently by two reviewers, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies was used to evaluate the risk of bias.
Results
Of 1491 articles initially identified, 35 studies met the inclusion criteria. These studies utilized various data types, including electronic medical records and clinical notes, and employed models like Bidirectional Encoder Representations from Transformers and Generative Pre-trained Transformers. High accuracy was observed in detecting conditions such as RA, SpAs and gout. The use of NLP also showed promise in managing diseases and predicting flares.
Conclusion
NLP showed significant potential in enhancing rheumatology by improving diagnostic accuracy and personalizing patient care. While applications in detecting diseases like RA and gout are well developed, further research is needed to extend these technologies to rarer and more complex clinical conditions. Overcoming current limitations through targeted research is essential for fully realizing NLP’s potential in clinical practice.
Keywords: large language models (LLMs), natural language processing (NLP), rheumatology, artificial intelligence (AI), disease detection
Key messages.
NLP models exhibit high accuracy in detecting conditions such as rheumatoid arthritis, spondyloarthropathies and gout from clinical texts.
LLMs show potential in addressing patient inquiries and enhancing education in rheumatology with high precision.
Further research is needed on NLP applications in rare rheumatic diseases and personalized treatment planning.
Introduction
Healthcare is rapidly evolving, driven by significant artificial intelligence (AI) advancements [1]. Among these, natural language processing (NLP) and especially large language models (LLMs) have emerged as transformative technologies [2, 3].
NLP and LLMs introduce methods for analysing unstructured clinical texts [2, 3]. These technologies can extract information from electronic health records (EHRs), improving patient care, research and administrative work [4–6]. The ability of LLMs to understand context and interpret complex medical terminology makes them valuable tools for clinicians and researchers [7, 8].
Rheumatology, a field characterized by diverse disorders, can benefit from these advancements [9]. Rheumatological conditions often involve multiple organ systems and present with overlapping symptoms, making accurate diagnosis challenging [10]. NLP offers the potential to extract relevant clinical data, enhance disease classification and support decision-making [4, 5, 11, 12].
Despite its promise, NLP adoption for rheumatology has been relatively slow [13, 14]. This lag is due in part to the complexity of the field and the need for highly accurate tools [13]. However, recent studies have demonstrated the feasibility of NLP in various aspects of rheumatology, including disease detection, patient management and education [5, 12, 15]. These studies highlight the potential for NLP to address some of the most pressing challenges in the field.
Our review aims to provide insights into the current state of NLP research in rheumatology and identify areas for future clinical application.
Materials and methods
Registration and protocol
This systematic literature review was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the registration code CRD42024509490 [16]. Our methodology adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [17].
Search strategy
We searched seven databases: PubMed, Embase, Web of Science, Scopus, Cochrane Library, IEEE Xplore and OVID-MEDLINE. The search covered studies published between 1 January 2002 and April 2024. The start date was chosen because it marks the announcement of the neural probabilistic language model, foundational for the application of NLP in medicine [18, 19]. Our focus was on the outcomes of integrating NLP and LLMs in rheumatology. We used keywords like ‘natural language processing,’ ‘NLP,’ ‘large language models’ and ‘LLMs’, along with specific model names and rheumatological terms like ‘GPT,’ ‘BERT,’ ‘rheumatoid arthritis’ and ‘gout’. We designed Boolean search strings tailored to each database. To maximize coverage, we supplemented our search with a manual reference screening of included studies and targeted searches on Google Scholar and medrxiv. Details of the specific Boolean strings used are provided in the supplementary materials (available at Rheumatology Advances in Practice online).
Study screening and selection
We included articles that directly evaluated the performance of NLP models in rheumatology applications and provided data about the performance, either qualitative or quantitative.
Our review encompasses original research articles and full conference articles [20]. The exclusion criteria were confined to review articles, case reports, commentaries, protocol studies, editorials and non-English publications, in addition to articles that did not directly evaluate the model performance.
For the initial screening, we used the Rayyan web application [21]. The initial screening and study selection, which were conducted according to predefined criteria, were independently performed by two reviewers (M.O. and E.K.). Discrepancies were resolved through discussion.
Data extraction
Data extraction was conducted by two researchers (M.O. and E.K.) using a standardized form to ensure consistent and accurate data capture. This included details such as author, publication year, sample size, data type, task type, disease interest, model used, results, performance metrics, conclusions and limitations. Any discrepancies in data extraction were resolved through discussion and a third reviewer was consulted when necessary.
Risk of bias assessment
To evaluate the quality and robustness of the methodologies in the included studies, the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies tool was used [22].
Results
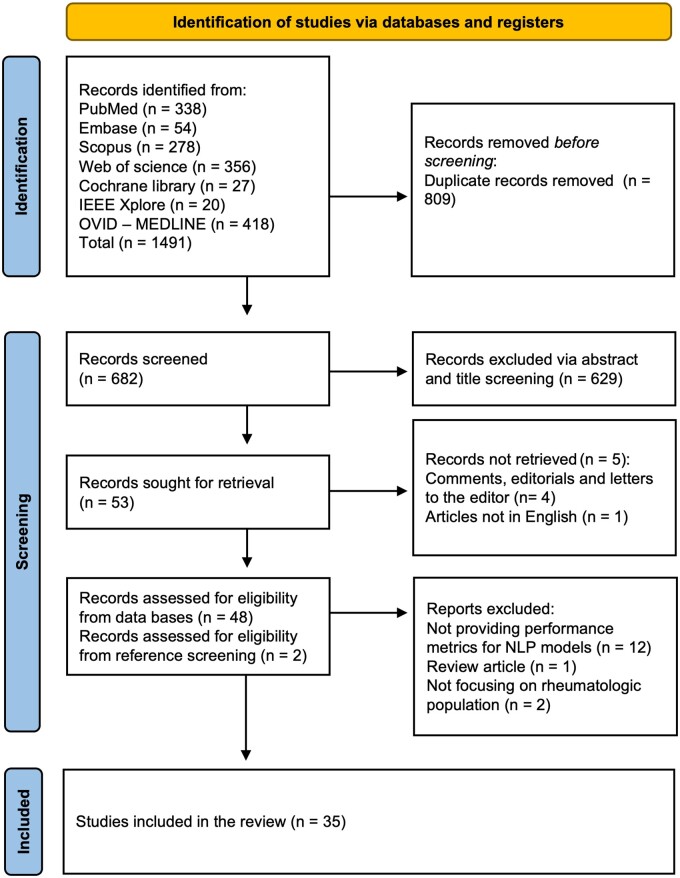
A total of 1491 articles were identified through the initial screening. After removing 809 duplicates, 682 articles remained for further evaluation. Title and abstract screening excluded 629 articles, leaving 53 articles for full-text review. From these, 34 studies met all inclusion criteria. By using reference checking and snowballing techniques, one additional study was identified, resulting in a final tally of 35 studies [5, 6, 12, 23–54]. A PRISMA flow chart visually represents the screening process in Fig. 1.
Figure 1.
PRISMA flow chart
Overview of the included studies
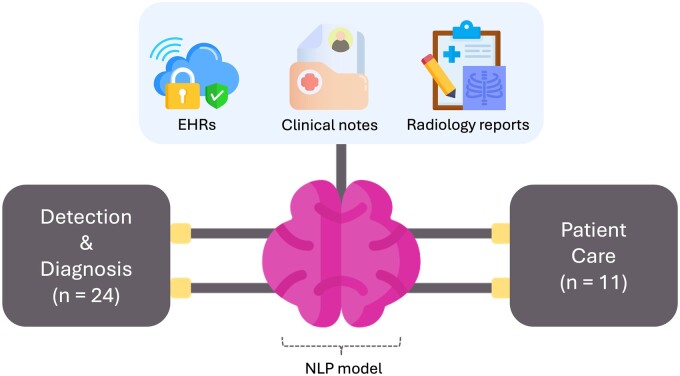
We included 35 studies [5, 6, 12, 23–54], spanning from 2010 to 2024. Analysed sample sizes ranged from a few hundred to >2 million patients. The studies utilized various data types, including electronic medical records (EMRs), structured and unstructured electronic health records (EHRs) data, clinical notes and radiology reports.
Employed models included advanced NLP techniques, ensemble models and specific LLM architectures like Bidirectional Encoder Representations from Transformers (BERT) and Generative Pre-trained Transformer (GPT).
We categorized the applications into two main groups: patient care and detection and diagnosis (Table 1). Eleven studies focused on patient care, evaluating models in answering patient questions, predicting flares, classifying disease severity and managing diseases. Twenty-four studies focused on detection and diagnosis, identifying diseases or flares from data and making diagnoses, such as identifying gout flares, detecting pain levels in OA and diagnosing RA.
Table 1.
Summary of the included studies
| Author, year [ref] | Data type and sample size | Model | Summary of most important results |
|---|---|---|---|
| Chen et al., 2023 [48] | EMR, data from >2 million patients | NLP (NER, POS) | Synonym-based pain-level detection tool accurately identified patients with moderate–severe pain due to OA |
| Saini et al., 2023 [29] | X-ray image reports, structured EHR data, 4508 patients | CNN, YOLO v4, Transformer, BERT | High performance in predicting knee OA severity and generating reports with AUROCs from 0.897 to 0.9582. |
| Benavent et al., 2024 [5] | Unstructured EHR data, 4337 patients | NLP-based system | High precision, recall and F1 scores (>0.80) for detecting clinical entities related to SpA |
| Li et al., 2022 [46] | EMRs, 1600 clinical notes | BERT | Improved NER in clinical notes with an F1 score of 0.936 |
| Krusche et al., 2024 [31] | Patient vignettes, 20 different real-world patient vignettes | GPT-4 | Comparable diagnostic accuracy to rheumatologists for IRDs, with top diagnosis accuracy of 35% |
| Madrid-García et al., 2023 [39] | Exam questions from Spanish access exam, 145 questions | GPT-4 | GPT-4 showed 93.71% accuracy in answering rheumatology questions |
| Irfan and Yaqoob 2023 [23] | Database of peer-reviewed articles and clinical guidelines | GPT-4 | Provided insights into SS, highlighting key characteristics and management details |
| Nelson et al., 2015 [49] | Medical text infusion notes, 115 patients, 2029 infliximab infusions | Custom rule-based NLP software | Improved sensitivity (0.858) and PPV (0.976) for identifying infliximab infusion dates and doses |
| Liu et al., 2023 [25] | Chinese EMRs, 1986 CEMRs | MC-BERT-BiLSTM-CRF, MC-BERT + FFNN | Achieved F1 scores of 92.96% for NER and 95.29% for relation extraction |
| Humbert-Droz et al., 2023 [30] | Clinical notes from the RISE registry, 854 628 patients | NLP pipeline (Spacy) | Sensitivity, PPV and F1 scores of 95%, 87% and 91%, respectively, for RA outcome measures extraction |
| Benavent et al., 2023 [6] | Free-text and structured clinical information, 758 patients | EHRead technology | High performance in identifying clinical variables for axSpA and PsA, precision of 0.798 and recall of 0.735 for PsA |
| VanSchaik et al., 2023 [53] | PubMed abstracts, 2350 abstracts | ELECTRA-based model | Extracted causal relationships with an F1 score of 0.91 |
| Walsh et al., 2020 [40] | Clinical notes, structured EHR data, 600 patients | NLP algorithms with random forest | AUROC of 0.96 for full algorithm in identifying axSpA |
| Yoshida et al., 2024 [42] | EHR notes and Medicare claims data, 500 patients | LASSO | Combined model showed an AUROC of 0.731 for identifying gout flares |
| Li et al., 2023 [52] | FAQ-based question-answering pairs, 176 questions | BERT, RoBERTa, ALBERT, MacBERT | Achieved top-1 precision of 0.551 and MRR of 0.660 in an RA question-answering system |
| Ye et al., 2024 [33] | Patient-generated rheumatology questions, 17 patients | GPT-4 | Patients rated AI responses similarly to physician responses; rheumatologists rated AI lower in comprehensiveness |
| Coskun et al., 2024 [23] | Questions on methotrexate use, 23 questions | GPT-4, GPT-3.5, BARD | GPT-4 achieved 100% accuracy in providing information on methotrexate use |
| Liao et al., 2010 [36] | Narrative and codified EMR data, 29 432 subjects | HITEx system | Improved RA classification accuracy with a PPV of 94% using narrative and codified data |
| Lin et al., 2015 [24] | Structured and unstructured EHR data, 5903 patients | Apache cTAKES, ML | PPV of 0.756, sensitivity of 0.919 and F1 score of 0.829 for identifying methotrexate-induced liver toxicity |
| Wang et al., 2017 [32] | Spontaneous reports, EMRs, 138 000 patients | MedEx, UMLS, MedDRA PT codes | Detected 152 signals for biologics and 147 for DMARDs from clinical notes |
| Uz and Umay, 2023 [34] | Structured EHR data and internet search data | ChatGPT | Reliability scores ranged from 4 to 7, with the highest for OA (5.62); usefulness scores highest for AS (5.87) |
| Luedders et al., 2023 [37] | Chest CT reports, 650 patients | Automated regular expressions | Improved PPV to 94.6% for RA-ILD identification |
| Osborne et al., 2024 [41] | Chief complaint text from emergency department, 8037 CCs | Rule-based, BERT-based algorithm | BERT-GF achieved an F1 score of 0.57 for detecting gout flares |
| Yang et al., 2024 [26] | Responses from ChatGPT and Bard, 20 treatments | GPT, BARD | ChatGPT had an 80% concordance rate with AAOS CPGs, while Bard had 60% |
| England et al., 2024 [38] | Clinical notes from EHRs, 7485 patients | NLP | 95.8% of NLP-derived FVC values were within 5% predicted of PFT equipment values |
| Love et al., 2011 [54] | EMR notes, billing codes, 2318 patients | NLP with random forest | PPV of 90% at sensitivity of 87% for PsA classification using NLP and coded data |
| Deng et al., 2024 [12] | Structured EHR data, clinical notes, 472 patients | MetaMap, logistic regression | Identified lupus nephritis phenotype with an F1 score of 0.79 at NU and 0.93 at VUMC |
| van Leeuwen et al., 2024 [50] | EHRs, 287 patients | AI tool, NLP | Sensitivity of 97.0% in training and 98.0% in validation centres for AAV identification |
| Román Ivorra et al., 2024 [47] | EHRs, 13 958 patients | EHRead, NLP, ML | Achieved precision of 79.4% for ILD detection and 76.4% for RA detection |
| Zhao et al., 2020 [43] | EHRs, 7853 patients | NLP, ICD codes, logistic regression | Sensitivity of 0.78, specificity of 0.94 and AUROC of 0.93 for identifying axSpA |
| Kerr et al., 2015 [45] | Clinical narrative data from EMRs, 2280 patients | NLP system | Compliance rates for gout QIs: QI 1, 92.1%; QI 2, 44.8%; QI 3, 7.7% |
| Redd et al., 2014 [44] | Structured and unstructured EHR data, 4272 patients | NLP, SVM | Precision of 0.814 and recall of 0.973 for identifying SSc patients at risk for SRC |
| Oliveira et al., 2024 [35] | Chief complaint notes from emergency department, 8037 CCs | RoBERTa-large, BioGPT | Achieved F1 scores of 0.8 (2019 dataset) and 0.85 (2020 dataset) for detecting gout flares |
| Gräf et al., 2022 [28] | Survey data, clinical vignettes, 132 vignettes | ADA | ADA’s diagnostic accuracy for IRD was higher compared with physicians (70% vs 54%) |
CCs: Clinical Cases; NER: named entity recognition; POS: parts of speech; CNN: convolutional neural network; YOLO: You Only Look Once; IRD: inflammatory rheumatic disease; FVC: forced vital capacity; QI: quality indicator; PFT: pulmonary function test; ADE: adverse drug event; RISE: Rheumatology Informatics System for Effectiveness; SRC: scleroderma renal crisis; GPA: granulomatosis with polyangiitis; MPA: microscopic polyangiitis; EGPA: eosinophilic granulomatosis with polyangiitis; ML: machine learning; HCPCS: Healthcare Common Procedure Coding System; LASSO: least absolute shrinkage and selection operator; MAP: maximum a posteriori; RoBERTa: A Robustly Optimized BERT Pretraining Approach; BioGPT: Biomedical Generative Pre-trained Transformer; NU: Northwestern University; VUMC: Vanderbilt University Medical Center; HITEx: Health Information Text Extraction; EHRead: Electronic Health Read; ADA: AI-based symptom checker; FFNN: Feedforward neural network.
The studies covered multiple conditions, including RA (12 studies), SpA (5 studies), gout (5 studies) and other conditions such as lupus, SSc and ANCA-associated vasculitis (AAV; 13 studies) (Fig. 2). Most of the included articles were published in quartile 1 journals (Supplementary Fig. S1, available at Rheumatology Advances in Practice online).
Figure 2.
A framework of the NLP model inputs and output categories
Risk of bias
The analysis of the risk of bias yielded mostly good and fair results using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Specifically, 20 studies were evaluated as having good quality and low risk of bias, 9 studies as having fair quality and fair risk of bias and 6 studies as having poor quality and high risk of bias. The poor evaluations were mainly due to the use of vignettes or question-based studies that did not fit well under the tool’s evaluation categories. Nonetheless, the overall results indicate a general trend of high quality and low overall risk of bias. A detailed evaluation for each study is provided in Supplementary Table S1 (available at Rheumatology Advances in Practice online).
A background on NLP for clinicians
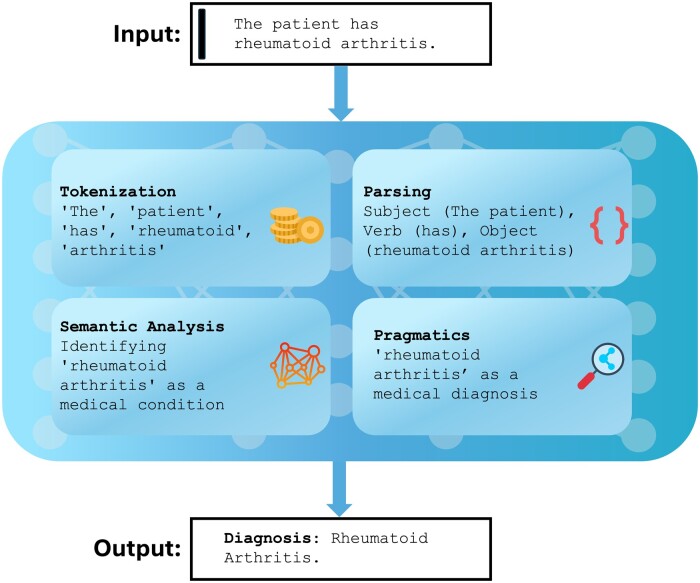
NLP allows machines to interpret and manipulate human language [55]. Key processes include tokenization (breaking text into words and phrases), parsing (analysing sentence structure), semantic analysis (interpreting meaning) and pragmatics (understanding context) [55]. NLP uses statistical analysis, machine learning and deep learning to perform tasks like translation, sentiment analysis and summarization [55, 56] (Fig. 3).
Figure 3.
A simple NLP framework in rheumatology
Transformers have revolutionized NLP by enabling parallel processing of input data, improving efficiency and speed [57]. They use self-attention mechanisms to weigh each part of the input independently, enhancing their ability to understand and generate contextually relevant responses [58]. This architecture underlies major models like OpenAI’s GPT series and Google’s BERT [58].
LLMs are trained on extensive text corpora to analyse and generate human-like text [2, 15]. They excel in applications such as automated dialogue systems, content creation and complex analytical tasks [2, 15]. OpenAI’s GPT series is known for generating coherent and context-aware text sequences, thanks to extensive pre-training and fine-tuning [31]. Another example is Meta’s LLaMA, which is an efficient, open-source model available in multiple configurations, while Google’s Gemini, formerly Bard, is designed for high-quality interactions using up-to-date content [59].
In AI, a ‘prompt’ is the input given to a language model to guide its output [2, 8]. Autoregression involves predicting the next word or sequence based on previous inputs, ensuring coherent and contextually appropriate text. This technique is crucial for tasks like text completion and machine translation [2, 8].
NLP in detecting and diagnosing rheumatic diseases
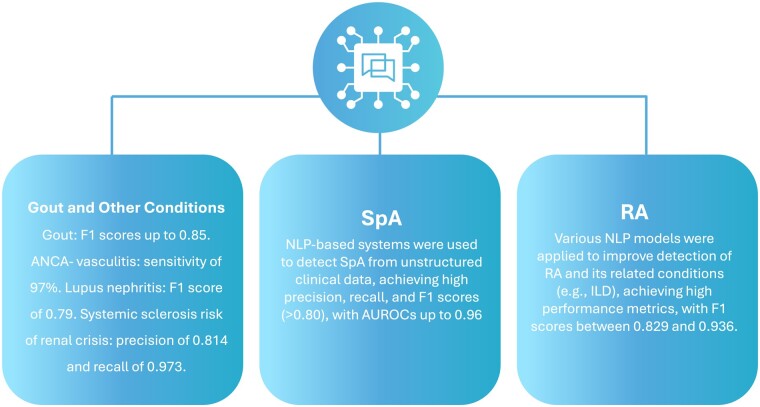
Overall, 24 studies fell under this application. Specifically, nine studies focused on RA, four on SpA and the others on diseases such as gout, SSc, OA and AAV (Table 1, Fig. 4).
Figure 4.
A summary of the applications and performance of NLP models in the detection across different conditions
RA
Various NLP models were used to improve detection and diagnosis of RA. These models were applied to tasks such as named entity recognition, adverse drug event detection and disease activity extraction, achieving high performance metrics with F1 scores up to 0.936, positive predictive values (PPVs) of up to 94% and sensitivities of up to 95%. For instance, Li et al. [46] used a BERT-based model to improve named entity recognition in clinical notes, achieving an F1 score of 0.936. Nelson et al. [49] demonstrated that NLP significantly improved sensitivity (86%) and PPV (98%) for identifying infliximab infusion dates and doses compared with using international Classification of Diseases (ICD) codes.
Liu et al. [25] used BERT for named entity and extraction from Chinese EMRs, achieving F1 scores of 93% for entity recognition and 95% for relation extraction.
Humbert-Droz et al. [30] developed an NLP pipeline that showed good internal and external validity for extracting RA disease activity and functional status scores, with sensitivity, PPV and F1 scores of 95%, 87% and 91%, respectively, in internal validation and 92%, 69% and 79%, respectively, in external validation.
Liao et al. [36] used the Health Information Text Extraction (HITEx) system, which improved the classification accuracy of RA subjects, compared with using ICD codes, achieving a PPV of 94% using both narrative and codified data. Lin et al. [24] combined Apache cTAKES for feature extraction with supervised machine learning, achieving a PPV of 76%, sensitivity of 92% and F1 score of 83% for identifying methotrexate-induced liver toxicity.
Wang et al. [32] used NLP tools to discover and validate adverse drug events, detecting 152 signals for biologics and 147 for DMARDs from clinical notes, that were not detected using other traditional tools. Luedders et al. [37] used automated regular expressions to enhance RA interstitial lung disease (RA-ILD) identification, achieving a PPV of 95% in the derivation cohort and 89% in the validation cohort.
Similarly, Román Ivorra et al. [47] used the EHRead technology to extract and standardize unstructured clinical information to estimate the prevalence of RA-ILD, achieving precisions of 79% for ILD detection and 76% for RA detection. England et al. [38] extracted forced vital capacity values from EHR notes, showing that 96% of NLP-derived values were within 5% of predicted pulmonary function test equipment values.
SpAs
Most of the studies focused on detecting SpAs from unstructured clinical data. For instance, Benavent et al. [5] used an NLP-based system to extract and identify clinical entities related to SpA, achieving high precision, recall and F1 scores (>0.80). Walsh et al. [40] developed three algorithms for identifying axial SpA (axSpA) from EHRs, with the full algorithm achieving an area under the receiver operating characteristics (AUROC) curve of 0.96, sensitivities of 85–95% and specificities of 78–94%. Zhao et al. [43] combined NLP with ICD codes and logistic regression models, achieving an AUROC of 0.93, sensitivity of 78% and specificity of 94% for identifying axSpA. In addition, Love et al. [54] focused on using NLP to classify PsA cases from EMRs. Their study showed that using NLP with EMR text notes significantly improved the performance of the prediction algorithm for PsA classification compared with using only coded data. Specifically, the AUROC) improved from 0.925 (coded data alone) to 0.950 (combined coded and NLP data), indicating a significant enhancement in classification accuracy.
Gout
All the studies focused on detecting gout flares using different data inputs and models. Zheng et al. [51] used NLP and machine learning to identify gout flares from unstructured EHR data, achieving a sensitivity of 82% and specificity of 92%. Yoshida et al. [42] combined NLP concepts with Medicare claims data, resulting in an AUROC of 0.731 for identifying gout flares. Osborne et al. [41] used a BERT-based algorithm to identify gout flares in emergency department patients, achieving an F1 score of 0.57. Oliveira et al. [35] compared different models for early detection of gout flares from chief complaint notes, with RoBERTa-large-PM-M3-Voc achieving an F1 score of 0.8 and BioGPT achieving an F1 score of 0.85.
Other conditions
Other studies addressed various rheumatologic conditions. Deng et al. [12] used MetaMap-based models to identify lupus nephritis phenotypes, achieving an F-measure of 0.79 at Northwestern Medicine and 0.93 at Vanderbilt University. Van Leeuwen et al. [50] used an AI tool incorporating NLP to identify AAV, achieving sensitivities of 97% and 98% in training and validation centres, respectively. Redd et al. [44] used NLP combined with a support vector machine (SVM) to detect SSc patients at risk for scleroderma renal crisis, achieving a precision of 0.814 and recall of 0.973.
Patient care
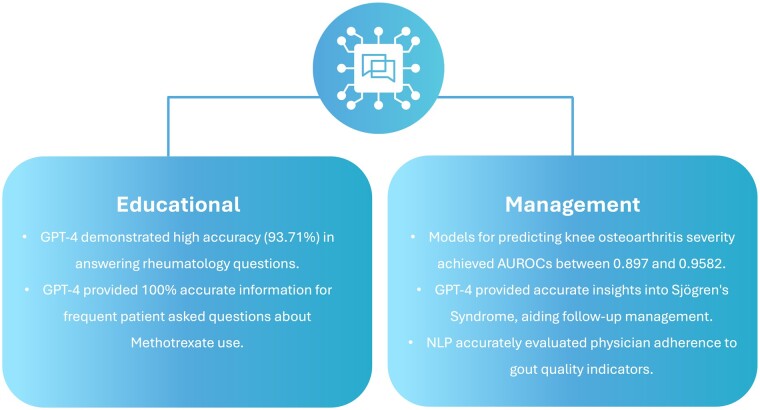
Patient care includes studies focusing on management, educational purposes for patients or practitioners and research. Under this category, there were 11 studies, divided into two main categories: management (plans, treatment, risk stratification, prediction) and education (answering questions, aiding research) (Table 1, Fig. 5).
Figure 5.
NLP model capabilities in performing high-level research questions under patient care applications
Management
Saini et al. [29] developed an ensemble model for knee OA severity prediction and report generation, achieving AUROCs from 0.897 to 0.958. Irfan et al. [27] used GPT-4 to provide insights into SS, highlighting key immunopathological and histopathological characteristics and providing follow-up management and differential diagnosis. Benavent et al. [6] used EHRead technology to explore the characteristics and management of patients with axSpA and PsA, achieving a precision of 0.798 and recall of 0.735 for PsA.
Ye et al. [33] compared AI-generated responses to rheumatology patient questions with physician responses. Patients rated AI responses similarly to physician responses, while rheumatologists rated AI responses lower in comprehensiveness and accuracy. Kerr et al. [45] used NLP to evaluate physician adherence to gout quality indicators (QIs), finding compliance rates of 92% for QI 1, 45% for QI 2 and 8% for QI 3. Rheumatology clinic visits were associated with greater compliance.
Educational
Madrid-García et al. [39] assessed the accuracy of GPT-4 in answering rheumatology questions, finding an accuracy of 94% and a median clinical reasoning score of 4.7. VanSchaik et al. [53] used an ELECTRA-based model to extract causal relationships from biomedical literature, achieving an F1 score of 0.91. Li et al. [52] used advanced models like BERT, RoBERTa, ALBERT and MacBERT for question matching in an RA question-answering system, achieving a top-1 precision of 55% and a mean reciprocal rank (MRR) of 0.660.
Coskun et al. [23] evaluated the accuracy of AI models in providing information on methotrexate use. GPT-4 achieved 100% accuracy, while GPT-3.5 scored 87%.
Uz and Umay [34] assessed the reliability and usefulness of ChatGPT for common rheumatic disease–related queries. Reliability scores ranged from 4 to 7, with the highest score for OA (5.6), and usefulness scores ranged from 4 to 7, with the highest for AS (5.9). Yang et al. [26] evaluated the concordance of ChatGPT and Bard with AAOS CPGs, finding that ChatGPT had an 80% concordance rate and Bard’s was 60%.
Discussion
NLP technology is starting to influence the management and diagnosis of rheumatic diseases. BERT and GPT, for instance, are showing promise in enhancing diagnostic accuracy for conditions such as RA and SpA [30, 54]. These models deliver results that suggest potential improvements over traditional diagnostic methods, offering better precision.
In clinical environments, NLP has begun to improve interactions between patients and healthcare providers and to augment educational resources for medical professionals [60, 61]. This technology challenges the idea that digital tools necessarily depersonalize care, indicating instead that they can foster more informed and engaging healthcare interactions. This is reflected in our review by studies such as that of Coskun et al. [23], which demonstrated the utility of GPT-4 in answering patient questions related to methotrexate use. Additionally, Venerito et al. [62] compared multiple LLMs, finding GPT-4 and Claude 2 performed well in answering clinical trivia, indicating their potential in clinical education and decision support, similar to the findings in our review regarding the performance of NLP models in educational applications. Maarseveen et al. [63] demonstrated the effectiveness of machine learning algorithms in accurately identifying patients with RA from unstructured text in EHRs. This approach showcases the potential of augmenting NLP models and classical machine learning in rheumatology to potentially enhance patient identification and facilitate large-scale observational studies across different healthcare systems. Ayer et al. [60] evaluated AI chatbot responses to patient questions, finding them to be of higher quality and more empathetic than physician responses. Another interesting area for educational use of LLMs was highlighted in recent findings by Haase et al. [64]. Their study showed that GPT-4 outperformed SLE experts in providing high-quality, empathetic responses to patient questions. This demonstrates GPT-4’s potential as a valuable tool for enhancing patient education and communication.
NLP also supports the development of personalized treatment plans and advanced disease management, providing alternatives to the traditional one-size-fits-all treatment approaches [65]. This emerging application invites a re-evaluation of established treatment paradigms. Our results suggest that NLP tools can effectively screen patients for comorbidities and associated diseases, such as detecting RA-ILD and extracting vital capacities of RA patients from large amounts of unstructured data [37, 38]. Additionally, these tools can predict or detect flares, enhancing their ability to provide timely and individualized interventions and treatments [29, 35].
Despite the promising results, there is a lack of research on certain rheumatic conditions, especially rare diseases such as Behçet’s disease. Conditions like SSc and lupus nephritis, although somewhat researched, are studied to a lesser extent than diseases like RA and SpA. However, current results suggest that integrating NLP can treat flairs by accurately predicting them, indicating an area for future exploration (Supplementary Table S2, available at Rheumatology Advances in Practice online). Expanding the scope of NLP research to cover less common rheumatic conditions and diverse patient demographics could increase the relevance and applicability of NLP tools, potentially challenging the prevailing focus on more prevalent conditions.
Several unmet clinical needs in rheumatology remain unaddressed by current NLP and LLM applications. For instance, preventing complications like falls in RA and cardiovascular disease in SLE is challenging [66, 67]. This technology could potentially contribute to risk stratification and personalized preventive interventions by analysing complex patient data and identifying high-risk individuals. Moreover, these models could aid in distinguishing between overlapping conditions like fibromyalgia and inflammatory arthritis, where patients often present with similar symptoms [68]. By integrating text analysis with clinical and laboratory data, LLMs might discern subtle patterns that could guide diagnosis and treatment decisions [68]. Furthermore, rare diseases like Behçet’s disease pose diagnostic challenges due to their heterogeneous presentations [69]. Advanced models integrating diverse data sources, including family history, demographics, clinical features and genetic markers like HLA-B51, could potentially improve diagnostic accuracy and facilitate early intervention [69].
For NLP to become integral to routine clinical practice, extensive clinical validation is necessary [61]. The current enthusiasm for the capabilities of NLP must be tempered with rigorous, evidence-based trials to bridge the gap between theoretical potential and practical utility. Moreover, the computational intensity required to run advanced NLP models is a significant barrier [70]. This challenge necessitates a balanced approach to technology adoption that considers existing infrastructural limits [71]. Nonetheless, the internet interface is widely available and easily usable, in addition to the use of application programming interfaces for streamlining different applications more efficiently [70, 71]. This could imply a future where these models can be relatively easily implemented and used.
Deploying NLP technologies also raises important ethical and privacy issues [72]. It is crucial to manage data responsibly and enforce stringent privacy measures to maintain trust and integrity within healthcare practices.
In conclusion, NLP shows significant potential to enhance rheumatology by improving diagnostic accuracy and personalizing patient care, particularly in detecting diseases and conditions from unstructured reports, especially for RA, SpA and gout. However, the realization of this potential is still in its early stages. Achieving the full benefits of NLP will require overcoming existing limitations through focused research, ethical commitment and ongoing technological enhancements.
Supplementary Material
Contributor Information
Mahmud Omar, Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel.
Mohammad E Naffaa, Rheumatology Unit, Galilee Medical Center, Nahariya, Israel.
Benjamin S Glicksberg, Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA; Division of Data-Driven and Digital Medicine (D3M), Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Hagar Reuveni, Division of Diagnostic Imaging, Sheba Medical Center, Affiliated to Tel-Aviv University, Ramat Gan, Israel.
Girish N Nadkarni, Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA; Division of Data-Driven and Digital Medicine (D3M), Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Eyal Klang, Charles Bronfman Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA; Division of Data-Driven and Digital Medicine (D3M), Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Supplementary material
Supplementary material is available at Rheumatology Advances in Practice online.
Data availability
The data underlying this article will be available upon reasonable request to the corresponding author.
Authors’ contributions
M.O. was responsible for data extraction, writing and validation. M.E.N. was responsible for data validation and editing. E.K. was responsible for data extraction, validation and editing. H.R. and B.S.G. were responsible for editing. G.N.N. was responsible for validation and oversight.
Funding
No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Beam AL, Drazen JM, Kohane IS. et al. Artificial intelligence in medicine. N Engl J Med 2023;388:1220–1. [DOI] [PubMed] [Google Scholar]
- 2. Thirunavukarasu AJ, Ting DSJ, Elangovan K. et al. Large language models in medicine. Nat Med 2023;29:1930–40. [DOI] [PubMed] [Google Scholar]
- 3. Wang J, Deng H, Liu B. et al. Systematic evaluation of research progress on natural language processing in medicine over the past 20 years: bibliometric study on PubMed. J Med Internet Res 2020;22:e16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ananthakrishnan AN, Cai T, Savova G. et al. Improving case definition of Crohn’s disease and ulcerative colitis in electronic medical records using natural language processing: a novel informatics approach. Inflamm Bowel Dis 2013;19:1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benavent D, Benavent-Núñez M, Marin-Corral J. et al. Natural language processing to identify and characterize spondyloarthritis in clinical practice. RMD Open 2024;10:e004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benavent D, Muñoz-Fernández S, De la Morena I. et al. Using natural language processing to explore characteristics and management of patients with axial spondyloarthritis and psoriatic arthritis treated under real-world conditions in Spain: SpAINET study. Ther Adv Musculoskelet Dis 2023;15:1759720X231220818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casey A, Davidson E, Poon M. et al. A systematic review of natural language processing applied to radiology reports. BMC Med Inform Decis Mak 2021;21:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nashwan AJ, AbuJaber AA.. Harnessing the power of large language models (LLMs) for electronic health records (EHRs) optimization. Cureus 2023;15:e42634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McMaster C, Bird A, Liew DFL. et al. Artificial intelligence and deep learning for rheumatologists. Arthritis Rheumatol 2022;74:1893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fiori G, Pignone A, Cerinic MM.. Overlap syndromes. Reumatizam 2002;49:12–5. [PubMed] [Google Scholar]
- 11. Van Vleck TT, Chan L, Coca SG. et al. Augmented intelligence with natural language processing applied to electronic health records for identifying patients with non-alcoholic fatty liver disease at risk for disease progression. Int J Med Inf 2019;129:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng Y, Pacheco JA, Ghosh A. et al. Natural language processing to identify lupus nephritis phenotype in electronic health records. BMC Med Inform Decis Mak 2024;22:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venerito V, Gupta L.. Large language models: rheumatologists’ newest colleagues? Nat Rev Rheumatol 2024;20:75–6. [DOI] [PubMed] [Google Scholar]
- 14. Hügle M, Omoumi P, van Laar JM, Boedecker J, Hügle T.. Applied machine learning and artificial intelligence in rheumatology. Rheumatol Adv Pract 2020;4:rkaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Venerito V, Bilgin E, Iannone F, Kiraz S.. AI am a rheumatologist: a practical primer to large language models for rheumatologists. Rheumatology (Oxford) 2023;62:3256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schiavo JH. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q 2019;38:171–80. [DOI] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bengio Y, Ducharme R, Vincent P, Janvin C.. A neural probabilistic language model. J Machine Learn Res 2003;3:1137–55. [Google Scholar]
- 19. LeCun Y, Bengio Y, Hinton G.. Deep learning. Nature 2015;521:436–44. [DOI] [PubMed] [Google Scholar]
- 20. Brietzke E, Gomes FA, Gerchman F, Freire RCR.. Should systematic reviews and meta-analyses include data from preprints? Trends Psychiatry Psychother 2023;45:e20210324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma LL, Wang YY, Yang ZH. et al. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res 2020;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coskun BN, Yagiz B, Ocakoglu G, Dalkilic E, Pehlivan Y.. Assessing the accuracy and completeness of artificial intelligence language models in providing information on methotrexate use. Rheumatol Int 2024;44:509–15. [DOI] [PubMed] [Google Scholar]
- 24. Lin C, Karlson EW, Dligach D. et al. Automatic identification of methotrexate-induced liver toxicity in patients with rheumatoid arthritis from the electronic medical record. J Am Med Inform Assoc 2015;22:e151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu F, Liu M, Li M. et al. Automatic knowledge extraction from Chinese electronic medical records and rheumatoid arthritis knowledge graph construction. Quant Imaging Med Surg 2023;13:3873–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang J, Ardavanis KS, Slack KE. et al. Chat generative pretrained transformer (ChatGPT) and bard: artificial intelligence does not yet provide clinically supported answers for hip and knee osteoarthritis. J Arthroplasty 2024;39:1184–90. [DOI] [PubMed] [Google Scholar]
- 27. Irfan B, Yaqoob A.. ChatGPT’s epoch in rheumatological diagnostics: a critical assessment in the context of Sjögren’s syndrome. Cureus 2023;15:e47754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gräf M, Knitza J, Leipe J. et al. Comparison of physician and artificial intelligence-based symptom checker diagnostic accuracy. Rheumatol Int 2022;42:2167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saini T, Ajad A, Niranjan Kumar A. Deep ensemble architecture for knee osteoarthritis severity prediction and report generation. In: 2023 5th International Conference on Recent Advances in Information Technology (RAIT), Dhanbad, India, 2023, pp. 1–6. https://ieeexplore.ieee.org/document/10126826 (27 May 2024, date last accessed).
- 30. Humbert-Droz M, Izadi Z, Schmajuk G. et al. Development of a natural language processing system for extracting rheumatoid arthritis outcomes from clinical notes using the national rheumatology informatics system for effectiveness registry. Arthritis Care Res 2023;75:608–15. [DOI] [PubMed] [Google Scholar]
- 31. Krusche M, Callhoff J, Knitza J, Ruffer N.. Diagnostic accuracy of a large language model in rheumatology: comparison of physician and ChatGPT-4. Rheumatol Int 2024;44:303–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Rastegar-Mojarad M, Liu S, Zhang H, Liu H.. Discovering adverse drug events combining spontaneous reports with electronic medical records: a case study of conventional DMARDs and biologics for rheumatoid arthritis. AMIA Jt Summits Transl Sci Proc 2017;2017:95–103. [PMC free article] [PubMed] [Google Scholar]
- 33. Ye C, Zweck E, Ma Z, Smith J, Katz S.. Doctor versus artificial intelligence: patient and physician evaluation of large language model responses to rheumatology patient questions in a cross-sectional study. Arthritis Rheumatol 2024;76:479–84. [DOI] [PubMed] [Google Scholar]
- 34. Uz C, Umay E.. “Dr ChatGPT”: is it a reliable and useful source for common rheumatic diseases? Int J Rheum Dis 2023;26:1343–9. [DOI] [PubMed] [Google Scholar]
- 35. Oliveira LL, Jiang X, Babu AN, Karajagi P, Daneshkhah A.. Effective natural language processing algorithms for early alerts of gout flares from chief complaints. Forecasting 2024;6:224–38. [Google Scholar]
- 36. Liao KP, Cai T, Gainer V. et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care Res 2010;62:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Luedders BA, Cope BJ, Hershberger D. et al. Enhancing the identification of rheumatoid arthritis-associated interstitial lung disease through text mining of chest computerized tomography reports. Semin Arthritis Rheum 2023;60:152204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. England BR, Roul P, Yang Y. et al. Extracting forced vital capacity from the electronic health record through natural language processing in rheumatoid arthritis-associated interstitial lung disease. Pharmacoepidemiol Drug Saf 2024;33:e5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Madrid-García A, Rosales-Rosado Z, Freites-Nuñez D. et al. Harnessing ChatGPT and GPT-4 for evaluating the rheumatology questions of the Spanish access exam to specialized medical training. Sci Rep 2023;13:22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walsh JA, Pei S, Penmetsa G. et al. Identification of axial spondyloarthritis patients in a large dataset: the development and validation of novel methods. J Rheumatol 2020;47:42–9. [DOI] [PubMed] [Google Scholar]
- 41. Osborne JD, Booth JS, O’Leary T. et al. Identification of gout flares in chief complaint text using natural language processing. AMIA Annu Symp Proc 2020;2020:973–82. [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshida K, Cai T, Bessette LG. et al. Improving the accuracy of automated gout flare ascertainment using natural language processing of electronic health records and linked Medicare claims data. Pharmacoepidemiol Drug Saf 2024;33:e5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao SS, Hong C, Cai T. et al. Incorporating natural language processing to improve classification of axial spondyloarthritis using electronic health records. Rheumatology (Oxford) 2020;59:1059–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Redd D, Frech TM, Murtaugh MA, Rhiannon J, Zeng QT.. Informatics can identify systemic sclerosis (SSc) patients at risk for scleroderma renal crisis. Comput Biol Med 2014;53:203–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kerr GS, Richards JS, Nunziato CA. et al. Measuring physician adherence with gout quality indicators: a role for natural language processing. Arthritis Care Res 2015;67:273–9. [DOI] [PubMed] [Google Scholar]
- 46. Li M, Liu F, Zhu J. et al. Model-based clinical note entity recognition for rheumatoid arthritis using bidirectional encoder representation from transformers. Quant Imaging Med Surg 2022;12:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Román Ivorra JA, Trallero-Araguas E, Lopez Lasanta M. et al. Prevalence and clinical characteristics of patients with rheumatoid arthritis with interstitial lung disease using unstructured healthcare data and machine learning. RMD Open 2024;10:e003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Y, Shu B, Moattari M, Zulkernine F, Queenan J, Barber D. SPaDe: a synonym-based pain-level detection tool for osteoarthritis. In: 2023 IEEE International Conference on Digital Health (ICDH), Chicago, IL, USA, 2023, pp. 118–20. https://ieeexplore.ieee.org/document/10224711 (2024 May 27, date last accessed)
- 49. Nelson SD, Lu C-C, Teng C-C. et al. The use of natural language processing of infusion notes to identify outpatient infusions. Pharmacoepidemiol Drug Saf 2015;24:86–92. [DOI] [PubMed] [Google Scholar]
- 50. van Leeuwen JR, Penne EL, Rabelink T, Knevel R, Teng YKO.. Using an artificial intelligence tool incorporating natural language processing to identify patients with a diagnosis of ANCA-associated vasculitis in electronic health records. Comput Biol Med 2024;168:107757. [DOI] [PubMed] [Google Scholar]
- 51. Zheng C, Rashid N, Wu Y-L. et al. Using natural language processing and machine learning to identify gout flares from electronic clinical notes. Arthritis Care Res 2014;66:1740–8. [DOI] [PubMed] [Google Scholar]
- 52. Li M, Shen X, Sun Y. et al. Using Semantic Text Similarity calculation for question matching in a rheumatoid arthritis question-answering system. Quant Imaging Med Surg 2023;13:2183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. VanSchaik JT, Jain P, Rajapuri A. et al. Using transfer learning-based causality extraction to mine latent factors for Sjögren’s syndrome from biomedical literature. Heliyon 2023;9:e19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Love TJ, Cai T, Karlson EW.. Validation of psoriatic arthritis diagnoses in electronic medical records using natural language processing. Semin Arthritis Rheum 2011;40:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sorin V, Barash Y, Konen E, Klang E.. Deep learning for natural language processing in radiology—fundamentals and a systematic review. J Am Coll Radiol 2020;17:639–48. [DOI] [PubMed] [Google Scholar]
- 56. Sorin V, Barash Y, Konen E, Klang E.. Deep-learning natural language processing for oncological applications. Lancet Oncol 2020;21:1553–6. [DOI] [PubMed] [Google Scholar]
- 57. Shamshad F, Khan S, Zamir SW. et al. Transformers in medical imaging: a survey. Med Image Anal 2023;88:102802. [DOI] [PubMed] [Google Scholar]
- 58. Soffer S, Glicksberg BS, Zimlichman E, Klang E.. BERT for the processing of radiological reports: an attention-based natural language processing algorithm. Acad Radiol 2022;29:634–5. [DOI] [PubMed] [Google Scholar]
- 59. Omar M, Nassar S, Hijaze K, Glicksberg BS, Nadkarni GN, Klang E.. Generating credible referenced medical research: a comparative study of Openai’s Gpt-4 and Google’s Gemini. 2024. https://papers.ssrn.com/abstract=4780940 (22 April 2024, date last accessed).
- 60. Ayers JW, Poliak A, Dredze M. et al. Comparing physician and artificial intelligence chatbot responses to patient questions posted to a public social media forum. JAMA Intern Med 2023;183:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Soto-Chávez MJ, Bustos MM, Fernández-Ávila DG, Muñoz OM.. Evaluation of information provided to patients by ChatGPT about chronic diseases in Spanish language. Digit Health 2024;10:20552076231224603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Venerito V, Puttaswamy D, Iannone F, Gupta L.. Large language models and rheumatology: a comparative evaluation. Lancet Rheumatol 2023;5:e574–8. [DOI] [PubMed] [Google Scholar]
- 63. Maarseveen TD, Meinderink T, Reinders MJT. et al. Machine learning electronic health record identification of patients with rheumatoid arthritis: algorithm pipeline development and validation study. JMIR Med Inform 2020;8:e23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Haase I, Xiong T, Rissmann A. et al. ChatSLE: consulting ChatGPT-4 for 100 frequently asked lupus questions. Lancet Rheumatol 2024;6:e196–9. [DOI] [PubMed] [Google Scholar]
- 65. Hossain E, Rana R, Higgins N. et al. Natural language processing in electronic health records in relation to healthcare decision-making: a systematic review. Comput Biol Med 2023;155:106649. [DOI] [PubMed] [Google Scholar]
- 66. Frostegård J. Systemic lupus erythematosus and cardiovascular disease. J Intern Med 2023;293:48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stanmore EK, Oldham J, Skelton DA. et al. Risk factors for falls in adults with rheumatoid arthritis: a prospective study. Arthritis Care Res 2013;65:1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Understanding fibromyalgia and its related disorders. Prim Care Companion J Clin Psychiatry 2008;10:133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Davatchi F, Chams-Davatchi C, Shams H. et al. Behcet’s disease: epidemiology, clinical manifestations, and diagnosis. Expert Rev Clin Immunol 2017;13:57–65. [DOI] [PubMed] [Google Scholar]
- 70. Tamang S, Humbert-Droz M, Gianfrancesco M. et al. Practical considerations for developing clinical natural language processing systems for population health management and measurement. JMIR Med Inform 2023;11:e37805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ray PP. ChatGPT: a comprehensive review on background, applications, key challenges, bias, ethics, limitations and future scope. Internet Things Cyber-Phys Syst 2023;3:121–54. [Google Scholar]
- 72. Naik N, Hameed BMZ, Shetty DK. et al. Legal and ethical consideration in artificial intelligence in healthcare: who takes responsibility? Front Surg 2022;9:862322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be available upon reasonable request to the corresponding author.