Abstract
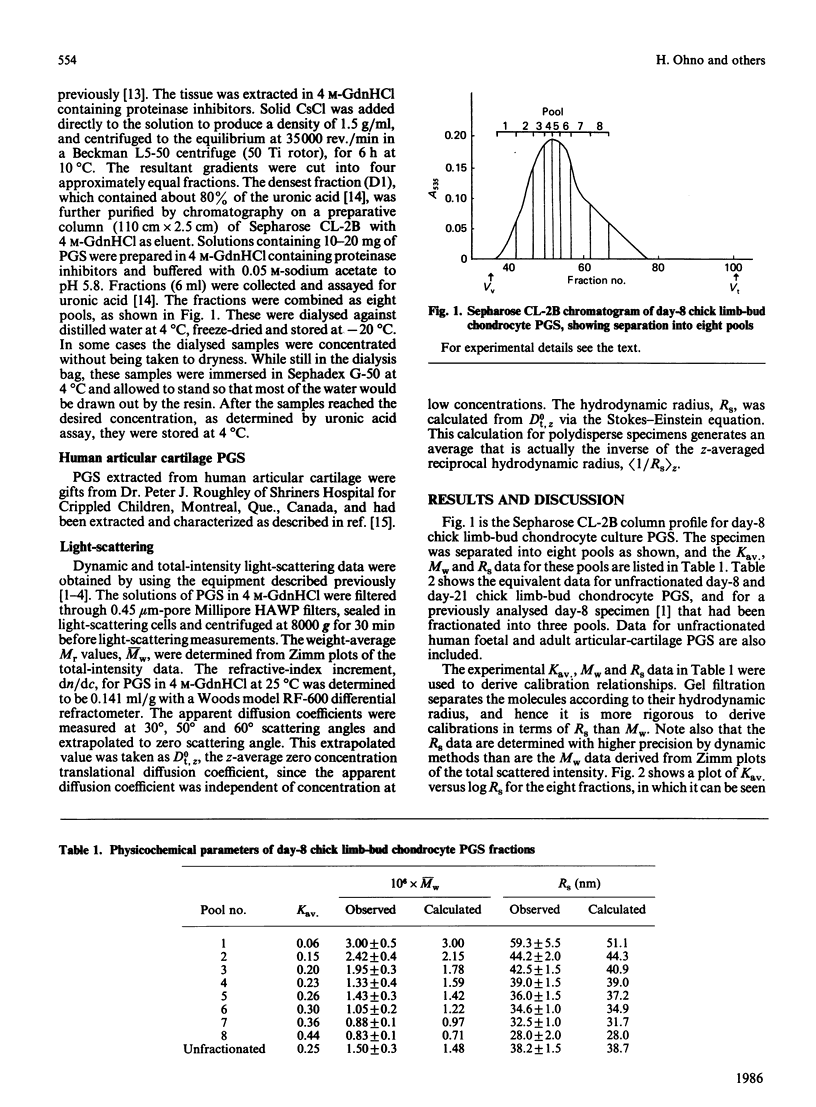
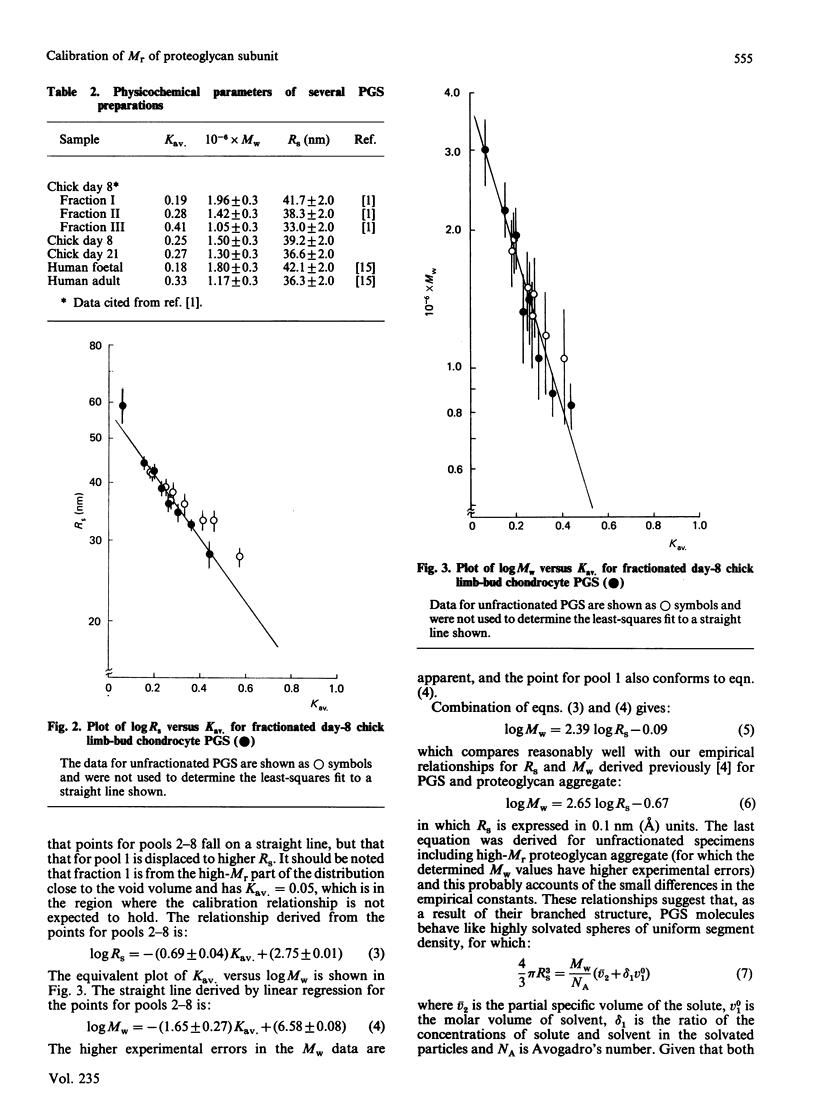
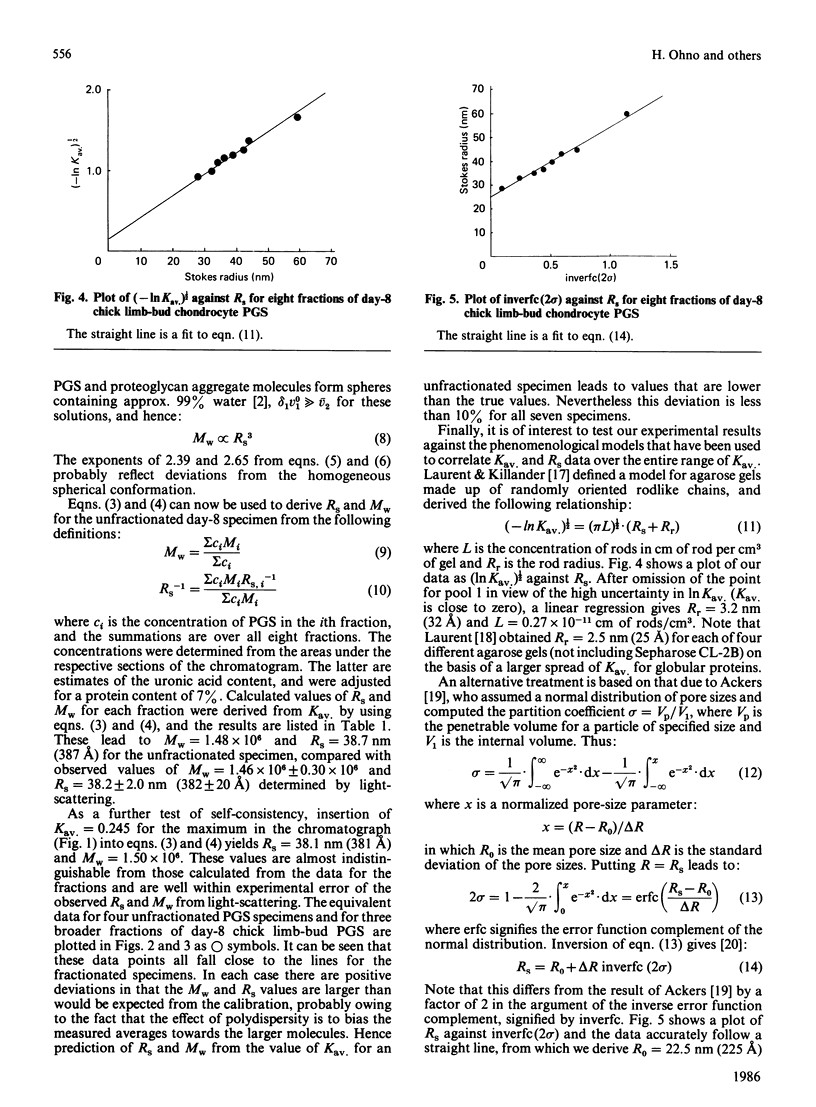
Calibration relationships were derived for cartilage proteoglycan subunit (PGS) that relate the inverse z-average hydrodynamic radius (Rs) and the weight-average Mr (Mw) to the partition coefficient (Kav.) for PGS when chromatographed on a Sepharose CL-2B column. PGS isolated from chick limb-bud chondrocyte cell cultures was fractionated chromatographically into eight pools, for which Mw and Rs were determined by total-intensity and dynamic light-scattering measurements. These data were found to be related to Kav. through the following empirical equations: log Mw = -(1.65 +/- 0.27)Kav. +(6.58 +/- 0.08); log Rs = -(0.69 +/- 0.04)Kav. +(2.75 +/- 0.01). Application of these relationships to the chromatographic data led to Mw = 1.48 X 10(6) and Rs = 38.7 nm (387 A) for the unfractionated specimens compared with values of Mw = 1.46 X 10(6) and Rs = 38.2 nm (382 A) determined by light-scattering. Our results were found to be consistent with previously proposed phenomenological models for the gel-filtration mechanism. Application of these calibration relationships to Kav. for several unfractionated specimens led to predicted values of Mw and Rs that are accurate to within 10%.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Caplan A. I. Effects of the nicotinamide-sensitive teratogen3-acetylpyridine on chick limb cells in culture. Exp Cell Res. 1970 Oct;62(2):341–355. doi: 10.1016/0014-4827(70)90564-1. [DOI] [PubMed] [Google Scholar]
- De Luca S., Heinegård D., Hascall V. C., Kimura J. H., Caplan A. I. Chemical and physical changes in proteoglycans during development of chick limb bud chondrocytes grown in vitro. J Biol Chem. 1977 Oct 10;252(19):6600–6608. [PubMed] [Google Scholar]
- Hardingham T. Proteoglycans: their structure, interactions and molecular organization in cartilage. Biochem Soc Trans. 1981 Dec;9(6):489–497. doi: 10.1042/bst0090489. [DOI] [PubMed] [Google Scholar]
- Hascall V. C. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. doi: 10.1002/jss.400070110. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Kimura J. H. Proteoglycans: isolation and characterization. Methods Enzymol. 1982;82(Pt A):769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Marroquin R., Wight T. N. Analysis of proteoglycans by high-performance liquid chromatography: a rapid micromethod for the separation of proteoglycans from tissue and cell culture. Anal Biochem. 1982 Oct;126(1):190–199. doi: 10.1016/0003-2697(82)90128-2. [DOI] [PubMed] [Google Scholar]
- Laurent T. C. Determination of the structure of agarose gels by gel chromatography. Biochim Biophys Acta. 1967 Mar 22;136(2):199–205. doi: 10.1016/0304-4165(67)90064-5. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Nakazawa K., Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem J. 1980 Oct 1;191(1):193–207. doi: 10.1042/bj1910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reihanian H., Jamieson A. M., Tang L. H., Rosenberg L. Hydrodynamic properties of proteoglycan subunit from bovine nasal cartilage. Self-association behavior and interaction with hyaluronate studied by laser light scattering. Biopolymers. 1979 Jul;18(7):1727–1747. doi: 10.1002/bip.1979.360180711. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Schwartz E. R., Stevens J., Schmidt D. E., Jr Proteoglycan analysis by high-performance liquid chromatography. Anal Biochem. 1981 Mar 15;112(1):170–175. doi: 10.1016/0003-2697(81)90276-1. [DOI] [PubMed] [Google Scholar]
- Shogren R. L., Blackwell J., Jamieson A. M., Carrino D. A., Pechak D., Caplan A. I. Light-scattering studies of chick limb bud proteoglycan aggregate. J Biol Chem. 1983 Dec 25;258(24):14741–14744. [PubMed] [Google Scholar]
- Shogren R., Jamieson A. M., Blackwell J., Carrino D. A., Caplan A. I. Light scattering studies of chick limb bud proteoglycans. J Biol Chem. 1982 Aug 10;257(15):8627–8629. [PubMed] [Google Scholar]


