Abstract
Background
Acute kidney injury (AKI) after spinal fusion is a significant morbidity that can lead to poor post-surgical outcomes. Identifying AKI risk factors and developing a risk model can raise surgeons’ awareness and allow them to take actions to mitigate the risks. The objective of the current study is to develop machine learning (ML) models to assess patient risk factors predisposing to AKI after posterior spinal instrumented fusion.
Methods
Data was collected from the IBM MarketScan Database (2009–2021) for patients >18 years old who underwent spinal fusion with posterior instrumentation (3–6 levels). AKI incidence (defined by the International Classification of Diseases codes) was recorded 90-day post-surgery. Risk factors for AKI were investigated and compared through several ML models including logistic regression, linear support vector machine (LSVM), random forest, extreme gradient boosting (XGBoost), and neural networks.
Results
Among the 141,697 patients who underwent fusion with posterior instrumentation (3–6 levels), the overall rate of 90-day AKI was 2.96%. We discovered that the logistic regression model and LSVM demonstrated the best predictions with area under the curve (AUC) values of 0.75. The most important AKI prediction features included chronic renal disease, hypertension, diabetes mellitus ± complications, older age (>50 years old), and congestive heart failure. Patients who did not have these five key risk factors had a 90-day AKI rate of 0.29%. Patients who had an increasing number of key risk factors subsequently had higher risks of postoperative AKI.
Conclusions
The analysis of the data with different ML models identified 5 key variables that are most closely associated with AKI: chronic renal disease, hypertension, diabetes mellitus ± complications, older age (>50 years old), and congestive heart failure. These variables constitute a simple risk calculator with additive odds ratio ranging from 3.38 (1 risk factor) to 91.10 (5 risk factors) over 90 days after posterior spinal fusion surgery. These findings can help surgeons risk-stratify their patients for AKI risk, and potentially guide post-operative monitoring and medical management.
Keywords: Spinal fusion, posterior spinal fusion surgery, acute kidney injury (AKI), risk factors, risk calculator
Highlight box.
Key findings
• A total of 141,697 patients who underwent multi-level instrumented spinal fusion were analyzed with machine learning models to create a 90-day acute kidney injury (AKI) risk model.
• 5 key variables most closely associated with AKI after surgery included: chronic renal disease, hypertension, diabetes without complications, older age (>50 years of age), and congestive heart failure.
What is known and what is new?
• AKI after spinal fusion is a significant morbidity that can lead to poor post-surgical outcomes.
• Identifying AKI risk factors and developing a risk stratification model can raise surgeons’ awareness and allow them to take actions to mitigate the risks.
What is the implication, and what should change now?
• Surgeons can utilize these findings to identify patients who may benefit from closer post-operative monitoring and targeted medical management strategies including increased fluid resuscitation and avoidance of potential nephrotoxic agents.
Introduction
Acute kidney injury (AKI) is a sudden, often reversible decrease in kidney function, usually measured as an increased serum creatinine level and/or reduced urine output (1). AKI after spine surgery can lead to significant morbidity, poor postoperative outcomes, and higher postoperative healthcare utilization (2). Identifying AKI risk factors and developing a risk model can raise surgeons’ awareness and allow them to take action to mitigate the risks.
Relatively few studies have attempted to evaluate perioperative risk factors associated with AKI after spine surgery. Studies have found preoperative hypertension (3)—but not intraoperative hypotension (4)—to potentially predispose to postoperative AKI. One retrospective study identified male sex, anemia, hypertension, and volatile anesthetics to be risk factors for AKI, concluding that total propofol-based intravenous anesthesia could be better for preventing kidney injury (5). Intraoperative hypothermia has been shown to be protective against postoperative AKI (6).
Only one study has attempted to create a predictive model for AKI after spine surgery, using a number of parameters including baseline glomerular filtration rates (GFR), Spinal Surgery Invasiveness Index, age, and preoperative hypertension that accounted for 64.4% of the variation in the postoperative GFRs (3). We present the largest study to date on this topic, incorporating machine learning (ML) models to stratify risk factors for AKI in a cohort of 141,697 patients after spinal fusion with posterior instrumentation. We secondarily present a simple tiered predictive calculator to help guide clinicians with patient risk stratification. We present this article in accordance with the STROBE reporting checklist (available at https://jss.amegroups.com/article/view/10.21037/jss-24-15/rc).
Methods
Data source
Patients were identified from the IBM MarketScan® Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefit databases (Ann Arbor, MI, USA) (https://www.merative.com/healthcare-analytics/truven-health-analytics). The database is a collection of medical insurance claims databases from over 300 employer-sponsored and Medicare supplemental plans, containing more than 240 million de-identified patient records. The database provides information on inpatient admissions, outpatient visits, and pharmaceutical encounters. The database was selected as it is one of the largest administrative claims databases and allows for longitudinal follow-up of continuously enrolled patients. As this was a retrospective cohort review of a national de-identified database, institutional review board (IRB) approval was not necessary. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Patient selection
The database was first queried for patients aged ≥18 years who underwent posterior spinal instrumentation (3–6 vertebral levels) between January 1, 2009, and December 31, 2021, as defined by the current procedural terminology (CPT) code ‘22842’ (posterior segmental instrumentation, e.g., pedicle fixation, dual rods with multiple hooks and sublaminar wires; 3 to 6 vertebral segments). Any procedures with associated traumas, malignancies, or infections were excluded via associated diagnosis code. Additionally, to ensure proper follow-up of the patient population, patients who were not continuously enrolled in the database for at least 6 months before surgery and 3 months after surgery were excluded. Finally, to limit confounder effects, patients with any episodes of AKI within 6 months prior to surgery were excluded.
Study variables and outcomes
Patient demographic information was collected from the database including age and sex. Ages were grouped into five categories as defined by the database: 18–34, 35–44, 45–54, 55–64, and 65+ years old. Comorbidity status was obtained using the Charlson Comorbidity Index (CCI). The CCI is a comorbidity measurement tool that is widely utilized to measure patients’ burden of diseases, which includes cardiovascular, neurologic, pulmonary, renal, and other chronic diseases (7). Additional comorbidities collected included obesity, smoking history, coronary artery disease (CAD), hypertension, hyperlipidemia, alcohol use disorder, depression, anxiety, atrial fibrillation, iron deficiency anemia, osteoporosis, valvular heart disease, a history of deep vein thrombosis (DVT) or pulmonary embolism (PE), and a chronic hypercoagulable state (e.g., diagnosis of protein C or S deficiency, Factor V Leiden, antiphospholipid antibody, lupus anticoagulant, or other thrombophilia).
The primary outcome for this study was the diagnosis of AKI within 90 days after surgery. Longitudinal tracking within the database allowed us to identify patients who had a 90-day AKI; as a result, patients were grouped as either having an AKI after surgery or no AKI after surgery. Comorbidities and complications were queried utilizing the ninth and tenth edition International Classification of Diseases (ICD) diagnostic codes (Table S1 and Table S2, respectively) (8).
Statistical analyses and predictive model construction
Descriptive statistics were generated based on demographics and CCI score between the two cohorts. Chi-squared tests were used to determine differences in categorical variables, and Student’s t-tests were used to analyze differences in continuous variables. To evaluate differences in each comorbidity collected, multivariate logistic regressions were performed, controlling for sex and age. Patients that had no AKI served as the reference group. All statistical analyses were conducted using R Studio (PBC, Boston, MA, USA). Statistical significance was defined as P<0.05 for all tests.
Five ML models were utilized to predict patient risk factors for AKI within 90 days after surgery: extreme gradient boosting (XGBoost) tree, logistic regression, random forest, linear support vector machine (LSVM), and neural networks. XGBoost (9) is an advanced implementation of a gradient boosting algorithm with a tree model as the base model. Multiple decision trees are trained to make predictions and identify feature importance. Logistic regression is a well-known method for building clinical prediction models utilizing general linear models (10). Random forest is a popular ML algorithm also utilizing decision tree models to construct classification tasks. LSVM is a robust classification technique that maps data to a high-dimensional feature space and incorporates a linear separator to classify data into separate categories. It is particularly suited for use with wide datasets (11). Neural networks are also a popular ML model that relies on interconnected nodes and hidden layers to accurately classify data.
Prior to ML usage, the data was randomly down-sampled to half of the patients in the no AKI group. This was done in order to balance the data, as prediction models with heavily weighted sample sizes in one cohort can create skewed results (12). For instance, since 97.04% of patients in our study population had no AKI within 90 days after surgery, a ML model that predicts “no AKI” every time will still have 97.04% of correct predictions; thus, the predictive values may not be represented accurately. Furthermore, in order to minimize overfitting of our models, due to the large number of potential variables, we performed feature selection utilizing Pearson’s correlation to remove variables that were not categorized as highly predictive of AKI within the dataset.
The data was then randomly partitioned in a 80:20 ratio of training and testing groups, where the testing data was evaluated after completion of the ML training process. Five-fold cross validation was used for the purposes of hyperparameter optimization. For each ML algorithm, four-fifths of the encounters within the 80% training split were randomly selected to train the corresponding model, and the remaining one-fifth was used as a validation set to determine model performance. This process gets repeated in total 5 times utilizing a new training and validation set. The combination of hyperparameters that performed the best across all five iterations was selected for incorporation into the final testing model, in which the entire 80% training split was trained on by the corresponding ML model before being tested on the 20% testing set. Figure 1 displays a work-flow of the methodology utilized for building the ML models with incorporation of cross validation.
Figure 1.
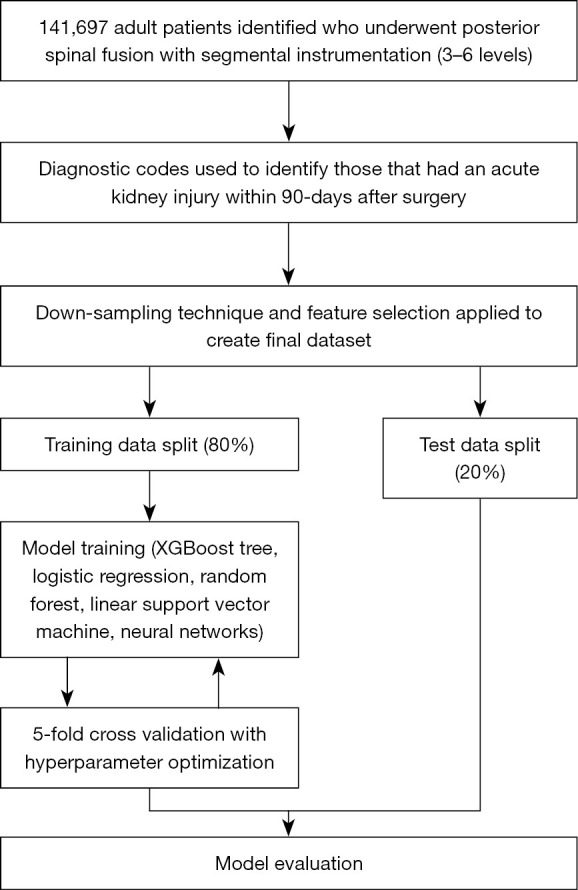
Methodology for building machine learning models to predict acute kidney injury. XGBoost, extreme gradient boosting.
In order to assess each ML prediction model, we computed the area under the receiving operating characteristic curve (AUROC) and derived sensitivity, specificity, positive predictive value, negative predictive value, diagnostic odds ratio (OR), positive likelihood ratio, and negative likelihood ratio from each confusion matrix. ML models were performed using SPSS Modeler version 18.4 (IBM, Chicago, IL, USA). Finally, the model with the highest AUROC and diagnostic OR was then utilized to quantify the risks for developing 90-day AKI based on the top five feature selection derived from the respective model. AKI rates were incrementally calculated for patients with none of the top five risk factors to patients with all of the top five risk factors. Multivariate logistic regressions, controlling for age, sex, and all collected comorbidities were then performed to identify the ORs for developing a 90-day AKI in patients with increasing numbers of top five risk factors. Patients that did not have any of the top five risk factors served as the reference group.
Results
Population demographics
A total of 141,697 adult patients who underwent posterior spinal fusion with segmental instrumentation (3–6 levels) were identified in the database from 2009 to 2021. Of the 141,697 patients, 4,192 patients (2.96%) were found to have an AKI within 90-days after surgery (Table 1). Patients that had a 90-day AKI were older (64.80 vs. 58.24 years; P<0.001), less likely to be female (41.39% vs. 53.65%; P<0.001), and had a higher CCI score (3.27 vs. 2.22; P<0.001) compared to patients that did not have a 90-day AKI.
Table 1. Baseline demographic data by 90-day AKI cohort.
| Characteristics | No AKI | AKI | P value |
|---|---|---|---|
| Total patients, n (%) | 137,505 (97.04) | 4,192 (2.96) | |
| Age (years), mean (SD) | 58.24 (11.87) | 64.80 (10.36) | <0.001 |
| Age group, n (%) | <0.001 | ||
| 18–34 years | 4,809 (3.50) | 17 (0.41) | |
| 35–44 years | 12,062 (8.77) | 98 (2.34) | |
| 45–54 years | 30,041 (21.85) | 491 (11.71) | |
| 55–64 years | 54,380 (39.55) | 1,635 (39.00) | |
| 65+ years | 36,213 (26.34) | 1,951 (46.54) | |
| Female patients, n (%) | 73,778 (53.65) | 1,735 (41.39) | <0.001 |
| CCI score, mean (SD) | 1.81 (2.22) | 3.89 (3.27) | <0.001 |
AKI, acute kidney injury; SD, standard deviation; CCI, Charlson Comorbidity Index.
Multivariate analyses of comorbidities
Comparisons of patient comorbidities between the AKI and no AKI groups are shown in Table 2. Utilizing multivariate logistic regressions controlling for age and sex, patients that experienced a 90-day AKI were more likely to have congestive heart failure (OR 2.61; P<0.001), rheumatic disease (OR 1.15; P<0.001), chronic renal disease (OR 5.00; P<0.001), chronic lung disease (OR 1.29; P<0.001), peripheral vascular disease (OR 1.61; P<0.001), cerebrovascular disease (OR 1.64; P<0.001), myocardial infarction (OR 1.85; P<0.001), peptic ulcer disease (OR 1.44; P<0.001), mild liver disease (OR 1.48; P<0.001), uncomplicated diabetes (OR 2.37; P<0.001), complicated diabetes (OR 3.09; P<0.001), solid malignant cancer (OR 1.35; P<0.001), hemiplegia (OR 1.59; P<0.001), metastatic cancer (OR 2.44; P<0.001), human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS) (OR 1.91; P=0.02), obesity (OR 1.83; P<0.001), CAD (OR 1.70; P<0.001), hypertension (OR 3.36; P<0.001), hyperlipidemia (OR 1.43; P<0.001), alcohol use disorder (OR 1.49; P<0.001), depression (OR 1.21; P<0.001), atrial fibrillation (OR 1.73; P<0.001), iron deficiency anemia (OR 2.06; P<0.001), valvular heart disease (OR 1.43; P<0.001), a history of DVT or PE (OR 2.08; P<0.001), and chronic hypercoagulability (OR 1.60; P<0.001). Based on individual multivariate logistic regressions, patients with chronic renal disease, hypertension, complicated diabetes, moderate/severe liver disease, and congestive heart failure had the highest ORs for a 90-day AKI, respectively.
Table 2. Ninety-day AKI rates with multivariate odds ratios by patient comorbidities.
| Comorbidity | No AKI (%) | AKI (%) | Odds ratio | 95% CI | P value |
|---|---|---|---|---|---|
| Congestive heart failure | 6.57 | 20.61 | 2.61 | 2.40–2.82 | <0.001 |
| Rheumatic disease | 20.63 | 23.03 | 1.15 | 1.07–1.24 | <0.001 |
| Chronic renal disease | 6.75 | 32.59 | 5.00 | 4.66–5.37 | <0.001 |
| Chronic lung disease | 27.62 | 34.49 | 1.29 | 1.21–1.38 | <0.001 |
| Peripheral vascular disease | 13.41 | 26.88 | 1.61 | 1.49–1.73 | <0.001 |
| Cerebrovascular disease | 13.42 | 26.67 | 1.64 | 1.52–1.76 | <0.001 |
| Myocardial infarction | 4.91 | 12.40 | 1.85 | 1.68–2.04 | <0.001 |
| Dementia | 0.54 | 1.24 | 1.31 | 0.98–1.75 | 0.06 |
| Peptic ulcer disease | 2.29 | 3.72 | 1.44 | 1.23–1.71 | <0.001 |
| Mild liver disease | 7.08 | 10.26 | 1.48 | 1.34–1.65 | <0.001 |
| Diabetes w/o complication | 22.22 | 45.49 | 2.37 | 2.22–2.52 | <0.001 |
| Diabetes w/ complication | 7.66 | 25.02 | 3.09 | 2.87–3.33 | <0.001 |
| Solid malignancy | 9.03 | 15.96 | 1.35 | 1.23–1.47 | <0.001 |
| Hemiplegia | 2.80 | 5.13 | 1.59 | 1.38–1.84 | <0.001 |
| Moderate/severe liver | 0.28 | 0.86 | 2.74 | 1.94–3.87 | <0.001 |
| Metastatic cancer (includes leukemia/lymphoma) | 1.28 | 3.79 | 2.44 | 2.06–2.89 | <0.001 |
| HIV/AIDS | 0.16 | 0.31 | 1.91 | 1.09–3.35 | 0.02 |
| Obesity | 27.69 | 38.17 | 1.83 | 1.71–1.95 | <0.001 |
| Smoking | 17.93 | 13.41 | 0.93 | 0.84–1.01 | 0.10 |
| Coronary artery disease | 20.28 | 39.15 | 1.70 | 1.59–1.82 | <0.001 |
| Hypertension | 67.11 | 90.63 | 3.36 | 3.02–3.74 | <0.001 |
| Hyperlipidemia | 59.59 | 73.83 | 1.43 | 1.33–1.53 | <0.001 |
| Alcohol use disorder | 3.39 | 4.84 | 1.49 | 1.29–1.72 | <0.001 |
| Depression | 27.83 | 27.39 | 1.21 | 1.13–1.29 | <0.001 |
| Anxiety | 13.57 | 11.98 | 1.06 | 0.96–1.16 | 0.25 |
| Atrial fibrillation | 5.12 | 13.02 | 1.73 | 1.57–1.90 | <0.001 |
| Iron deficiency anemia | 6.09 | 12.48 | 2.06 | 1.87–2.27 | <0.001 |
| Osteoporosis | 8.87 | 10.99 | 1.08 | 0.97–1.20 | 0.15 |
| Valvular heart disease | 12.96 | 22.99 | 1.43 | 1.37–1.60 | <0.001 |
| History of DVT/PE | 1.58 | 3.53 | 2.08 | 1.75–2.47 | <0.001 |
| Chronic hypercoagulable state | 1.72 | 2.53 | 1.60 | 1.31–1.95 | <0.001 |
Multivariate logistic regression controlled for by age, sex (reference is No AKI group). Diabetes w/o complication includes diabetes w/o cardiovascular disease, nerve or eye damage, or skin conditions. AKI, acute kidney injury; CI, confidence interval; HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome; DVT, deep vein thrombosis; PE, pulmonary embolism.
Predictive model parameters and assessment
Variable rankings from the five ML models can be seen in Table 3. In all models, chronic renal disease was selected as the most important variable for predicting a 90-day AKI after posterior fusion with spinal instrumentation. Furthermore, hypertension, congestive heart failure, and complicated diabetes were consistently featured by the different models (≥3) as top predictors of 90-day AKI. The models with the highest AUROC were the logistic regression and LSVM, which together predicted that patients with a chronic renal disease, hypertension, diabetes ± complications, older age (>50 years old), and congestive heart failure were the most important variables for predicting an AKI within 90 days after surgery.
Table 3. Top five important variables for risk of 90-day acute kidney injury by model.
| Model | AUROC | Variable 1 | Variable 2 | Variable 3 | Variable 4 | Variable 5 |
|---|---|---|---|---|---|---|
| XGBoost tree | 0.62 | Chronic renal disease | Diabetes w/ complications | Male sex | Congestive heart failure | Cerebrovascular disease |
| Logistic regression | 0.75 | Chronic renal disease | Hypertension | Diabetes w/o complications | Older age* | Congestive heart failure |
| Random forest | 0.68 | Chronic renal disease | Chronic pulmonary disease | Rheumatic disease | Obesity | Valvular heart disease |
| Linear support vector machine | 0.75 | Chronic renal disease | Diabetes w/ complications | Congestive heart failure | Hypertension | Diabetes w/o complications |
| Neural networks | 0.74 | Chronic renal disease | Hypertension | Diabetes w/ complications | Alcohol use disorder | Chronic hypercoagulability |
*, older age defined as patients >50 years old. AUROC, area under receiver operating characteristic curve.
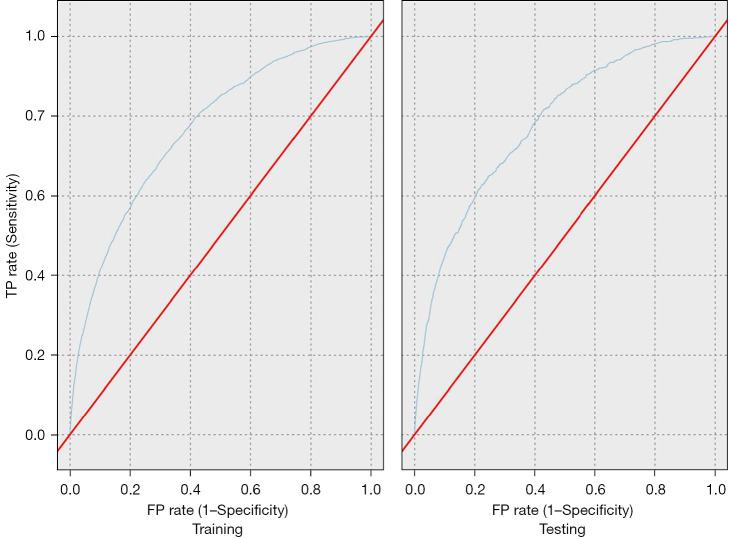
Assessment of the testing sets from each ML model is shown in Table 4. Three of the five models had an AUROC of >0.70. All of the models demonstrated strong specificity for predicting patients with a 90-day AKI but demonstrated weak sensitivity. The logistic regression model was selected as the model with the highest accuracy due to having the largest AUROC (0.75) with a high diagnostic OR (11.78). The AUROC for both the training and testing splits of the logistic regression model are displayed in Figure 2.
Table 4. Confusion matrices by machine learning model.
| Method | XGBoost tree | Logistic regression | Random forest | Linear support vector machine | Neural network |
|---|---|---|---|---|---|
| AUROC | 0.62 | 0.75 | 0.68 | 0.75 | 0.74 |
| Sensitivity | 9.65% | 1.05% | 3.37% | 0.00% | 1.63% |
| Specificity | 97.71% | 99.91% | 99.29% | 100.00% | 99.86% |
| Positive predictive value | 18.36% | 39.13% | 20.28% | 0.00% | 38.89% |
| Negative predictive value | 95.29% | 94.97% | 95.06% | 94.93% | 95.00% |
| Diagnostic odds ratio | 4.56 | 11.78 | 5.37 | N/A | 11.82 |
| Positive likelihood ratio | 4.21 | 12.03 | 4.76 | 0.00 | 11.91 |
| Negative likelihood ratio | 0.92 | 0.99 | 0.97 | 1.00 | 0.99 |
AUROC, area under receiver operating characteristic curve; N/A, not available.
Figure 2.
Receiver operating curves of the training and testing splits for the logistic regression model. TP, true positive; FP, false positive.
AKI risk stratification
In order to understand the AKI risks associated with the ML model rankings, patients with any top five risk factors for AKI from the logistic regression model were compared to patients that did not have any of the respective comorbidities (Table 5). Patients with no top five risk factors (n=16,139) had a 90-day AKI rate of 0.29%. Patients with any one of the top five risk factors (n=34,687) had a 90-day AKI rate of 0.96%, which was associated with a 3.38 greater OR of developing an AKI compared to patients with no risk factors (P<0.001). Furthermore, patients with any two of the top five risk factors (n=55,434) had a 90-day AKI rate of 2.42%, which was associated with 8.67 greater OR after multivariate logistic regression (P<0.001). Patients with any three of the top five risk factors (n=27,094) had a 90-day AKI rate of 5.06%, which was associated with 18.66 greater OR compared to patients with no risk factors (P<0.001). Patients with four of the top five risk factors (n=6,828) had a 90-day AKI rate of 11.57%, which represented 45.77 greater OR compared to patients with no risk factors (P<0.001). Finally, patients with all five risk factors (n=1,515) had a 90-day AKI rate of 20.66%. This represented a 91.10 greater OR for AKI compared to patients with none of the top risk factors (P<0.001).
Table 5. Risk calculator for 90-day AKI based on the number of comorbidities within the top 5 per the logistic regression model.
| No. of risk factors in patients | No. of patients | 90-day AKI rate (%) | Odds ratio | 95% CI | P value |
|---|---|---|---|---|---|
| 0 | 16,139 | 0.29% | Reference | ||
| 1 | 34,687 | 0.96% | 3.38 | 2.48–4.60 | <0.001 |
| 2 | 55,434 | 2.42% | 8.67 | 6.45–11.62 | <0.001 |
| 3 | 27,094 | 5.06% | 18.66 | 13.90–25.05 | <0.001 |
| 4 | 6,828 | 11.57% | 45.77 | 33.95–61.71 | <0.001 |
| 5 | 1,515 | 20.66% | 91.10 | 66.49–124.83 | <0.001 |
Top 5 risk factors included chronic renal disease, hypertension, diabetes without complications, older age, and congestive heart failure. AKI, acute kidney injury; CI, confidence interval.
Discussion
This represents the largest cohort of 141,697 patients who underwent posterior spinal instrumented fusion (3–6 levels) analyzed with predictive ML and logistic regression models to identify key risk factors for developing AKI postoperatively. In our study, the logistic regression and LSVM models demonstrated the best prediction capabilities with area under the curve (AUC) values of 0.75. According to this model, the most important risk factors for developing AKI 90 days postoperatively were chronic renal disease, hypertension, diabetes mellitus ± complications, age >50 years, and congestive heart failure (Table 5). These five key variables formulate a simplified AKI risk calculator with additive ORs ranging from 3.38 (1 risk factor) to 91.10 (5 risk factors).
There is a dearth of literature specifically evaluating risk of AKI following spine surgery. Some studies have attempted to utilize multivariate logistic regression analysis to identify risk factors for AKI after spine surgery, similarly identifying hypertension as one of the most important variables (3,5). The analysis by Naik et al. developed a regression equation to predict postoperative GFR, which similarly factored in age and baseline GFR (i.e., chronic renal disease) (3). Their model could only account for 64.4% of the variation in their sample of 726 patients. Our model incorporated the largest cohort of patients to date of 141,697 and was able to generate a more accurate regression model that accounted for hypertension, age >50 years, and chronic renal disease. The overall 90-day rate of AKI in our patient cohort after posterior spinal instrumented fusion was 2.96%, which is very similar to the overall rates published by other studies: 1.0–4.0% (3-5,13).
To our knowledge, this is the first study to utilize ML methodologies to attempt to develop a risk stratification model to predict AKI after spine surgery. Prior studies have attempted this in the field of cardiothoracic surgery (14,15). Some of the models utilized in this study, including LSVM and neural networks, approached an AUC of ~0.75 of our logistic regression model. Similar risk factors were identified by these models, including chronic renal disease, congestive heart failure, hypertension, diabetes mellitus, and age >50 years. Although logistic regression in our study reported the highest diagnostic OR, the ML prediction models still showed value in our study.
Overall, ML is useful in the context of understanding AKI risk after spinal surgery because it incorporates non-linear methods and complex decision models that can adapt with repeated iterations in order to identify patterns for predicting outcomes. As a result, ML can identify important trends that may be beyond the scope when utilizing traditional analyses, especially when working with large, complex datasets. For example, Tables 1,2 demonstrate baseline differences in demographics and comorbidities through univariate and multivariate analyses. Due to the large baseline sample population, most of the P values are below 0.001. It is difficult to contextualize which variables most contribute to AKI. As a result, ML techniques are useful for incorporating all the covariates into models in order to partition through which variables are more contributory for AKI; thus, the findings from this study can potentially help guide by clinicians in postoperative medical management strategies.
There are several limitations to this study. First, we are limited to the data available to us through the source IBM MarketScan Database. For instance, we did not have access to specific variables such as perioperative medication usage. We also lacked specific intraoperative variables, such as intraoperative hypotension or blood loss that could contribute to postoperative AKI. However, one study has shown that intraoperative hypotension during spinal fusion may not necessarily correlate with postoperative AKI (4). The database also does not provide details on anesthesia used or total time of operation. While little is known about the influence of operative time on risk of AKI, one study has shown that sevoflurane anesthesia may be more associated with postoperative AKI compared to propofol (16). Future studies should incorporate these variables in identifying risk factors for AKI after spinal surgery. Additionally, the model represented in this study is designed to allow surgeons and clinicians to risk stratify patients based on preoperative factors. We were unable to cross-reference CPT code 22842 with fusion codes to stratify our dataset across cervical, thoracic, and lumbar procedures. However, we removed trauma, infection, and malignancy diagnoses, allowing us to assume posterior spinal instrumentation would refer to a degenerative fusion. Another limitation of the study is the overall low AKI rate, which likely contributed to the overall low sensitivity values of our models. An up-sampling technique may be beneficial in future studies to better capture patients with AKI, although this method contains risks of overfitting and data noise. Finally, given our singular database, there could be limitations on the generalizability of our results. We recommend further study to externally validate our model and its results.
Conclusions
We present the largest cohort of 141,697 patients who underwent spinal fusion with posterior instrumentation (3–6 levels) studied with ML and logistic regression methodologies to construct a risk stratification tool that incorporates five key factors with additive ORs: chronic renal disease, hypertension, diabetes mellitus ± complications, congestive heart failure, and age >50 years. Clinicians and surgeons can utilize the results of this study to risk stratify patients preoperatively and identify those who may benefit from closer monitoring postoperatively and targeted medical management strategies (e.g., fluid resuscitation, avoidance of nephrotoxic agents such as ketorolac or vancomycin) for AKI.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study utilized a publicly available database with de-identified data; therefore, institutional review board approval was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jss.amegroups.com/article/view/10.21037/jss-24-15/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jss.amegroups.com/article/view/10.21037/jss-24-15/coif). The authors have no conflicts of interest to declare.
References
- 1.Goyal A, Daneshpajouhnejad P, Hashmi M, et al. Acute Kidney Injury. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 2.Ilyas H, Golubovsky JL, Chen J, et al. Risk factors for 90-day reoperation and readmission after lumbar surgery for lumbar spinal stenosis. J Neurosurg Spine 2019;31:20-6. 10.3171/2019.1.SPINE18878 [DOI] [PubMed] [Google Scholar]
- 3.Naik BI, Colquhoun DA, McKinney WE, et al. Incidence and risk factors for acute kidney injury after spine surgery using the RIFLE classification. J Neurosurg Spine 2014;20:505-11. 10.3171/2014.2.SPINE13596 [DOI] [PubMed] [Google Scholar]
- 4.Blue R, Gutierrez A, Ahmad HS, et al. Intraoperative Hypotension Is Not Correlated With Acute Kidney Injury During Spinal Fusion Surgery. Int J Spine Surg 2022;16:1061-7. 10.14444/8367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han J, Oh AY, Koo CH, et al. Effects of Anesthetic Technique on the Occurrence of Acute Kidney Injury after Spine Surgery: A Retrospective Cohort Study. J Clin Med 2021;10:5653. 10.3390/jcm10235653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh TK, Ryu JH, Sohn HM, et al. Intraoperative Hypothermia Is Associated with Reduced Acute Kidney Injury After Spine Surgery Under General Anesthesia: A Retrospective Observational Study. J Neurosurg Anesthesiol 2020;32:63-9. 10.1097/ANA.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 8.Heo KY, Bonsu JM, Muffly BT, et al. Complications Rates Among Revision Total Knee Arthroplasty Patients Diagnosed With COVID-19 Postoperatively. J Arthroplasty 2024;39:766-771.e2. 10.1016/j.arth.2023.09.028 [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Ichikawa D, Ueno T, et al. XGBoost, a Machine Learning Method, Predicts Neurological Recovery in Patients with Cervical Spinal Cord Injury. Neurotrauma Rep 2020;1:8-16. 10.1089/neur.2020.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deo RC. Machine Learning in Medicine. Circulation 2015;132:1920-30. 10.1161/CIRCULATIONAHA.115.001593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IBM. IBM SPSS Modeler 18.3 User’s Guide. Available online: https://www.ibm.com/docs/it/SS3RA7_18.3.0/pdf/ModelerUsersGuide.pdf
- 12.Pittman B, Buta E, Krishnan-Sarin S, et al. Models for analyzing zero-inflated and overdispersed count data: an application to cigarette and marijuana use. Nicotine Tob Res 2018;22:1390-8. 10.1093/ntr/nty072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin SJ, Park YS, Kim SH, et al. Effect of Prone Positional Apparatus on the Occurrence of Acute Kidney Injury After Spine Surgery. World Neurosurg 2019;128:e597-602. 10.1016/j.wneu.2019.04.216 [DOI] [PubMed] [Google Scholar]
- 14.Luo XQ, Kang YX, Duan SB, et al. Machine Learning-Based Prediction of Acute Kidney Injury Following Pediatric Cardiac Surgery: Model Development and Validation Study. J Med Internet Res 2023;25:e41142. 10.2196/41142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrosyan Y, Mesana TG, Sun LY. Prediction of acute kidney injury risk after cardiac surgery: using a hybrid machine learning algorithm. BMC Med Inform Decis Mak 2022;22:137. 10.1186/s12911-022-01859-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu X, Feng Z. Analysis of Risk Factors for Perioperative Acute Kidney Injury and Management Strategies. Front Med (Lausanne) 2021;8:751793. 10.3389/fmed.2021.751793 [DOI] [PMC free article] [PubMed] [Google Scholar]