Abstract
The potential roles of human herpesvirus 8 (HHV-8) cytokines in HHV-8 pathogenesis were investigated by determining the expression of the HHV-8 chemokines viral macrophage inflammatory protein 1A (vMIP-1A) and vMIP-1B in primary effusion lymphoma (PEL)-derived cell lines and examining the signaling activities of these chemokines and HHV-8-encoded vIL-6 in these cells. Secreted vMIP-1A and vMIP-1B were detected in biologically significant concentrations following tetradecanoyl phorbol acetate treatment, which induces productive replication. vIL-6 and vMIP-1A, added exogenously to cultures of four different PEL cell lines, induced the expression of vascular endothelial growth factor type B (VEGF-B) and VEGF-A, respectively. These cells were found to express VEGF receptor 1 (Flt-1) protein, and signaling by recombinant VEGF-A165 was demonstrated for two of the PEL cell lines, indicating the potential for autocrine, as well as paracrine, effects of viral cytokine-induced VEGF. In addition, vMIP-1A and vMIP-1B, but not VEGF-A165, were found to inhibit chemically induced apoptosis in PEL cells. Our data suggest that vIL-6 and vMIP-1A may influence PEL through VEGF autocrine and paracrine signaling that promotes PEL cell growth and extravascular effusion and that vMIP-1A and vMIP-1B can act independently of VEGF as antiapoptotic factors.
Human herpesvirus 8 (HHV-8) is associated with Kaposi's sarcoma, primary effusion lymphoma (PEL), and multicentric Castleman's disease. The role of HHV-8 in these diseases is unclear, but candidate viral transforming proteins and encoded factors that may contribute to viral pathogenesis have been identified (reviewed in references 8 and 28). In PEL, expression of several HHV-8 genes has been detected. Thus, the genes for LANA (open reading frame 73 [ORF73] product), kaposin (ORF K12 product), cyclin D (ORF72 product), vFLIP (ORF K13 product), and vIL-6 (ORF K2 product), in addition to some other genes, are expressed, to various degrees, in PEL cell lines or effusions that have been investigated (12, 27, 29, 32, 35, 37). Significantly, it has recently been reported that vIL-6 appears to play a mitogenic role, together with PEL-produced interleukin-10 (IL-10), in PEL, allowing the accelerated growth of PEL cells cultured at low serum concentrations (24). Further, it has been demonstrated that human IL-6 (hIL-6), secreted by PEL cells, stimulates clonal growth of PEL cells, although a similar effect of viral IL-6 (vIL-6) in the assays used was not observed (6), and that hIL-6 accelerates the growth of PEL cells in inoculated mice (20).
vIL-6 and the three chemokines (viral macrophage inflammatory protein 1A [vMIP-1A], vMIP-1B, and vBCK [new names vCCL1, vCCL2, and vCCL3, respectively, have been approved by the HHV-8/chemokine research community and will supercede previous v-chemokine names]) encoded by HHV-8 have been reported to induce angiogenesis (3, 9, 24, 27, 29, 38). Vascular endothelial growth factor (VEGF) can be induced by vIL-6 in in vitro and in vivo experimental systems (3) and is a key factor in promoting angiogenesis and important for the tumor-promoting effects of vIL-6 and the development of PEL-like disease in murine model systems (2, 3). The potential role in PEL of the three chemokines encoded by HHV-8 has not been investigated. Two of the viral chemokines, vMIP-1A (also called vMIP-I) and vMIP-1B (also called vMIP-II), have been demonstrated to signal through CCR8 to induce chemotaxis of Th2 cells, but not Th1 cells, and it has been suggested that these cytokines may perform immune evasion functions (16, 18, 36). However, it is also possible that they serve to attract lymphocytes to mediate viral dissemination, as has been implied by studies with viral chemokine-deficient murine cytomegalovirus recombinants used in vivo (19, 34). Whether the HHV-8 chemokines can be synthesized and secreted and can signal in PEL has not been investigated. If VEGF proteins are induced by the viral chemokines and/or vIL-6 and the induced VEGF species have autocrine effects through PEL-expressed VEGF receptors (VEGFRs), one of which (VEGFR-1) is known to be expressed at the mRNA level in at least some PEL cells (2), this could be relevant to PEL disease.
Here we have investigated the production of the viral chemokines vMIP-1A and vMIP-1B by PEL cells and the abilities of these proteins and vIL-6 (shown previously to be produced by PEL cells [2, 4, 24]) to induce VEGF expression from these cells. The data presented suggest that at least two of the viral cytokines, vIL-6 and vMIP-1A, may play mitogenic or antiapoptotic roles in PEL and/or induce PEL cell migration via induction of VEGF and VEGF autocrine and paracrine signaling and that vMIP-1A and vMIP-1B mediate antiapoptotic functions independently of VEGF. These effects may be relevant to HHV-8 pathogenesis.
MATERIALS AND METHODS
Cell culture.
HBL-6, BC-2, BC-3, and JSC-1 PEL cells were grown at 37°C in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics. For VEGF induction assays, PEL cells were seeded at a density of 107/ml in serum-free medium and left for 12 h prior to addition of recombinant viral cytokines or negative controls (glutathione S-transferase [GST] and intein). Medium was harvested after 18 h for detection of VEGF proteins by Western analysis. Human embryonic kidney (HEK) 293T cells were grown in Dulbecco modified Eagle medium containing 10% fetal bovine serum; these were transfected (by the calcium phosphate procedure) with cytokine expression plasmids to allow synthesis and secretion of the cytokines.
Cell viability assays and detection of apoptosis.
For determinations of potential antiapoptotic effects of viral cytokines on dexamethasone-induced PEL cell apoptosis, BC-3 or HBL-6 cells were seeded at 104 per well of a microassay plate in 200 μl of fresh medium, transfected-cell-conditioned medium containing dexamethasone (20 nM), or fresh medium containing dexamethasone (20 nM) and either vMIP-1A or vMIP-1B peptide (50 to 100 ng/ml) or rI309 (50 ng/ml). Conditioned media (harvested 48 h posttransfection) were derived from HEK 293T cells transfected with pSG5 (empty vector), pSG5-vMIP-1A, or pSG5-I309 (5 μg/5 × 106 cells). After 24 h of treatment, PEL cells were pelleted by centrifugation and 200 μl of MTT solution (0.5 mg/ml) was added to the cells, followed, after 3 h of incubation at 37°C, by addition of 200 μl of isopropanol. Color intensities were measured at 560 nm in a plate spectrophotometer. All assays were performed in triplicate. Dexamethasone-induced apoptosis of BC-3 cells and antiapoptotic effects of vMIP-1A and vMIP-1B were confirmed by using fluorescein isothiocyanate (FITC)-conjugated annexin V (catalog no. sc-4252-FL; Santa Cruz Biotechnology, Santa Cruz, Calif.). Following centrifugation, cells were resuspended in annexin V binding buffer (10 mM HEPES [pH 7.4], 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2) containing annexin V-FITC at 2 μg/ml, incubated on ice (in the dark) for 10 min, and then examined by UV and white light microscopy to calculate the percentage of cells (fluorescent) undergoing apoptosis.
Plasmids and oligonucleotides.
The vIL-6 ORF was also cloned as a BamHI fragment into bacterial expression vector pGEX4T1 (Pharmacia Biotech, Piscataway, N.J.) to generate GST–vIL-6 fusion protein (41) (see below). The coding sequences of vMIP-1A and vMIP-1B were cloned as NcoI and SmaI fragments into bacterial expression vector pTYB4 (New England Biolabs, Beverly, Mass.) to generate intein-chemokine fusion proteins (see below). Oligonucleotide primers directed to the 5′ and 3′ coding regions of VEGF-A were used to amplify, by reverse transcription (RT)-PCR, and clone as an EcoRI-BamHI fragment into pSG5 coding sequences of VEGF-A165 (40). The sequences of the VEGF-A primers are ggaatTCGGGCCTCCGAAACCA (VEGFA.P1; the complementary sequence, capitalized, corresponds to positions 41 to 57 of GenBank entry M32977) and acggaTCCTGCCCGGCTCACCG (VEGFA.P2; positions 643 to 627 of GenBank entry M32977).
Western analysis and immunoprecipitations.
vMIP-1A and vMIP-1B were immunoprecipitated from PEL cell culture media (400 μl) by using rabbit antisera (to peptides corresponding to amino acids 78 to 90 and 77 to 89, respectively) and protein A-agarose. Precipitated material was analyzed by Western blot assay to detect the viral chemokines. Western blotting was carried out essentially as described previously (41), by using horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology catalog no. sc-2004) and chemiluminescence assay to the detect filter-bound primary antibody. For the detection of VEGFRs in PEL cells, cells were lysed in 0.5% NP-40–40 mM Tris-HCl (pH 7.4)–150 mM NaCl and samples of cleared supernatants were analyzed by Western blotting using antibodies to VEGFR-1 (Santa Cruz Biotechnology catalog no. sc-316) or VEGFR-2 (catalog no. AF357; R & D Systems, Minneapolis, Minn.). Paxillin was immunoprecipitated from PEL cell lysates by incubation with paxillin-specific antibody (catalog no. P13520; Transduction Laboratories, Lexington, Ky.) and protein G-agarose (4°C, overnight), followed by centrifugation. Detection in sedimented material of total paxillin and phosphorylated paxillin was undertaken by Western blotting using anti-paxillin or anti-phosphotyrosine (catalog no. 05-321; Upstate Biotechnology, Lake Placid, N.Y.) primary antibody. VEGF-A and VEGF-B proteins in concentrated PEL cell culture media (500 μl) were detected by Western analysis using primary antibodies to VEGF-A and VEGF-B obtained from Santa Cruz Biotechnology (catalog no. sc-507) and R & D Systems (catalog no. AF751), respectively.
Recombinant viral cytokines.
GST–vIL-6 fusion protein was purified from isopropyl-β-d-thiogalactopyranoside (IPTG)-induced pGEX4T1-vIL-6-transformed bacteria by passage of sonicated cell extracts over a Sepharose 4B-glutathione column (Pharmacia Biotech, Piscataway, N.J.). Unbound material was removed by washing the column with 5 to 10 bed volumes of phosphate-buffered saline. Bound GST–vIL-6 was eluted with 5 bed volumes of elution buffer (50 mM Tris-HCl [pH 8.0], 10 mM glutathione [reduced]), and fractions were collected. vMIP-1A– and vMIP-1B–intein fusion proteins were purified from IPTG-induced pTYB4-vMIP-1A- and pTYB4-vMIP-1B-transformed bacteria by passage of sonicated cell extracts over chitin columns (New England Biolabs) and washing with 15 bed volumes of washing buffer (20 mM HEPES [pH 8.0], 500 mM NaCl, 0.1 mM EDTA, 0.1% Triton X-100). Recovery of the chemokines was effected by cleavage of the fusion proteins by incubation of the columns in cleavage buffer (30 mM dithiothreitol, 20 mM HEPES [pH 8.0], 50 mM NaCl, 0.1 mM EDTA) at 4°C overnight, followed by elution with cleavage buffer. Proteins were checked for purity and integrity by Coomassie staining of sodium dodecyl sulfate-polyacrylamide gels and Western blotting using antisera specific for vIL-6 (41), vMIP-1A (rabbit antiserum to amino acids 78 to 90 peptide), and vMIP-1B (rabbit antiserum to amino acids 77 to 89 peptide).
RESULTS
Viral chemokine production by PEL cells.
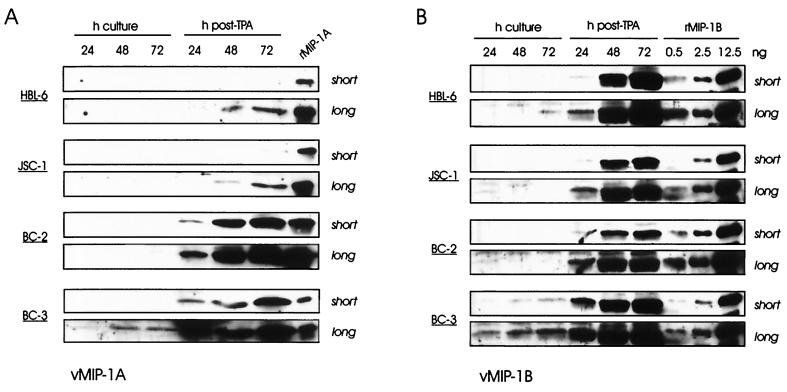
To provide insight into the possibility that the viral chemokines might play a role in PEL, we looked to see if we could detect vMIP-1A and vMIP-1B in the medium of different PEL cell cultures. Four different PEL cell lines, HBL-6, BC-2, BC-3 (EBV-negative), and JSC-1 (5, 11, 13, 21), were seeded at a density of 105 cells/ml, and medium samples were taken at different time points for detection of secreted vMIP-1A and vMIP-1B by Western analysis following immunoprecipitation (concentration) of the respective proteins. Rabbit antisera used for immunoprecipitation and detection of vMIP-1A and vMIP-1B were raised against peptides corresponding to residues 78 to 90 and 77 to 89, respectively; these were shown to be specific for their target proteins, with no detectable cross-reactivity (data not shown). Parallel samples were also taken from tetradecanoyl phorbol acetate (TPA)-treated PEL cell cultures, in which lytic replication of HHV-8 was induced, with consequent elevated expression of lytic genes, including those for the viral chemokines. Known amounts of recombinant vMIP-1A and vMIP-1B, derived by dithiothreitol cleavage of chitin-purified intein fusion proteins made in bacteria, were loaded onto the same gels as the culture media for direct comparisons of signal intensities on the Western blots for approximate quantitation.
The results of these experiments are shown in Fig. 1A and B. It is notable that v-MIP-1A and vMIP-1B could be detected in the media of BC-3 and HBL-6/BC-3 PEL cell cultures, respectively, in the absence of TPA induction, accumulating to levels ranging from around 0.1 ng/ml (vMIP-1A/BC-3) to 5 ng/ml (vMIP-1B/BC-3). However, the viral chemokines were induced to much higher levels in all PEL cell cultures by TPA. These data suggest that vMIP-1A and vMIP-1B are expressed as lytic genes, although their expression could conceivably occur in the absence of productive replication. It is possible that the levels of viral chemokines produced in vivo, from spontaneous lytic or abortive lytic replication, may reach the biologically relevant levels observed for vMIP-1B in uninduced BC-3 cultures; higher levels of vIL-6 in fresh PEL tissue than in PEL cell cultures suggest that this might be case (4, 12, 27, 37). It is worth noting, however, that higher levels (over 30-fold) of secreted vIL-6 expression by uninduced JSC-1 cells relative to BC-3 cultures did not correlate with the relative levels of viral chemokine expression by the two PEL cell lines (data not shown).
FIG. 1.
Expression of vMIP-1A and vMIP-1B in uninduced and TPA-induced PEL cell lines. Cells were seeded at a density of 105/ml in fresh medium either with or without added TPA (40 ng/ml), and samples were taken 24, 48, and 72 h thereafter. Medium samples (400 μl) were probed by Western analysis of immunoprecipitated material for vMIP-1A (A) or vMIP-1B (B) by using peptide antisera specific for each and horseradish peroxidase-conjugated anti-rabbit-IgG secondary antibody for detection by chemiluminescence assay. Known amounts of recombinant vMIP-1A (rMIP-1A) or vMIP-1B (rMIP-1B) were run alongside the samples to allow quantitation (the amount of recombinant vMIP-1A used was 0.4 ng). The estimated sizes of the detected proteins, from comparisons with the migration of molecular size markers, were around 8 kDa, as expected.
Induction by vIL-6 and vMIP-1A of VEGF in PEL cells.
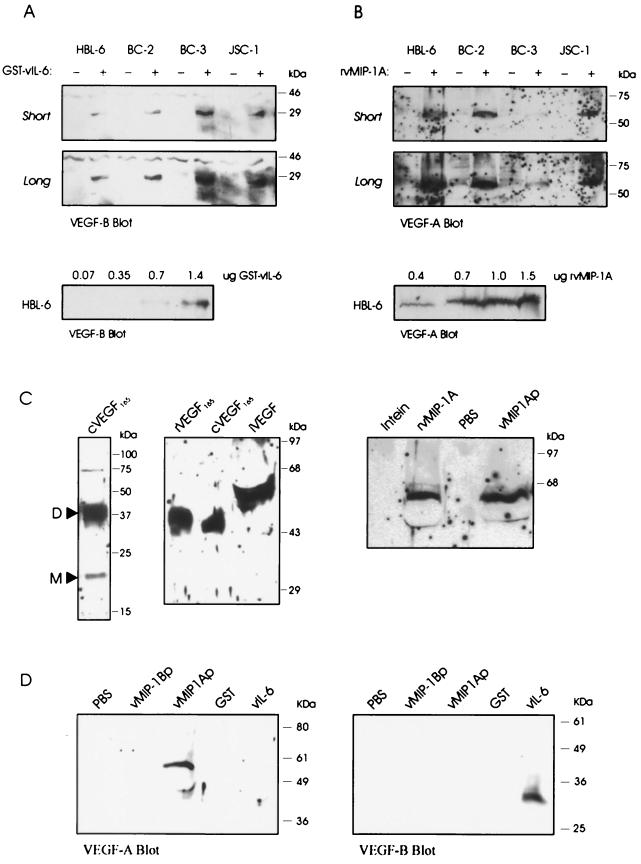
To investigate the possibility that vIL-6, vMIP-1A, and vMIP-1B might induce VEGF in PEL cells, as has been demonstrated for vIL-6 in other cell types (3), we probed for the secretion of VEGF-A and VEGF-B in the presence and absence of added viral cytokines. To be sure of the specificity of these effects, we used purified recombinant proteins derived from bacteria. vIL-6 was used as a GST fusion protein as described previously (41), while vMIP-1A and vMIP-1B were generated as viral chemokine-intein fusion proteins, by expression of pTYB4-cloned ORFs lacking their signal sequences, which were then cleaved to release the native chemokines (amino acids 26 to 95 and 24 to 94, respectively, with added N-terminal methionine and C-terminal pTYB4-derived PG residues). As shown in Fig. 2A, addition of GST–vIL-6 to different PEL cell cultures (HBL-6, BC-2, BC-3, and JSC-1) led to increased levels of VEGF-B secreted into the culture media, as detected by Western analysis using VEGF-B antiserum, while GST alone (negative control) had no effect. Using serial dilutions of GST–vIL-6 applied to HBL-6 cultures, we found that around 1 μg/ml was required for detectable VEGF-B induction, consistent with previous data concerning recombinant vIL-6-mediated signal transduction and support of myeloma cell growth (10, 41). This amount is approximately 103 times higher than the concentration of eukaryotically expressed vIL-6 required for activity (41) and could be due to differences in posttranslational modification and protein folding, for example. The size of vIL-6-induced VEGF-B was around 29 kDa, indicating that it represented the glycosylated VEGF-B186 isoform (32 kDa) rather than the smaller VEGF-B167 species (21 kDa) (30, 31). VEGF-A was not detected in the medium of vIL-6 treated cultures (shown for HBL-6 cells in Fig. 2D).
FIG. 2.
Induction of VEGF proteins in PEL cells. (A) Purified GST (−) or GST–vIL-6 (+) protein (2 μg/ml) was added to PEL cell cultures, and media were harvested 18 h later. Western analysis of concentrated samples of media (obtained from 0.5-ml samples of culture media by vacuum reduction) detected VEGF-B in GST–vIL-6-treated cultures (short and long exposures of the blot are shown). A 0.7-μg/ml concentration of GST–vIL-6 was able to induce VEGF-B (bottom). (B) Analogous experiments using a 1-μg/ml concentration of vMIP-1A (+) (derived from cleaved intein–vMIP-1A fusion protein) or intein (−) demonstrated that vMIP-1A was able to induce VEGF-A in PEL cell cultures and that a 0.4-μg/ml concentration of the recombinant chemokine was sufficient to detect this activity. (C) VEGF-A protein isoform induced by vMIP-1A. The left blot shows a Western analysis of VEGF165 protein secreted from HEK 293T cells transfected with pSG5-based expression vectors containing RT-PCR-amplified VEGF165 cDNA sequences (cVEGF165). Monomeric (M) and dimeric (D, predominant) forms of the proteins were detected. The VEGF-A isoform induced by vMIP-1A in HBL-6 cells migrated more slowly than the dimeric forms of the cVEGF165 and commercially obtained recombinant VEGF165 (rVEGF165) (middle blot), suggesting that the vMIP-1A-induced form (iVEGF) corresponds to VEGF-A206 (23). A 1-μg/ml concentration of chemically synthesized vMIP-1A peptide (vMIP-1Ap) was also able to induce expression (in HBL-6 cells) of VEGF-A; the size of the protein was the same as that induced by our recombinant vMIP-1A (1 μg/ml) (rMIP-1A). (D) Synthetic vMIP-1B peptide (vMIP-1Bp) was tested alongside vMIP-1A peptide (vMIP-1Ap) and GST–vIL-6 (vIL-6) for the ability to induce HBL-6 VEGF-A or VEGF-B expression and secretion. Each viral cytokine was used at a concentration of 1 μg/ml.
Conversely, treatment of the PEL cell cultures with vMIP-1A led to increases in secreted VEGF-A (Fig. 2B) but not VEGF-B (shown for HBL-6 in Fig. 2D). In dose-response experiments (Fig. 2B, bottom), 400 ng/ml, the lowest concentration used, gave reduced but detectable activity in HBL-6 cells. The size of induced VEGF-A, estimated at around 60 kDa, could correspond to a dimer of VEGF-A206 (23). While this isoform is known to associate with cell surface-expressed heparin sulfate proteoglycans, the protein is also secreted and can be detected in cell-free form. The vMIP-1A-induced VEGF-A species was found to migrate more slowly than dimeric forms of recombinant VEGF165 produced in bacteria or cDNA-derived VEGF165 expressed in transfected HEK 293T cells and was also induced in HBL-6 cells by commercially obtained vMIP-1A peptide (amino acids 25 to 95 of Met-initiated ORF K6; Technogen) (Fig. 2C). The latter result demonstrates the equivalent functions of bacterium-derived vMIP-1A and synthetic vMIP-1A, despite their different N and C termini.
Recombinant vMIP-1B from bacteria had no detectable effect on VEGF-A or VEGF-B expression (data not shown), indicating that vMIP-1B could not induce VEGF expression in PEL cells. However, to exclude the possibility that this result was due to the recombinant protein being functionally defective, we also used biologically active vMIP-1B peptide (Technogen) in additional experiments with HBL-6 cultures. The results (Fig. 2D) showed no evidence of vMIP-1B induction of VEGF, although vMIP-1A (peptide) and vIL-6 were able to induce VEGF-A and VEGF-B, respectively, in these experiments.
VEGFR-1 expression in PEL cells.
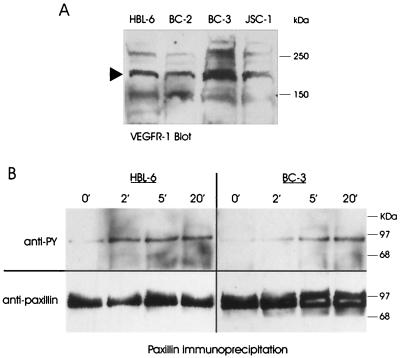
The finding that vIL-6 and vMIP-1A induced VEGF expression in PEL cells raised the possibility of autocrine signaling by VEGF-A and VEGF-B through PEL-expressed VEGFRs. To determine whether this was likely to be the case, we examined PEL cell extracts for expression of VEGFR-1 (targeted by VEGF-A and VEGF-B) and VEGFR-2 (targeted by VEGF-A) by Western analysis. Antisera specific for each of the receptors were used to detect the respective proteins in extracts derived from HBL-6, BC-2, BC-3, and JSC-1 cells. Strong immunoreactive bands of around 180 kDa, the size of VEGFR-1, were detected in all PEL cell extracts (Fig. 3A), but VEGFR-2 was not detected by this approach (data not shown). That each of the PEL cell lines was positive for VEGFR-1, able to bind and support signaling by both VEGF-A and VEGF-B, indicates that PEL cells generally are responsive to these vIL-6- and vMIP-1A-induced proteins.
FIG. 3.
Detection of VEGFR-1 expression and VEGF-A signaling in PEL cells. (A) Western analysis of PEL cell lysates using VEGFR-1 specific antiserum. VEGFR-1 protein of the expected size of 180 kDa (arrow) was detected in all PEL cell extracts. (B) Immunoprecipitation and Western analysis were used to detect phosphorylated (top blots) and total paxillin, a target of VEGFR-1 signaling, in HBL-6 and BC-3 cells following addition of recombinant VEGF165 (0.1 μg/ml). Aliquots of each culture were taken before addition of recombinant VEGF165 (0′) and at 2-, 5-, and 20-min time points following recombinant VEGF165 treatment. After detection of phosphopaxillin with anti-phosphotyrosine (anti-PY) antibody, blots were stripped and probed with anti-paxillin antibody to confirm equal loading of the lanes.
VEGF-A signaling in PEL cells.
To determine directly whether PEL cells are able to support signaling by VEGF, we measured the phosphorylation status of a target of VEGF signaling, the focal adhesion protein paxillin (1), in the absence and presence of exogenously added recombinant VEGF-A165. Paxillin was immunoprecipitated by using an antibody specific for this protein and then analyzed by Western blotting using detection antibodies specific for paxillin (to measure the total protein present) or phosphotyrosine (to determine changes in phosphorylation status in response to VEGF-A165). The results of these assays (Fig. 3B) demonstrate that in HBL-6 and BC-3 cells, at least (other cell lines were not investigated), VEGF-A165 is indeed able to induce phosphorylation of paxillin. These data provide direct evidence of VEGF-A signaling in PEL cells, indicate that the VEGFR-1 protein that we detected in PEL cells is functional, and suggest the possibility of VEGF autocrine signaling in PEL. Clearly, such signaling could be influenced by vIL-6 and vMIP-1A, which induce the expression of VEGF species that are able to interact functionally with VEGFR-1.
Viral chemokines protect cells from chemically induced apoptosis.
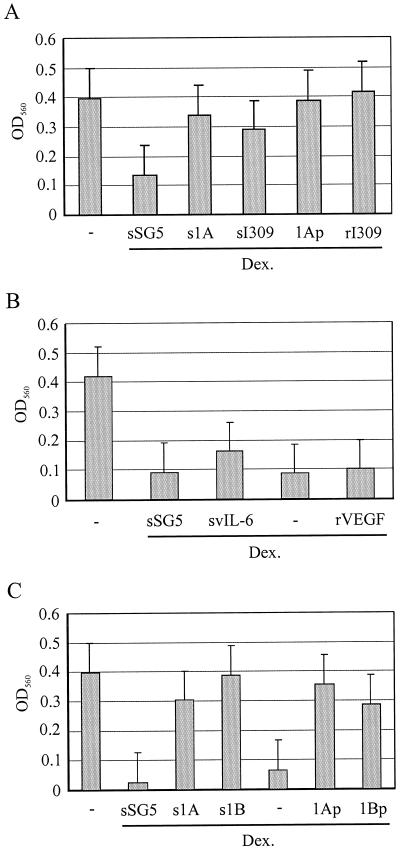
To test the possible effects of vMIP-1A and vIL-6 as antiapoptotic agents on PEL cells, functions possibly mediated via VEGF, we examined their abilities to protect PEL cells from dexamethasone-induced apoptosis. For these experiments, BC-3 cells were seeded at 104 per well in 96-well plates in either the absence or the presence of dexamethasone (20 nM) and conditioned medium from HEK 293T cells transfected with either pSG5 (empty vector), pSG5-vMIP-1A, or pSG5-I309 (encoding the CCR8 agonist I309). Parallel cultures were treated with dexamethasone and either synthetic vMIP-1A peptide (Technogen) or recombinant I309 (R & D Systems). Triplicate cultures (wells) were used for each of the conditions. After 24 h, cells were centrifuged, the medium was discarded, and MTT assays were performed (see Materials and Methods), with the optical density at 560 nm reflecting the numbers of viable cells. The results of these experiments are shown in Fig. 4A. HEK 293T transfected-cell medium containing vMIP-1A, but not medium from pSG5-transfected cultures, was able to mediate protective effects, as was I309-containing medium derived from transfected-cell cultures. Essentially the same results were obtained with the purified synthetic and recombinant proteins, demonstrating these to be the active agents. Conditioned media from pSG5-vIL-6-transfected HEK 293T cells and recombinant VEGF-A165 effected either marginal (vIL-6) or no protection against the effects of dexamethasone (Fig. 5B). The negative result for VEGF-A indicates that this cytokine and VEGFR-1-mediated signaling are not involved in the observed antiapoptotic effects of vMIP-1A. Analogous experiments with HBL-6 cells were undertaken using vMIP-1B peptide alongside vMIP-1A (positive control), and the results of these experiments (Fig. 4C) demonstrated that vMIP-1B also was able to mediate protection against the effects of dexamethasone. The activities on BC-3 and/or HBL-6 cells of I309, vMIP-1A, and vMIP-1B, all of which have been demonstrated to signal through CCR8 (16, 33, 36, 39), indicate that these PEL cells may express CCR8 and that this receptor may mediate antiapoptotic signaling by these chemokines.
FIG. 4.
Protective effects of viral cytokines. (A) PEL cells (BC-3, 104) were aliquoted into wells of microtiter plates in 200 μl of fresh medium (−), transfected HEK 293T cell-conditioned medium (negative control [pSG5], vMIP-1A, and I309) containing dexamethasone (Dex.; 20 nM), or fresh medium containing dexamethasone and either synthetic vMIP-1A peptide (1Ap; 50 ng/ml) or rI309 (50 ng/ml). The results obtained for vMIP-1A and I309 conditioned media (s1A and sI309), relative to those obtained with the negative control (sSG5), indicate that these chemokines have protective effects. vMIP-1A peptide and rI309 showed the same activities. (B) Similar experiments were undertaken by using vIL-6-conditioned medium (derived from HEK 293T cells transfected with pSG5-vIL-6) and recombinant VEGF165 (50 ng/ml), but no clear protective activity was detected. (C) Protective effects were detected for synthetic vMIP-1B peptide (1Bp; 100 ng/ml), whose activity was comparable to that of the vMIP-1A peptide (100 ng/ml), in HBL-6 cultures. All data presented are averages of triplicate samples for each treatment. OD560, optical density at 560 nm.
FIG. 5.
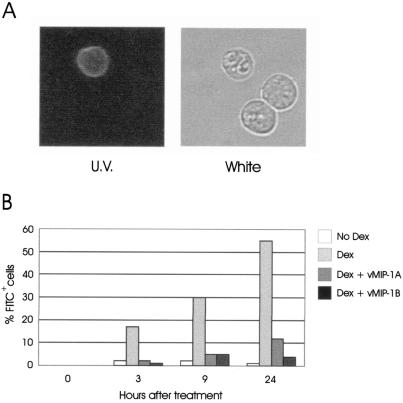
Confirmation of antiapoptotic effects of viral chemokines. (A) Induction of apoptosis in a representative PEL cell line, BC-3, by dexamethasone (Dex.) was confirmed by using FITC-conjugated annexin V. An example of a positively staining cell (observed under UV light) with apparent altered nuclear morphology (observed under white light) is shown, together with healthy cells in the same population. (B) At different time points, annexin V-staining (apoptotic) cells in untreated cultures or dexamethasone-treated cultures containing vMIP-1A or vMIP-1B peptide (50 ng/ml) or no chemokine were counted and expressed as a percentage of the total number of cells (three fields were counted; n = 200 to 300).
Additional experiments were performed to demonstrate the induction of apoptosis in PEL cells by dexamethasone and to confirm the antiapoptotic activities of vMIP-1A and vMIP-1B. For these experiments, we used FITC-conjugated annexin V, which recognizes phosphatidylserine residues that are exposed on the cell surface during early stages of apoptosis (26), to identify apoptotic cells in dexamethasone-treated or untreated BC-3 cultures. An example of the annexin V-FITC staining pattern induced by dexamethasone is shown in Fig. 5A. Dexamethasone induced apoptosis in a substantial proportion (17% at 3 h and up to 55% at later times) of cells within treated cultures, with annexin V staining of only a very small proportion (1 to 2%) of BC-3 cells in untreated, parallel cultures (Fig. 5B). Addition of vMIP-1A or vMIP-1B to dexamethasone-treated cultures led to markedly reduced numbers of cells staining with annexin V-FITC, relative to cultures treated with dexamethasone only (Fig. 5B). For example, at 9 h, 5% of cells in the viral chemokine-containing cultures were FITC positive, compared to 30% of those in the control culture. These data are consistent with the data from the MTT assays (Fig. 4) and demonstrate that vMIP-1A and vMIP-1B are able to protect BC-3 cells from dexamethasone-induced apoptosis.
DISCUSSION
HHV-8 encodes multiple cytokines that are able to promote mitogenesis (vIL-6) and angiogenesis (vIL-6, vMIP-1A, vMIP-1B, and vBCK) (3, 9, 10, 24, 27, 29, 38). VEGF can be induced by vIL-6 in in vitro and in vivo experimental systems (3) and is a key factor in promoting angiogenesis and important for the tumor-promoting effects of vIL-6 and the development of PEL-like disease in murine model systems (2, 3). These findings prompted us to examine the expression of the HHV-8 cytokines in PEL cells and their influences on VEGF expression in these cells. In addition, the potential antiapoptotic effects of the viral cytokines on PEL cells were investigated, as IL-6 and some chemokines are known to mediate such effects on other cell types. We were able to detect by Western analysis vMIP-1A and vMIP-1B, in addition to vIL-6 (data not shown), proteins in PEL cell culture media (at least when the cultures were treated with TPA), although we could not detect vBCK by analogous procedures, despite the ability of our vBCK antiserum to detect recombinant vBCK (data not shown). Our data show for the first time that the vMIP-1A and vMIP-1B proteins are synthesized and secreted by PEL cells and that these proteins mediate protective effects against dexamethasone-induced apoptosis. Further, we were able to demonstrate that both vIL-6 and vMIP-1A induce the expression of VEGF (VEGF-B and VEGF-A, respectively) in PEL cells and that PEL cells express VEGFR-1, suggesting the possibility of in vivo autocrine regulation by vIL-6- and vMIP-1A-induced VEGF.
VEGF is likely to play a crucial role in the development of HHV-8-associated diseases by acting as a mediator of angiogenesis and vascular permeability, factors that are central to the development of Kaposi's sarcoma and PEL and that may be involved in multicentric Castleman's disease. Also, VEGF has been implicated in viral cytokine-mediated angiogenic activities observed in experimental systems (2, 3, 9, 38). Although VEGF-A has been reported to be induced by vIL-6 (3), our data show clearly that VEGF-B, and not secreted isoforms of VEGF-A, is induced by vIL-6 in PEL cells, and size estimates indicate that of the two isoforms of VEGF-B, VEGF-B186 is the form that is induced. In contrast, vMIP-1A induced secretion of VEGF-A by PEL cells; here, VEGF-A206 is likely to be the isoform that we detected. Attempts to identify VEGF isoforms induced by the viral chemokines by RT-PCR using primers to common 5′ and 3′ exons of the various mRNA species, allowed detection in both untreated and viral cytokine-treated PEL cells of VEGF-A121, VEGF-A145, and VEGF-A165, as reported previously for several PEL cell lines (2), but mRNAs for other VEGF-A isoforms or VEGF-B mRNAs were not detected in either the absence or the presence of viral cytokines (data not shown). The reasons for the lack of detection of VEGF-A and VEGF-B mRNAs corresponding to the sizes of the respective proteins induced by vMIP-1A and vIL-6 are uncertain, but it may be due to sequence-specific difficulties with RT and/or PCR of the mRNAs and cDNAs in question. The lack of detection of VEGF-A121, VEGF-A145, and VEGF-A165 proteins in our PEL cell cultures suggests the operation of posttranslational regulatory mechanisms that are known to be important for control of VEGF expression. The particular mechanisms and functional significance of induction of specific VEGF-A and VEGF-B isoforms by vMIP-1A and vIL-6 remain to be determined, however. Notwithstanding, our detection in PEL cells of VEGFR-1 (Flt-1) receptors, which are able to respond to signaling by the different VEGF-A and VEGF-B isoforms, and signaling by recombinant VEGF-A165 in these cells (most likely through VEGFR-1) suggest the possibility of autocrine signaling by PEL-produced VEGF species.
The biological significance of autocrine signaling by VEGF proteins in PEL cells is open to speculation, but it is conceivable that mitogenic functions in addition to chemotactic activities associated with VEGFR-1 (7, 14) could be effected to influence disease. Findings of Aoki and Tosato (2) that VEGF-A neutralizing antibodies inhibit PEL growth, in addition to the development of lymphomatous effusions, in inoculated mice are consistent with this hypothesis. Although it is notable that no effects of VEGF-A neutralization on the growth of PEL cells in culture were found in this study, it is possible that intracellular autocrine signaling (not susceptible to blocking by neutralizing antibodies) occurs in cultured PEL cells, as appears to be the case in at least one other system (22). VEGFR-1 has been associated mainly with cell migration rather than mitogenesis, but VEGFR-1 is able to mediate mitogenic responses in at least some cell types (15, 25), and VEGFR-1 is dramatically increased in rat retinal pigment epithelial cells transformed by constitutive expression of VEGF (17). It is possible that VEGFR-1-mediated antiapoptotic effects may also be relevant to PEL; it has been demonstrated that in primary human trophoblast cells, placental growth factor, which signals through VEGFR-1 (expressed in trophoblast cells), can inhibit apoptosis induced by growth factor withdrawal (17). However, we were unable to detect antiapoptotic activity of recombinant VEGF-A165 in dexamethasone-treated BC-3 cells. VEGFR-1-mediated PEL cell migration could conceivably play a role in disease by allowing PEL cells to respond to VEGF secreted either by other PEL cells or by distinct cell types, perhaps enhancing cell migration through vessel walls. Thus, PEL-expressed VEGFR-1 could potentially be involved in PEL disease via autocrine or paracrine signaling by VEGF-A or VEGF-B through effects on mitogenesis, cell survival, or cell migration.
The antiapoptotic activities of vMIP-1A and vMIP-1B that we have identified have not previously been noted for these chemokines, and it is of potential biological significance that the viral chemokines are active on PEL cells. These activities are independent of VEGF, as recombinant VEGF-A165 was unable to mediate protection against dexamethasone-induced apoptosis and vMIP-1B was unable to induce VEGF-A or VEGF-B in our experiments. We do not know the identity of the PEL-expressed chemokine receptor(s) targeted by vMIP-1A and vMIP-1B, but CCR8 is a likely candidate, as both viral chemokines are known to signal through this receptor, and I309, a CCR8 agonist, was also able to mediate protective effects on dexamethasone-treated PEL cells (Fig. 4A). Whether the same or a different receptor mediates vMIP-1A induction of VEGF-A is an open question, but the apparent inability of vMIP-1B to induce VEGF-A might indicate the latter.
In summary, the data presented in this report show for the first time that the vMIP-1A and vMIP-1B proteins are synthesized and secreted by PEL cells; that both vIL-6 and vMIP-1A induce the expression of VEGF (VEGF-B and VEGF-A, respectively) in PEL cells; that PEL cells express VEGFR-1 and support VEGF signaling, suggesting the possibility of in vivo autocrine regulation by vIL-6- and vMIP-1A-induced VEGF; and that vMIP-1A and vMIP-1B are able to protect PEL cells from chemically induced apoptosis. These findings demonstrate the ability of vIL-6, vMIP-1A, and vMIP-1B to mediate signal transduction in PEL cells and indicate that autocrine signaling by these viral cytokines and induced VEGF species may be relevant to PEL disease.
ACKNOWLEDGMENTS
This work was supported by grants CA76445 from the National Institutes of Health and MBC-89095 from the American Cancer Society.
Footnotes
In memory of Bill Burns.
REFERENCES
- 1.Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J Biol Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- 2.Aoki Y, Tosato G. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood. 1999;94:4247–4254. [PubMed] [Google Scholar]
- 3.Aoki Y, Jaffe E S, Chang Y, Jones K, Teruya-Feldstein J, Moore P S, Tosato G. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood. 1999;93:4034–4043. [PubMed] [Google Scholar]
- 4.Aoki Y, Yarchoan R, Braun J, Iwamoto A, Tosato G. Viral and cellular cytokines in AIDS-related malignant lymphomatous effusions. Blood. 2000;96:1599–1601. [PubMed] [Google Scholar]
- 5.Arvanitakis L, Mesri E A, Nador R G, Said J W, Asch A S, Knowles D M, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- 6.Asou H, Said J W, Yang R, Munker R, Park D J, Kamada N, Koeffler H P. Mechanisms of growth control of Kaposi's sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood. 1998;91:2475–2481. [PubMed] [Google Scholar]
- 7.Barleon B, Sozzani S, Zhou D, Weich H A, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 8.Boshoff C, Chang Y. Kaposi's sarcoma-associated herpesvirus: a new DNA tumor virus. Annu Rev Med. 2001;52:453–470. doi: 10.1146/annurev.med.52.1.453. [DOI] [PubMed] [Google Scholar]
- 9.Boshoff C, Endo Y, Collins P D, Takeuchi Y, Reeves J D, Schweickart V L, Saini M A, Sasaki T, Williams T J, Gray P W, Moore P S, Chang Y, Weiss R A. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 10.Burger R, Neipel F, Fleckenstein B, Savino R, Ciliberto G, Kalden J R, Gramatzki M. Human herpesvirus 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood. 1998;91:1858–1863. [PubMed] [Google Scholar]
- 11.Cannon J S, Ciufo D, Hawkins A L, Griffin C A, Borowitz M J, Hayward G S, Ambinder R F. A new primary effusion lymphoma-derived cell line yields highly infectious Kaposi's sarcoma herpesvirus supernatant. J Virol. 2000;74:10187–10193. doi: 10.1128/jvi.74.21.10187-10193.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannon J S, Nicholas J, Ornstein J M, Mann R B, Murray P G, Browning P J, DiGiuseppe J A, Cesarman E, Hayward G S, Ambinder R F. Heterogeneity of viral IL-6 expression in HHV-8-associated disease. J Infect Dis. 1999;180:824–828. doi: 10.1086/314956. [DOI] [PubMed] [Google Scholar]
- 13.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 14.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 15.Couper L L, Bryant S R, Eldrup-Jorgensen J, Bredenberg C E, Lindner V. Vascular endothelial growth factor increases the mitogenic response to fibroblast growth factor-2 in vascular smooth muscle cells in vivo via expression of fms-like tyrosine kinase-1. Circ Res. 1997;81:932–939. doi: 10.1161/01.res.81.6.932. [DOI] [PubMed] [Google Scholar]
- 16.Dairaghi D J, Fan R A, McMaster B E, Hanley M R, Schall T J. HHV-8-encoded vMIP-I selectively engages chemokine receptor CCR8. Agonist and antagonist profiles of viral chemokines. J Biol Chem. 1999;274:21569–21574. doi: 10.1074/jbc.274.31.21569. [DOI] [PubMed] [Google Scholar]
- 17.Desai J, Holt-Shore V, Torry R J, Caulde M R, Torry D S. Signal transduction and biological function of placental growth factor in primary human trophoblast. Biol Reprod. 1999;60:887–892. doi: 10.1095/biolreprod60.4.887. [DOI] [PubMed] [Google Scholar]
- 18.Endres M J, Garlisi C G, Xiao H, Shan L, Hedrick J A. The Kaposi's sarcoma-related herpesvirus (KSHV)-encoded chemokine vMIP-I is a specific agonist for the CC chemokine receptor (CCR)8. J Exp Med. 1999;189:1993–1998. doi: 10.1084/jem.189.12.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleming P, Davis-Poynter N, Degli-Esposti M, Densley E, Papadimitriou J, Shellam G, Farrell H. The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity. J Virol. 1999;73:6800–6809. doi: 10.1128/jvi.73.8.6800-6809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foussat A, Wijdenes J, Bouchet L, Gaidano G, Neipel F, Balabanian K, Galanaud P, Couderc J, Emilie D. Human interleukin-6 is in vivo an autocrine growth factor for human herpesvirus-8-infected malignant B lymphocytes. Eur Cytokine Netw. 1999;10:501–508. [PubMed] [Google Scholar]
- 21.Gaidano G, Cechova K, Chang Y, Moore P S, Knowles D M, Dalla-Favera R. Establishment of AIDS-related lymphoma cell lines from lymphomatous effusions. Leukemia. 1996;10:1237–1240. [PubMed] [Google Scholar]
- 22.Guerrin M, Scotet E, Malecaze F, Houssaint E, Plouet J. Overexpression of vascular endothelial growth factor induces cell transformation in cooperation with fibroblast growth factor 2. Oncogene. 1997;14:463–471. doi: 10.1038/sj.onc.1200846. [DOI] [PubMed] [Google Scholar]
- 23.Houck K A, Ferrara N, Winer J, Cachianes G, Li B, Leung D W. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 24.Jones K D, Aoki Y, Chang Y, Moore P S, Yarchoan R, Tosato G. Involvement of interleukin-10 (IL-10) and viral IL-6 in the spontaneous growth of Kaposi's sarcoma herpesvirus-associated infected primary effusion lymphoma cells. Blood. 1999;94:2871–2879. [PubMed] [Google Scholar]
- 25.Landgren E, Schiller P, Cao Y, Claesson-Welsh L. Placenta growth factor stimulates MAP kinase and mitogenicity but not phospholipase C-gamma and migration of endothelial cells expressing Flt 1. Oncogene. 1998;16:359–367. doi: 10.1038/sj.onc.1201545. [DOI] [PubMed] [Google Scholar]
- 26.Martin S J, Reutelingsperger C P, McGahon A J, Rader J A, van Schie R C, LaFace D M, Green D R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 28.Neipel F, Fleckenstein B. The role of HHV-8 in Kaposi's sarcoma. Semin Cancer Biol. 1999;9:151–164. doi: 10.1006/scbi.1999.0129. [DOI] [PubMed] [Google Scholar]
- 29.Nicholas J, Ruvolo V R, Burns W H, Sandford G, Wan X, Ciufo D, Hendrickson S B, Guo H G, Hayward G S, Reitz M S. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 30.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Sakesela O, Orpana A, Pettersson R F, Alitalo K, Eriksson U. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olofsson B, Pajusola K, von Euler G, Chilov D, Alitalo K, Eriksson U. Genomic organization of the mouse and human genes for vascular endothelial growth factor B (VEGF-B) and characterization of a second splice isoform. J Biol Chem. 1996;271:19310–19317. doi: 10.1074/jbc.271.32.19310. [DOI] [PubMed] [Google Scholar]
- 32.Parravicini C, Chandran B, Corbellino M, Berti E, Paulli M, Moore P S, Chang Y. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am J Pathol. 2000;156:743–749. doi: 10.1016/S0002-9440(10)64940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roos R S, Loetscher M, Legler D F, Clark-Lewis I, Baggiolini M, Moser B. Identification of CCR8, the receptor for the human CC chemokine I-309. J Biol Chem. 1997;272:17251–17254. doi: 10.1074/jbc.272.28.17251. [DOI] [PubMed] [Google Scholar]
- 34.Saederup N, Lin Y C, Dairaghi D J, Schall T J, Mocarski E S. Cytomegalovirus-encoded beta chemokine promotes monocyte-associated viremia in the host. Proc Natl Acad Sci USA. 1999;96:10881–10886. doi: 10.1073/pnas.96.19.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sozzani S, Luini W, Bianchi G, Allavena P, Wells T N, Napolitano M, Bernardini G, Vecchi A, D'Ambrosio D, Mazzeo D, Sinigaglia F, Santoni A, Maggi E, Romagnani S, Mantovani A. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood. 1998;92:4036–4039. [PubMed] [Google Scholar]
- 37.Staskus K A, Sun R, Miller G, Racz P, Jaslowski A, Metroka C, Brett-Smith H, Haas A T. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. J Virol. 1999;73:4181–4187. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stine J T, Wood C, Hill M, Epp A, Raport C J, Schweickart V L, Endo Y, Sasaki T, Simmons G, Boshoff C, Clapham P, Chang Y, Moore P, Gray P W, Chantry D. KSHV-encoded CC chemokine vMIP-III is a CCR4 agonist, stimulates angiogenesis, and selectively chemoattracts TH2 cells. Blood. 2000;95:1151–1157. [PubMed] [Google Scholar]
- 39.Tiffany H L, Lautens L L, Gao J L, Pease J, Locati M, Combadiere C, Modi W, Bonner T I, Murphy P M. Identification of CCR8: a human monocyte and thymus receptor for the CC chemokine I-309. J Exp Med. 1997;186:165–170. doi: 10.1084/jem.186.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes J C, Abraham J A. The human gene for vascular endothelial growth factor. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 41.Wan X, Wang H, Nicholas J. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J Virol. 1999;73:8268–8278. doi: 10.1128/jvi.73.10.8268-8278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]