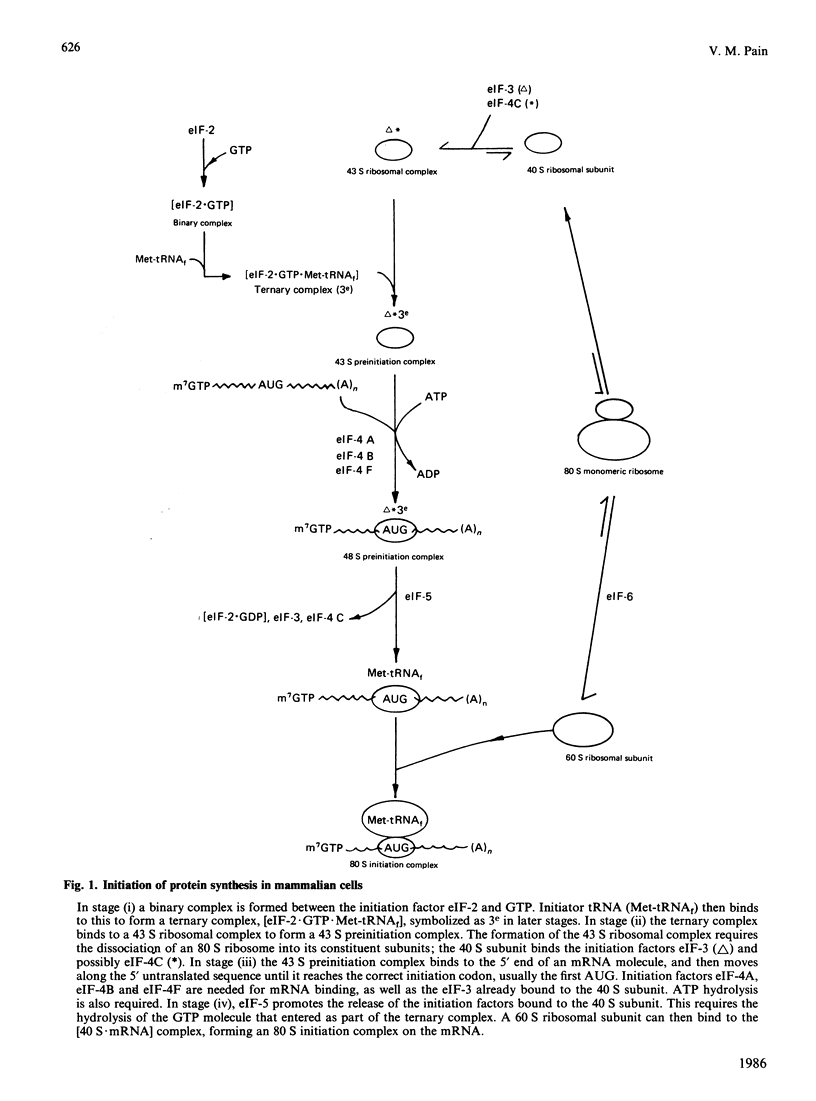
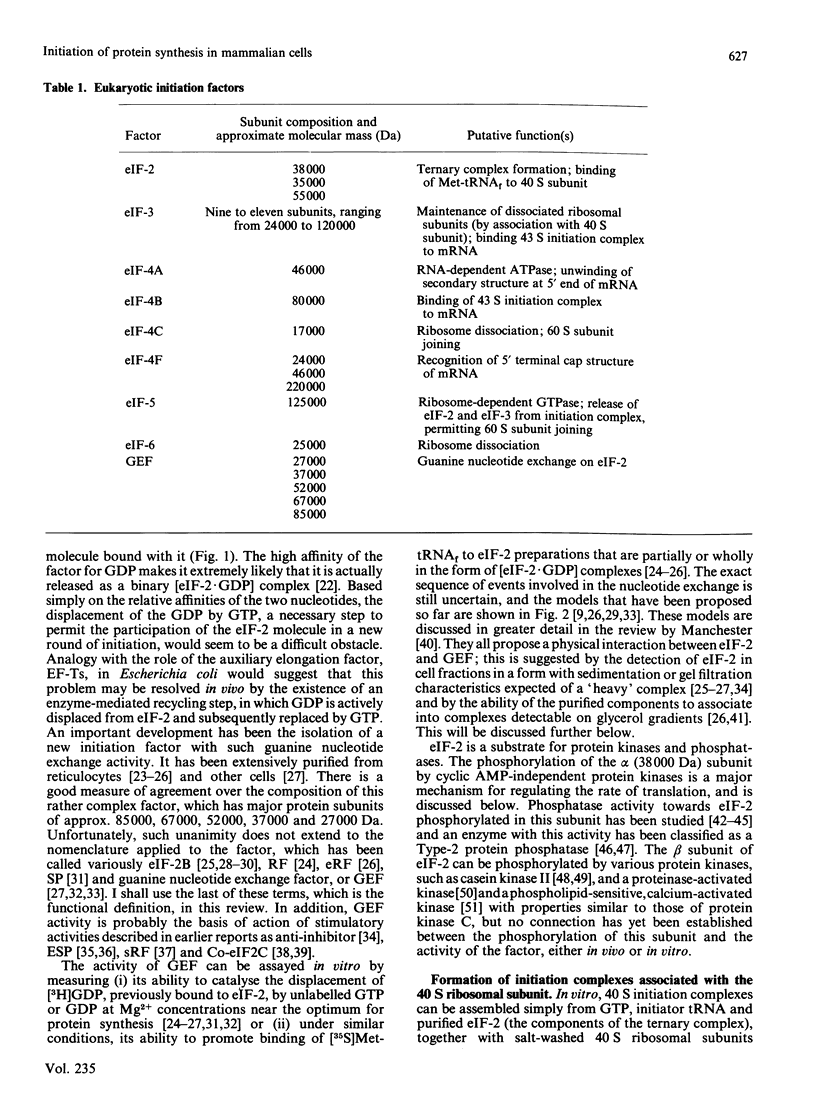
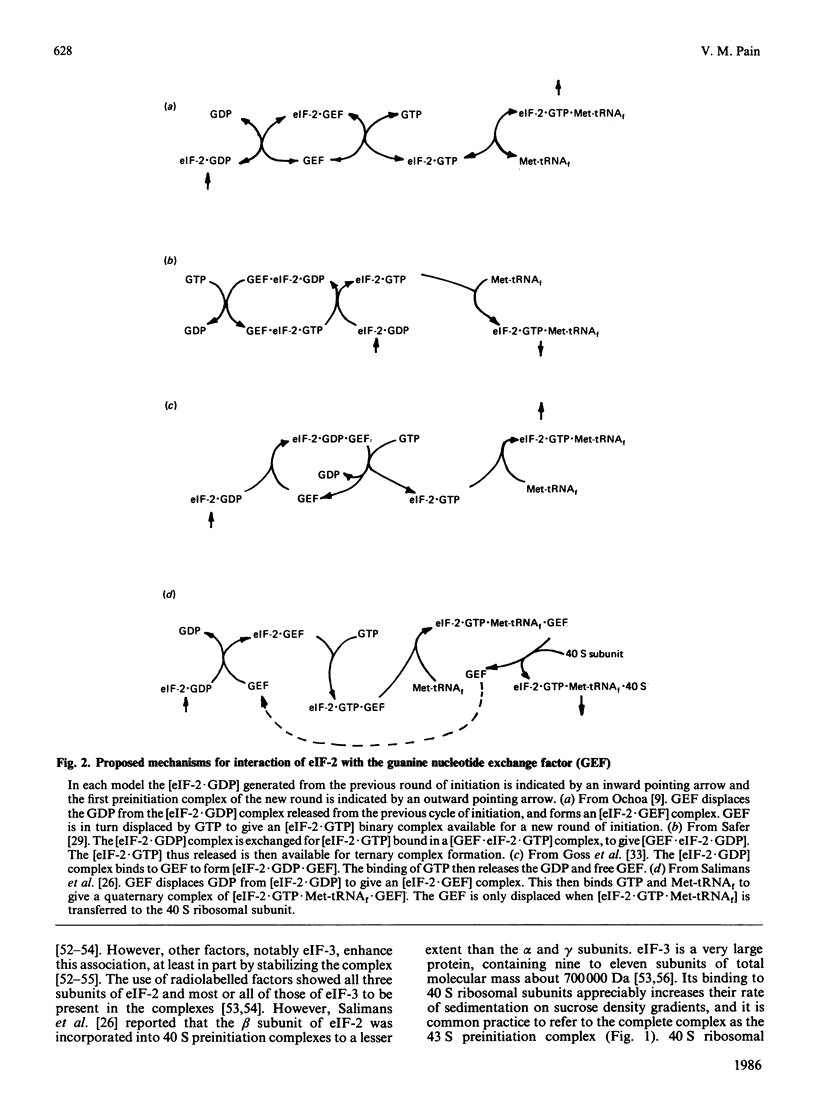
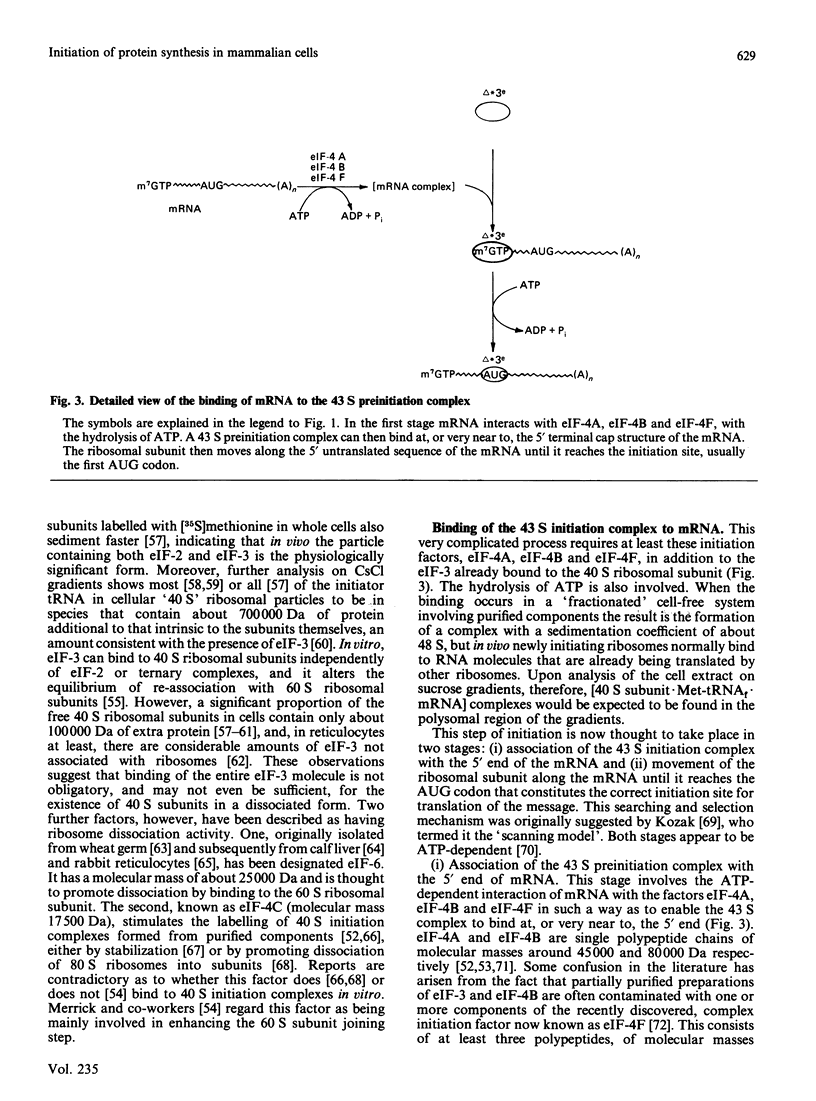
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesz H., Goumans H., Haubrich-Morree T., Voorma H. O., Benne R. Purification and characterization of a protein factor that reverses the inhibition of protein synthesis by the heme-regulated translational inhibitor in rabbit reticulocyte lysates. Eur J Biochem. 1979 Aug 1;98(2):513–520. doi: 10.1111/j.1432-1033.1979.tb13212.x. [DOI] [PubMed] [Google Scholar]
- Austin S. A., Kay J. E. Translational regulation of protein synthesis in eukaryotes. Essays Biochem. 1982;18:79–120. [PubMed] [Google Scholar]
- Barrieux A., Rosenfeld M. G. Characterization of GTP-dependent Met-tRNAf binding protein. J Biol Chem. 1977 Jun 10;252(11):3843–3847. [PubMed] [Google Scholar]
- Benne R., Hershey J. W. Purification and characterization of initiation factor IF-E3 from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3005–3009. doi: 10.1073/pnas.73.9.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Hershey J. W. The mechanism of action of protein synthesis initiation factors from rabbit reticulocytes. J Biol Chem. 1978 May 10;253(9):3078–3087. [PubMed] [Google Scholar]
- Benne R., Salimans M., Goumans H., Amesz H., Voorma H. O. Regulation of protein synthesis in rabbit reticulocyte lysates. Phosphorylation of eIF-2 does not inhibit its capacity to recycle. Eur J Biochem. 1980 Mar;104(2):501–509. doi: 10.1111/j.1432-1033.1980.tb04452.x. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D., Sonenberg N., Baltimore D. Poliovirus mutant that does not selectively inhibit host cell protein synthesis. Mol Cell Biol. 1985 Nov;5(11):2913–2923. doi: 10.1128/mcb.5.11.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M., Lucas-Lenard J. Regulation of protein synthesis in vesicular stomatitis virus-infected mouse L-929 cells by decreased protein synthesis initiation factor 2 activity. J Virol. 1982 Mar;41(3):781–791. doi: 10.1128/jvi.41.3.781-791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Pain V. M., Wong S. T., Henshaw E. C. Phosphorylation inhibits guanine nucleotide exchange on eukaryotic initiation factor 2. Nature. 1982 Mar 4;296(5852):93–95. doi: 10.1038/296093a0. [DOI] [PubMed] [Google Scholar]
- Crouch D., Safer B. Purification and properties of eIF-2 phosphatase. J Biol Chem. 1980 Aug 25;255(16):7918–7924. [PubMed] [Google Scholar]
- Crouch D., Safer B. The association of eIF-2 with Met-tRNAi or eIF-2B alters the specificity of eIF-2 phosphatase. J Biol Chem. 1984 Aug 25;259(16):10363–10368. [PubMed] [Google Scholar]
- Daniels-McQueen S., Detjen B. M., Grifo J. A., Merrick W. C., Thach R. E. Unusual requirements for optimum translation of polio viral RNA in vitro. J Biol Chem. 1983 Jun 10;258(11):7195–7199. [PubMed] [Google Scholar]
- Das A., Bagchi M. K., Ghosh-Dastidar P., Gupta N. K. Protein synthesis in rabbit reticulocytes. A study of peptide chain initiation using native and beta-subunit-depleted eukaryotic initiation factor 2. J Biol Chem. 1982 Feb 10;257(3):1282–1288. [PubMed] [Google Scholar]
- Das A., Bagchi M., Roy R., Ghosh-Dastidar P., Gupta N. K. Protein synthesis in rabbit reticulocytes XXXI: Purification of Co-eIF-2C and studies of its roles in peptide chain initiation. Biochem Biophys Res Commun. 1982 Jan 15;104(1):89–98. doi: 10.1016/0006-291x(82)91944-1. [DOI] [PubMed] [Google Scholar]
- Das A., Ralston R. O., Grace M., Roy R., Ghosh-Dastidar P., Das H. K., Yaghmai B., Palmieri S., Gupta N. K. Protein synthesis in rabbit reticulocytes: mechanism of protein synthesis inhibition by heme-regulated inhibitor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5076–5079. doi: 10.1073/pnas.76.10.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Kinetics of dephosphorylation of eIF-2(alpha P) and reutilization of mRNA. J Biol Chem. 1985 Mar 10;260(5):3135–3139. [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Phosphorylation of initiation factor eIF-2 alpha, binding of mRNA to 48 S complexes, and its reutilization in initiation of protein synthesis. J Biol Chem. 1983 Dec 10;258(23):14556–14562. [PubMed] [Google Scholar]
- DePaoli-Roach A. A., Roach P. J., Pham K., Kramer G., Hardesty B. Phosphorylation of glycogen synthase and of the beta subunit of eukaryotic initiation factor two by a common protein kinase. J Biol Chem. 1981 Sep 10;256(17):8871–8874. [PubMed] [Google Scholar]
- Delaunay J., Ranu R. S., Levin D. H., Ernst V., London I. M. Characterization of a rat liver factor that inhibits initiation of protein synthesis in rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2264–2268. doi: 10.1073/pnas.74.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Etchison D., Hershey J. W. Protein synthesis eukaryotic initiation factors 4A and 4B are not altered by poliovirus infection of HeLa cells. J Biol Chem. 1983 Jun 10;258(11):7236–7239. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Heat shock-induced translational alterations in HeLa cells. Initiation factor modifications and the inhibition of translation. J Biol Chem. 1984 Oct 10;259(19):11882–11889. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Identification and quantitation of levels of protein synthesis initiation factors in crude HeLa cell lysates by two-dimensional polyacrylamide gel electrophoresis. J Biol Chem. 1983 Jun 10;258(11):7228–7235. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Abundance, synthesis, and turnover rates. J Biol Chem. 1985 May 10;260(9):5486–5492. [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Regulation of initiation factors during translational repression caused by serum depletion. Covalent modification. J Biol Chem. 1985 May 10;260(9):5493–5497. [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Edery I., Lee K. A., Sonenberg N. Functional characterization of eukaryotic mRNA cap binding protein complex: effects on translation of capped and naturally uncapped RNAs. Biochemistry. 1984 May 22;23(11):2456–2462. doi: 10.1021/bi00306a021. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E. Poliovirus-induced inhibition of host-cell protein synthesis. Cell. 1982 Mar;28(3):435–436. doi: 10.1016/0092-8674(82)90195-7. [DOI] [PubMed] [Google Scholar]
- Erni B., Staehelin T. Initiation of mammalian protein synthesis: dynamic properties of the assembly process in vitro. Biochim Biophys Acta. 1983 Sep 9;740(4):373–378. doi: 10.1016/0167-4781(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Leroux A., London I. M. Site-specific phosphorylation of the alpha subunit of eukaryotic initiation factor eIF-2 by the heme-regulated and double-stranded RNA-activated eIF-2 alpha kinases from rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1286–1290. doi: 10.1073/pnas.77.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. Evidence that glucose 6-phosphate regulates protein synthesis initiation in reticulocyte lysates. J Biol Chem. 1978 Oct 25;253(20):7163–7172. [PubMed] [Google Scholar]
- Ernst V., Levin D. H., London I. M. In situ phosphorylation of the alpha subunit of eukaryotic initiation factor 2 in reticulocyte lysates inhibited by heme deficiency, double-stranded RNA, oxidized glutathione, or the heme-regulated protein kinase. Proc Natl Acad Sci U S A. 1979 May;76(5):2118–2122. doi: 10.1073/pnas.76.5.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Fout S. Human rhinovirus 14 infection of HeLa cells results in the proteolytic cleavage of the p220 cap-binding complex subunit and inactivates globin mRNA translation in vitro. J Virol. 1985 May;54(2):634–638. doi: 10.1128/jvi.54.2.634-638.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Hansen J., Ehrenfeld E., Edery I., Sonenberg N., Milburn S., Hershey J. W. Demonstration in vitro that eucaryotic initiation factor 3 is active but that a cap-binding protein complex is inactive in poliovirus-infected HeLa cells. J Virol. 1984 Sep;51(3):832–837. doi: 10.1128/jvi.51.3.832-837.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Milburn S. C., Edery I., Sonenberg N., Hershey J. W. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982 Dec 25;257(24):14806–14810. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Hunt T., Jackson R. J. Analysis of phosphorylation of protein synthesis initiation factor eIF-2 by two-dimensional gel electrophoresis. Eur J Biochem. 1978 Sep 1;89(2):517–521. doi: 10.1111/j.1432-1033.1978.tb12556.x. [DOI] [PubMed] [Google Scholar]
- Flaim K. E., Liao W. S., Peavy D. E., Taylor J. M., Jefferson L. S. The role of amino acids in the regulation of protein synthesis in perfused rat liver. II. Effects of amino acid deficiency on peptide chain initiation, polysomal aggregation, and distribution of albumin mRNA. J Biol Chem. 1982 Mar 25;257(6):2939–2946. [PubMed] [Google Scholar]
- Goss D. J., Parkhurst L. J., Mehta H. B., Woodley C. L., Wahba A. J. Studies on the role of eukaryotic nucleotide exchange factor in polypeptide chain initiation. J Biol Chem. 1984 Jun 25;259(12):7374–7377. [PubMed] [Google Scholar]
- Goumans H., Thomas A., Verhoeven A., Voorma H. O., Benne R. The role of eIF-4C in protein synthesis initiation complex formation. Biochim Biophys Acta. 1980 Jun 27;608(1):39–46. doi: 10.1016/0005-2787(80)90131-8. [DOI] [PubMed] [Google Scholar]
- Grankowski N., Lehmusvirta D., Kramer G., Hardesty B. Partial purification and characterization of reticulocyte phosphatase with activity for phosphorylated peptide initiation factor 2. J Biol Chem. 1980 Jan 10;255(1):310–317. [PubMed] [Google Scholar]
- Grifo J. A., Abramson R. D., Satler C. A., Merrick W. C. RNA-stimulated ATPase activity of eukaryotic initiation factors. J Biol Chem. 1984 Jul 10;259(13):8648–8654. [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Leis J. P., Morgan M. A., Shatkin A. J., Merrick W. C. Characterization of eukaryotic initiation factor 4A, a protein involved in ATP-dependent binding of globin mRNA. J Biol Chem. 1982 May 10;257(9):5246–5252. [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Gross M., Rabinovitz M. Partial purification of a translational repressor mediating hemin control of globin synthesis and implication of results on the site of inhibition. Biochem Biophys Res Commun. 1973 Feb 5;50(3):832–838. doi: 10.1016/0006-291x(73)91320-x. [DOI] [PubMed] [Google Scholar]
- Gross M., Redman R., Kaplansky D. A. Evidence that the primary effect of phosphorylation of eukaryotic initiation factor 2(alpha) in rabbit reticulocyte lysate is inhibition of the release of eukaryotic initiation factor-2.GDP from 60 S ribosomal subunits. J Biol Chem. 1985 Aug 5;260(16):9491–9500. [PubMed] [Google Scholar]
- Harmon C. S., Proud C. G., Pain V. M. Effects of starvation, diabetes and acute insulin treatment on the regulation of polypeptide-chain initiation in rat skeletal muscle. Biochem J. 1984 Nov 1;223(3):687–696. doi: 10.1042/bj2230687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E., Brown-Luedi M. L., Hershey J. W. Alterations in initiation factor activity from poliovirus-infected HeLa cells. J Biol Chem. 1979 Nov 10;254(21):10973–10978. [PubMed] [Google Scholar]
- Henshaw E. C., Panniers R. Translational systems prepared from the Ehrlich ascites tumor cell. Methods Enzymol. 1983;101:616–629. doi: 10.1016/0076-6879(83)01042-3. [DOI] [PubMed] [Google Scholar]
- Hirsch C. A., Cox M. A., van Venrooij W. J., Henshaw E. C. The ribosome cycle in mammalian protein synthesis. II. Association of the native smaller ribosomal subunit with protein factors. J Biol Chem. 1973 Jun 25;248(12):4377–4385. [PubMed] [Google Scholar]
- Howe J. G., Hershey J. W. Translational initiation factor and ribosome association with the cytoskeletal framework fraction from HeLa cells. Cell. 1984 May;37(1):85–93. doi: 10.1016/0092-8674(84)90303-9. [DOI] [PubMed] [Google Scholar]
- Hunt T. False starts in translational control of gene expression. Nature. 1985 Aug 15;316(6029):580–581. doi: 10.1038/316580a0. [DOI] [PubMed] [Google Scholar]
- Hunt T., Herbert P., Campbell E. A., Delidakis C., Jackson R. J. The use of affinity chromatography on 2'5' ADP-sepharose reveals a requirement for NADPH, thioredoxin and thioredoxin reductase for the maintenance of high protein synthesis activity in rabbit reticulocyte lysates. Eur J Biochem. 1983 Mar 15;131(2):303–311. doi: 10.1111/j.1432-1033.1983.tb07263.x. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Campbell E. A., Herbert P., Hunt T. The preparation and properties of gel-filtered rabbit-reticulocyte lysate protein-synthesis systems. Eur J Biochem. 1983 Mar 15;131(2):289–301. doi: 10.1111/j.1432-1033.1983.tb07262.x. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Herbert P., Campbell E. A., Hunt T. The roles of sugar phosphates and thiol-reducing systems in the control of reticulocyte protein synthesis. Eur J Biochem. 1983 Mar 15;131(2):313–324. doi: 10.1111/j.1432-1033.1983.tb07264.x. [DOI] [PubMed] [Google Scholar]
- Jen G., Thach R. E. Inhibition of host translation in encephalomyocarditis virus-infected L cells: a novel mechanism. J Virol. 1982 Jul;43(1):250–261. doi: 10.1128/jvi.43.1.250-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat D., Chappell M. R. Competition between globin messenger ribonucleic acids for a discriminating initiation factor. J Biol Chem. 1977 Apr 25;252(8):2684–2690. [PubMed] [Google Scholar]
- Kelly F. J., Jefferson L. S. Control of peptide-chain initiation in rat skeletal muscle. Development of methods for preparation of native ribosomal subunits and analysis of the effect of insulin on formation of 40 S initiation complexes. J Biol Chem. 1985 Jun 10;260(11):6677–6683. [PubMed] [Google Scholar]
- Kimchi A., Zilberstein A., Schmidt A., Shulman L., Revel M. The interferon-induced protein kinase PK-i from mouse L cells. J Biol Chem. 1979 Oct 10;254(19):9846–9853. [PubMed] [Google Scholar]
- Konieczny A., Safer B. Purification of the eukaryotic initiation factor 2-eukaryotic initiation factor 2B complex and characterization of its guanine nucleotide exchange activity during protein synthesis initiation. J Biol Chem. 1983 Mar 10;258(5):3402–3408. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Role of ATP in binding and migration of 40S ribosomal subunits. Cell. 1980 Nov;22(2 Pt 2):459–467. doi: 10.1016/0092-8674(80)90356-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T. V., Bommer U. A., Babkina G. T., Karpova G. G. GTP interacts with the gamma-subunit of eukaryotic initiation factor eIF-2. FEBS Lett. 1984 Oct 1;175(2):313–316. doi: 10.1016/0014-5793(84)80758-9. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Edery I., Sonenberg N. Isolation and structural characterization of cap-binding proteins from poliovirus-infected HeLa cells. J Virol. 1985 May;54(2):515–524. doi: 10.1128/jvi.54.2.515-524.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Sonenberg N. Inactivation of cap-binding proteins accompanies the shut-off of host protein synthesis by poliovirus. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3447–3451. doi: 10.1073/pnas.79.11.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S., Jackson R. J., Hunt T. Control of protein synthesis in reticulocyte lysates by haemin. Nat New Biol. 1973 Jan 31;241(109):150–152. doi: 10.1038/newbio241150a0. [DOI] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Leroux A., London I. M. Regulation of protein synthesis by phosphorylation of eukaryotic initiation factor 2 alpha in intact reticulocytes and reticulocyte lysates. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2147–2151. doi: 10.1073/pnas.79.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. H., Petryshyn R., London I. M. Characterization of double-stranded-RNA-activated kinase that phosphorylates alpha subunit of eukaryotic initiation factor 2 (eIF-2 alpha) in reticulocyte lysates. Proc Natl Acad Sci U S A. 1980 Feb;77(2):832–836. doi: 10.1073/pnas.77.2.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M. A., Osborne J. C., Jr, Safer B., Powell G. M., Merrick W. C. Characteristics of eukaryotic initiation factor 2 and its subunits. J Biol Chem. 1980 Feb 10;255(3):1189–1193. [PubMed] [Google Scholar]
- Maitra U., Stringer E. A., Chaudhuri A. Initiation factors in protein biosynthesis. Annu Rev Biochem. 1982;51:869–900. doi: 10.1146/annurev.bi.51.070182.004253. [DOI] [PubMed] [Google Scholar]
- Manchester K. L. Evaluation and significance of kinetic parameters governing function of protein synthesis initiation factors eIF-2 and eIF-2B. FEBS Lett. 1985 Mar 11;182(1):15–19. doi: 10.1016/0014-5793(85)81144-3. [DOI] [PubMed] [Google Scholar]
- Matts R. L., Levin D. H., London I. M. Effect of phosphorylation of the alpha-subunit of eukaryotic initiation factor 2 on the function of reversing factor in the initiation of protein synthesis. Proc Natl Acad Sci U S A. 1983 May;80(9):2559–2563. doi: 10.1073/pnas.80.9.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matts R. L., London I. M. The regulation of initiation of protein synthesis by phosphorylation of eIF-2(alpha) and the role of reversing factor in the recycling of eIF-2. J Biol Chem. 1984 Jun 10;259(11):6708–6711. [PubMed] [Google Scholar]
- Mengod G., Trachsel H. Eukaryotic protein synthesis initiation factor eIF-3: determination of concentration and association with ribosomes in rabbit reticulocyte and HeLa cell lysates. Biochim Biophys Acta. 1985 Jun 24;825(2):169–174. doi: 10.1016/0167-4781(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Ernst V., Levin D. H., London I. M. Effects of glucose 6-phosphate and hemin on activation of heme-regulated eIF-2 alpha kinase in gel-filtered reticulocyte lysates. J Biol Chem. 1984 Jul 10;259(13):8529–8533. [PubMed] [Google Scholar]
- Mitsui K., Datta A., Ochoa S. Removal of beta subunit of the eukaryotic polypeptide chain initiation factor 2 by limited proteolysis. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4128–4132. doi: 10.1073/pnas.78.7.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- Morley S. J., Jackson R. J. Preparation and properties of an improved cell-free protein synthesis system from mammalian liver. Biochim Biophys Acta. 1985 May 24;825(1):45–56. doi: 10.1016/0167-4781(85)90078-8. [DOI] [PubMed] [Google Scholar]
- Mosenkis J., Daniels-McQueen S., Janovec S., Duncan R., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Shutoff of host translation by encephalomyocarditis virus infection does not involve cleavage of the eucaryotic initiation factor 4F polypeptide that accompanies poliovirus infection. J Virol. 1985 May;54(2):643–645. doi: 10.1128/jvi.54.2.643-645.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa S. Regulation of protein synthesis initiation in eucaryotes. Arch Biochem Biophys. 1983 Jun;223(2):325–349. doi: 10.1016/0003-9861(83)90598-2. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Clemens M. J. Assembly and breakdown of mammalian protein synthesis initiation complexes: regulation by guanine nucleotides and by phosphorylation of initiation factor eIF-2. Biochemistry. 1983 Feb 15;22(4):726–733. doi: 10.1021/bi00273a003. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Henshaw E. C. Initiation of protein synthesis in Ehrlich ascites tumour cells. Evidence for physiological variation in the association of methionyl-tRNAf with native 40-S ribosomal subunits in vivo. Eur J Biochem. 1975 Sep 15;57(2):335–342. doi: 10.1111/j.1432-1033.1975.tb02306.x. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Lewis J. A., Huvos P., Henshaw E. C., Clemens M. J. The effects of amino acid starvation on regulation of polypeptide chain initiation in Ehrlich ascites tumor cells. J Biol Chem. 1980 Feb 25;255(4):1486–1491. [PubMed] [Google Scholar]
- Panniers R., Henshaw E. C. A GDP/GTP exchange factor essential for eukaryotic initiation factor 2 cycling in Ehrlich ascites tumor cells and its regulation by eukaryotic initiation factor 2 phosphorylation. J Biol Chem. 1983 Jul 10;258(13):7928–7934. [PubMed] [Google Scholar]
- Panniers R., Henshaw E. C. Mechanism of inhibition of polypeptide chain initiation in heat-shocked Ehrlich ascites tumour cells. Eur J Biochem. 1984 Apr 2;140(1):209–214. doi: 10.1111/j.1432-1033.1984.tb08088.x. [DOI] [PubMed] [Google Scholar]
- Panniers R., Stewart E. B., Merrick W. C., Henshaw E. C. Mechanism of inhibition of polypeptide chain initiation in heat-shocked Ehrlich cells involves reduction of eukaryotic initiation factor 4F activity. J Biol Chem. 1985 Aug 15;260(17):9648–9653. [PubMed] [Google Scholar]
- Pato M. D., Adelstein R. S., Crouch D., Safer B., Ingebritsen T. S., Cohen P. The protein phosphatases involved in cellular regulation. 4. Classification of two homogeneous myosin light chain phosphatases from smooth muscle as protein phosphatase-2A1 and 2C, and a homogeneous protein phosphatase from reticulocytes active on protein synthesis initiation factor eIF-2 as protein phosphatase-2A2. Eur J Biochem. 1983 May 2;132(2):283–287. doi: 10.1111/j.1432-1033.1983.tb07360.x. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Photochemical cross-linking of cap binding proteins to eucaryotic mRNAs: effect of mRNA 5' secondary structure. Mol Cell Biol. 1985 Nov;5(11):3222–3230. doi: 10.1128/mcb.5.11.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. T., Merrick W. C., Safer B. Binding and release of radiolabeled eukaryotic initiation factors 2 and 3 during 80 S initiation complex formation. J Biol Chem. 1979 Apr 10;254(7):2509–2516. [PubMed] [Google Scholar]
- Peterson D. T., Safer B., Merrick W. C. Role of eukaryotic initiation factor 5 in the formation of 80 S initiation complexes. J Biol Chem. 1979 Aug 25;254(16):7730–7735. [PubMed] [Google Scholar]
- Proud C. G., Clemens M. J., Pain V. M. Regulation of binding of initiator tRNA to eukaryotic initiation factor eIF-2. Effects of the haem-controlled repressor on the kinetics of ternary complex formation. FEBS Lett. 1982 Nov 8;148(2):214–220. doi: 10.1016/0014-5793(82)80810-7. [DOI] [PubMed] [Google Scholar]
- Proud C. G., Pain V. M. Purification and phosphorylation of initiation factor eIF-2 from rabbit skeletal muscle. FEBS Lett. 1982 Jun 21;143(1):55–59. doi: 10.1016/0014-5793(82)80273-1. [DOI] [PubMed] [Google Scholar]
- Ralston R. O., Das A., Dasgupta A., Roy R., Palmieri S., Gupta N. K. Protein synthesis in rabbit reticulocytes: characteristics of a ribosomal factor that reverses inhibition of protein synthesis in heme-deficient lysates. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4858–4862. doi: 10.1073/pnas.75.10.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: preparation of efficient protein synthesis lysates and the purification and characterization of the heme-regulated translational inhibitory protein kinase. Methods Enzymol. 1979;60:459–484. doi: 10.1016/s0076-6879(79)60045-9. [DOI] [PubMed] [Google Scholar]
- Ray B. K., Brendler T. G., Adya S., Daniels-McQueen S., Miller J. K., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Role of mRNA competition in regulating translation: further characterization of mRNA discriminatory initiation factors. Proc Natl Acad Sci U S A. 1983 Feb;80(3):663–667. doi: 10.1073/pnas.80.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. K., Lawson T. G., Kramer J. C., Cladaras M. H., Grifo J. A., Abramson R. D., Merrick W. C., Thach R. E. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J Biol Chem. 1985 Jun 25;260(12):7651–7658. [PubMed] [Google Scholar]
- Raychaudhuri P., Chaudhuri A., Maitra U. Formation and release of eukaryotic initiation factor 2 X GDP complex during eukaryotic ribosomal polypeptide chain initiation complex formation. J Biol Chem. 1985 Feb 25;260(4):2140–2145. [PubMed] [Google Scholar]
- Raychaudhuri P., Stringer E. A., Valenzuela D. M., Maitra U. Ribosomal subunit antiassociation activity in rabbit reticulocyte lysates. Evidence for a low molecular weight ribosomal subunit antiassociation protein factor (Mr = 25,000). J Biol Chem. 1984 Oct 10;259(19):11930–11935. [PubMed] [Google Scholar]
- Reichel P. A., Merrick W. C., Siekierka J., Mathews M. B. Regulation of a protein synthesis initiation factor by adenovirus virus-associated RNA. Nature. 1985 Jan 17;313(5999):196–200. doi: 10.1038/313196a0. [DOI] [PubMed] [Google Scholar]
- Rice A. P., Duncan R., Hershey J. W., Kerr I. M. Double-stranded RNA-dependent protein kinase and 2-5A system are both activated in interferon-treated, encephalomyocarditis virus-infected HeLa cells. J Virol. 1985 Jun;54(3):894–898. doi: 10.1128/jvi.54.3.894-898.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Trachsel H., Leong K., Baltimore D. Inhibition of translation by poliovirus: inactivation of a specific initiation factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2732–2736. doi: 10.1073/pnas.75.6.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Spremulli L. L. Purification and characterization of a ribosome dissociation factor (eukaryotic initiation factor 6) from wheat germ. J Biol Chem. 1979 Sep 25;254(18):8796–8800. [PubMed] [Google Scholar]
- Safer B. 2B or not 2B: regulation of the catalytic utilization of eIF-2. Cell. 1983 May;33(1):7–8. doi: 10.1016/0092-8674(83)90326-4. [DOI] [PubMed] [Google Scholar]
- Safer B., Jagus R. Control of eIF-2 phosphatase activity in rabbit reticulocyte lysate. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1094–1098. doi: 10.1073/pnas.76.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimans M. M., van Heugten H. A., van Steeg H., Voorma H. O. The effect of serum deprivation on the initiation of protein synthesis in mouse neuroblastoma cells. Biochim Biophys Acta. 1985 Jan 29;824(1):16–26. doi: 10.1016/0167-4781(85)90024-7. [DOI] [PubMed] [Google Scholar]
- Salimans M., Goumans H., Amesz H., Benne R., Voorma H. O. Regulation of protein synthesis in eukaryotes. Mode of action of eRF, an eIF-2-recycling factor from rabbit reticulocytes involved in GDP/GTP exchange. Eur J Biochem. 1984 Nov 15;145(1):91–98. doi: 10.1111/j.1432-1033.1984.tb08526.x. [DOI] [PubMed] [Google Scholar]
- Salimans M., Posno M., Benne R., Voorma H. O. Regulation of protein synthesis in eukaryotes. Eukaryotic initiation factor eIF-2 and eukaryotic recycling factor eRF from neuroblastoma cells. Biochim Biophys Acta. 1985 Aug 21;825(4):384–392. doi: 10.1016/0167-4781(85)90065-x. [DOI] [PubMed] [Google Scholar]
- Sameshima M., Izawa M. Properties and synthesis of multiple components in native small ribosomal subunits of mouse ascites tumor cells. Biochim Biophys Acta. 1975 Feb 10;378(3):405–414. doi: 10.1016/0005-2787(75)90185-9. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Duncan R., Knutson G. S., Hershey J. W. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eIF-2 alpha in interferon-treated, reovirus-infected mouse L929 fibroblasts in vitro and in vivo. J Biol Chem. 1984 Nov 10;259(21):13451–13457. [PubMed] [Google Scholar]
- Sarkar G., Edery I., Sonenberg N. Photoaffinity labeling of the cap-binding protein complex with ATP/dATP. Differential labeling of free eukaryotic initiation factor 4A and the eukaryotic initiation factor 4A component of the cap-binding protein complex with [alpha-32P]ATP/dATP. J Biol Chem. 1985 Nov 5;260(25):13831–13837. [PubMed] [Google Scholar]
- Schatzman R. C., Grifo J. A., Merrick W. C., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase phosphorylates the beta subunit of eukaryotic initiation factor 2 (eIF-2). FEBS Lett. 1983 Aug 8;159(1-2):167–170. doi: 10.1016/0014-5793(83)80439-6. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Safer B., Munemitsu S. M., Samuel C. E., Shenk T. Adenovirus VAI RNA prevents phosphorylation of the eukaryotic initiation factor 2 alpha subunit subsequent to infection. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4321–4325. doi: 10.1073/pnas.82.13.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Siekierka J., Manne V., Mauser L., Ochoa S. Polypeptide chain initiation in eukaryotes: reversibility of the ternary complex-forming reaction. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1232–1235. doi: 10.1073/pnas.80.5.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Manne V., Ochoa S. Mechanism of translational control by partial phosphorylation of the alpha subunit of eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1984 Jan;81(2):352–356. doi: 10.1073/pnas.81.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mariano T. M., Reichel P. A., Mathews M. B. Translational control by adenovirus: lack of virus-associated RNAI during adenovirus infection results in phosphorylation of initiation factor eIF-2 and inhibition of protein synthesis. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1959–1963. doi: 10.1073/pnas.82.7.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mauser L., Ochoa S. Mechanism of polypeptide chain initiation in eukaryotes and its control by phosphorylation of the alpha subunit of initiation factor 2. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2537–2540. doi: 10.1073/pnas.79.8.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J., Mitsui K. I., Ochoa S. Mode of action of the heme-controlled translational inhibitor: relationship of eukaryotic initiation factor 2-stimulating protein to translation restoring factor. Proc Natl Acad Sci U S A. 1981 Jan;78(1):220–223. doi: 10.1073/pnas.78.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. E., Henshaw E. C. Binding of Met-tRNAf to native 40 S ribosomal subunits in Ehrlich ascites tumor cells. J Biol Chem. 1975 Sep 10;250(17):6880–6884. [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer E. A., Chaudhuri A., Maitra U. Purified eukaryotic initiation factor 2 from calf liver consists of two polypeptide chains of 48,000 and 38,000 daltons. J Biol Chem. 1979 Aug 10;254(15):6845–6848. [PubMed] [Google Scholar]
- Stringer E. A., Chaudhuri A., Valenzuela D., Maitra U. Rabbit reticulocyte initiation factor 2 contains two polypeptide chains of molecular weights 48,000 and 38,000. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3356–3359. doi: 10.1073/pnas.77.6.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson C., Akusjärvi G. Adenovirus VA RNAI mediates a translational stimulation which is not restricted to the viral mRNAs. EMBO J. 1985 Apr;4(4):957–964. doi: 10.1002/j.1460-2075.1985.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Tahara S. M., Traugh J. A. Differential activation of two protease-activated protein kinases from reticulocytes by a Ca2+-stimulated protease and identification of phosphorylated translational components. Eur J Biochem. 1982 Aug;126(2):395–399. doi: 10.1111/j.1432-1033.1982.tb06793.x. [DOI] [PubMed] [Google Scholar]
- Thomas A., Goumans H., Voorma H. O., Benne R. The mechanism of action of eukaryotic initiation factor 4C in protein synthesis. Eur J Biochem. 1980;107(1):39–45. doi: 10.1111/j.1432-1033.1980.tb04621.x. [DOI] [PubMed] [Google Scholar]
- Thomas N. S., Matts R. L., Levin D. H., London I. M. The 60 S ribosomal subunit as a carrier of eukaryotic initiation factor 2 and the site of reversing factor activity during protein synthesis. J Biol Chem. 1985 Aug 15;260(17):9860–9866. [PubMed] [Google Scholar]
- Thomas N. S., Matts R. L., Petryshyn R., London I. M. Distribution of reversing factor in reticulocyte lysates during active protein synthesis and on inhibition by heme deprivation or double-stranded RNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6998–7002. doi: 10.1073/pnas.81.22.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J Mol Biol. 1977 Nov;116(4):755–767. doi: 10.1016/0022-2836(77)90269-8. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Ranu R. S., London I. M. Purification of the heme-reversible form of the translational inhibitory protein kinase. Methods Enzymol. 1979;60:485–495. doi: 10.1016/s0076-6879(79)60046-0. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Staehelin T. Initiation of mammalian protein synthesis. The multiple functions of the initiation factor eIF-3. Biochim Biophys Acta. 1979 Dec 17;565(2):305–314. doi: 10.1016/0005-2787(79)90207-7. [DOI] [PubMed] [Google Scholar]
- Tuazon P. T., Merrick W. C., Traugh J. A. Site-specific phosphorylation if initiation factor 2 by three cyclic nucleotide-independent protein kinases. J Biol Chem. 1980 Nov 25;255(22):10954–10958. [PubMed] [Google Scholar]
- Valenzuela D. M., Chaudhuri A., Maitra U. Eukaryotic ribosomal subunit anti-association activity of calf liver is contained in a single polypeptide chain protein of Mr = 25,500 (eukaryotic initiation factor 6). J Biol Chem. 1982 Jul 10;257(13):7712–7719. [PubMed] [Google Scholar]
- Voorma H. O., Goumans H., Amesz H., Benne R. The control of the rate of initiation of eukaryotic protein synthesis. Curr Top Cell Regul. 1983;22:51–70. doi: 10.1016/b978-0-12-152822-5.50006-3. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Gill G. N. Nucleotide regulation of a eukaryotic protein synthesis initiation complex;. Biochim Biophys Acta. 1975 May 1;390(2):231–245. doi: 10.1016/0005-2787(75)90344-5. [DOI] [PubMed] [Google Scholar]
- Wong S. T., Mastropaolo W., Henshaw E. C. Differential phosphorylation of soluble versus ribosome-bound eukaryotic initiation factor 2 in the Ehrlich ascites tumor cell. J Biol Chem. 1982 May 10;257(9):5231–5238. [PubMed] [Google Scholar]
- Zardeneta G., Kramer G., Hardesty B. Structure and function of peptide initiation factor 2: differential loss of activities during proteolysis and generation of a terminal fragment containing the phosphorylation sites of the alpha subunit. Proc Natl Acad Sci U S A. 1982 May;79(10):3158–3161. doi: 10.1073/pnas.79.10.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Ochoa S. Further studies on the mode of action of the heme-controlled translational inhibitor: stimulating protein acts at level of binary complex formation. Proc Natl Acad Sci U S A. 1979 May;76(5):2163–2164. doi: 10.1073/pnas.76.5.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., Janssen A. P., Hoeymakers J. H., de Man B. M. On the heterogeneity of native ribosomal subunits in Ehrlich-ascites-tumor cells cultured in vitro. Eur J Biochem. 1976 May 1;64(2):429–435. doi: 10.1111/j.1432-1033.1976.tb10319.x. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Sillekens P. T., van Eekelen C. A., Reinders R. J. On the association of mRNA with the cytoskeleton in uninfected and adenovirus-infected human KB cells. Exp Cell Res. 1981 Sep;135(1):79–91. doi: 10.1016/0014-4827(81)90301-3. [DOI] [PubMed] [Google Scholar]


