Abstract
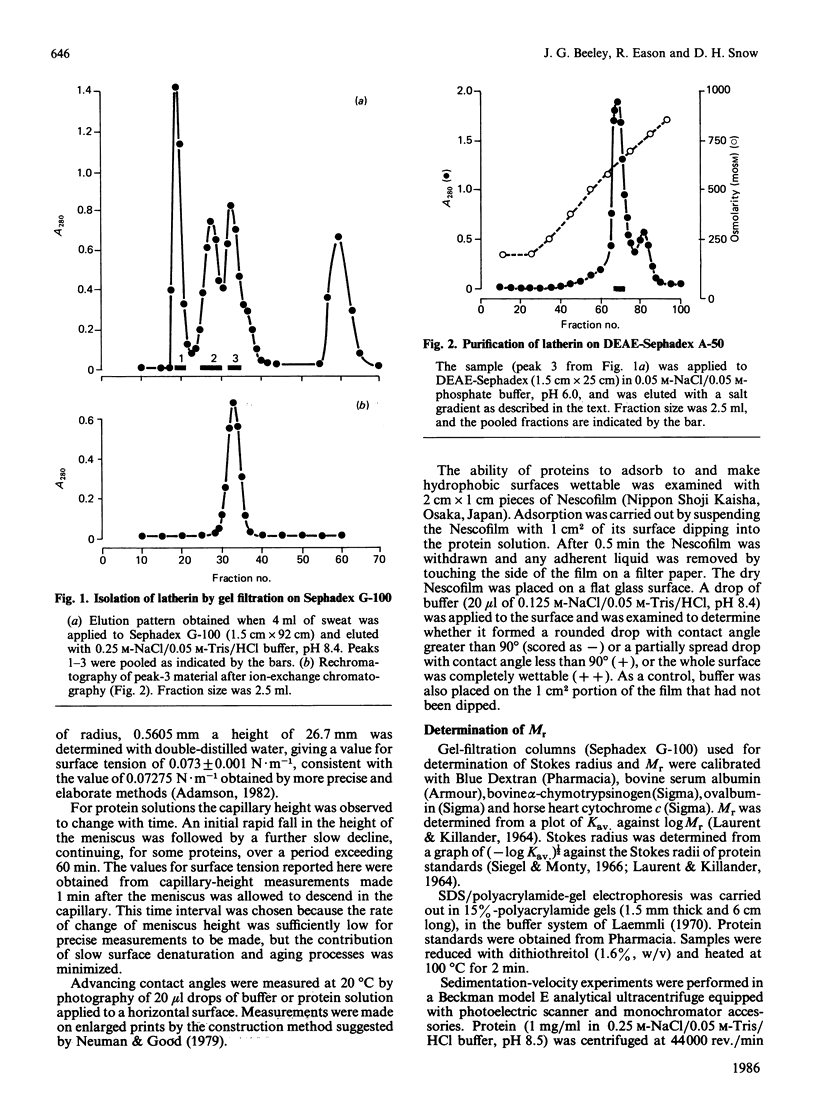
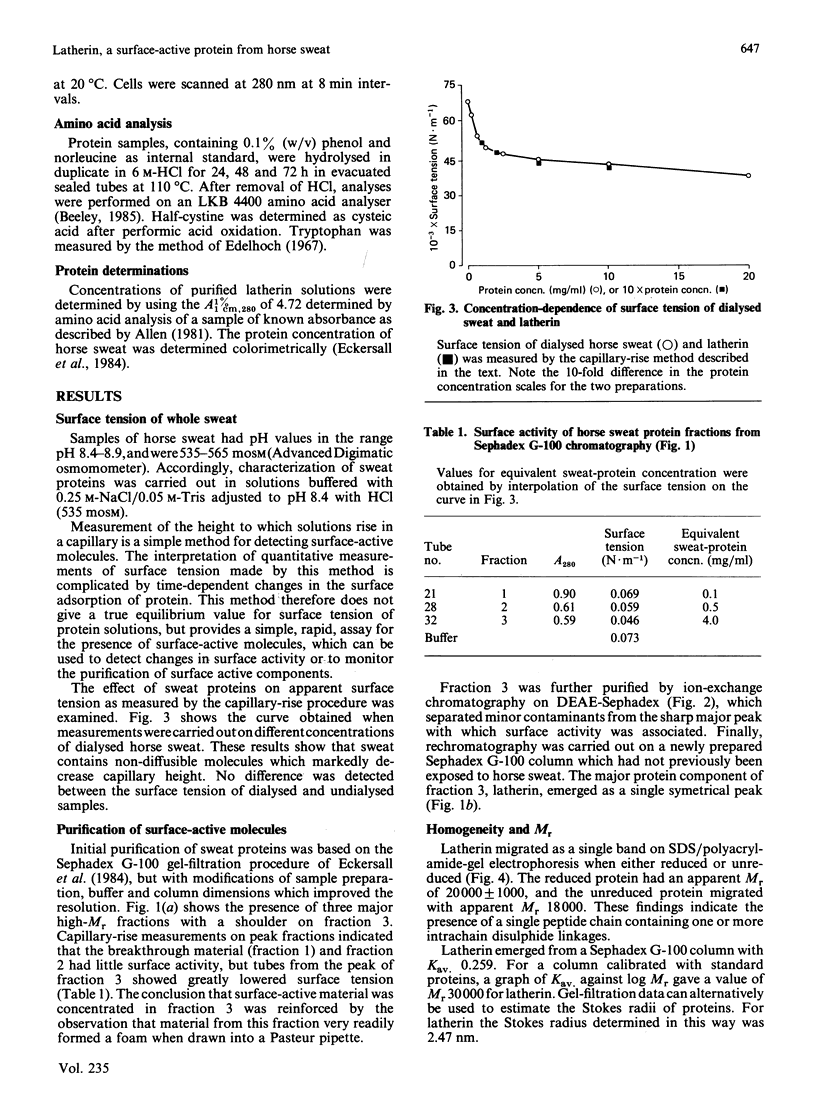
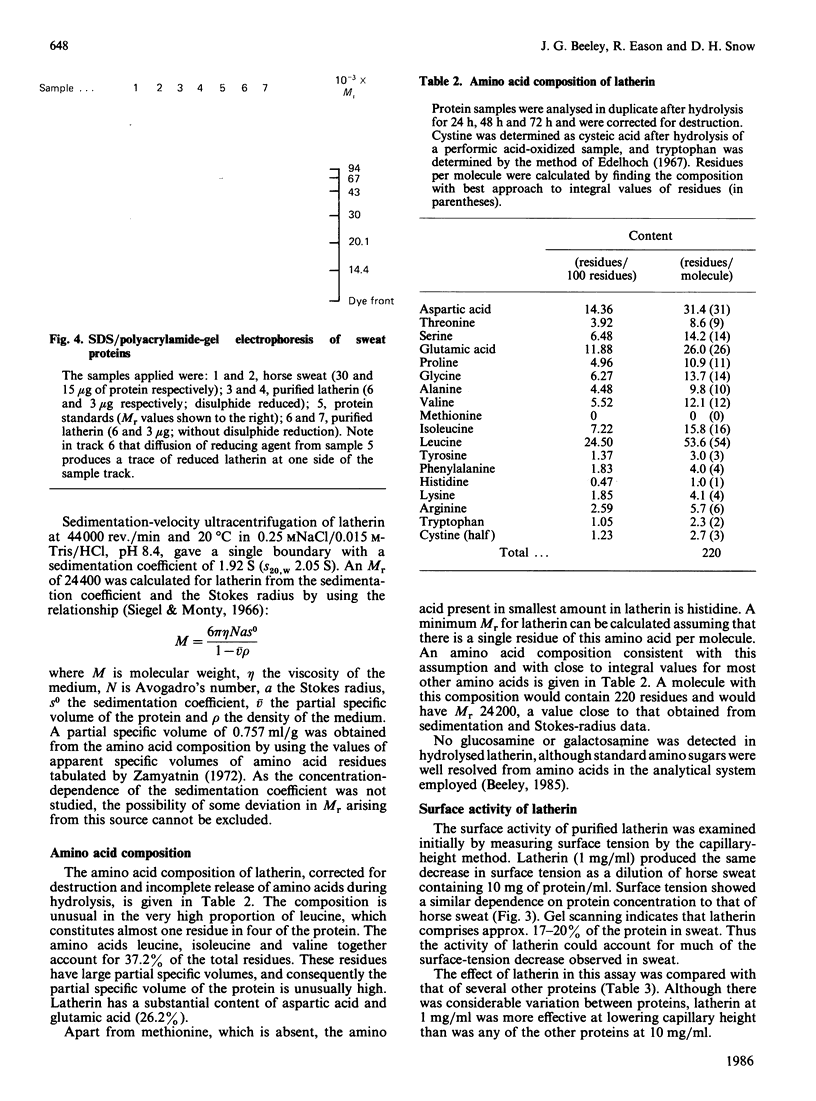
A protein, latherin, with unusual surface activity was isolated from horse sweat by gel filtration and ion-exchange chromatography. The protein has a Stokes radius, determined by gel filtration, of 2.47 nm, and in the ultracentrifuge sediments as a single species with S20,W 2.05 S, indicating an Mr of 24,400. On SDS/polyacrylamide-gel electrophoresis the molecule behaves as a single peptide chain of apparent Mr 20,000. Latherin contains a high proportion of hydrophobic amino acids (37.2%), and the leucine content (24.5%) is exceptionally high. The unusual composition of the protein may account for apparent anomalies in the Mr of latherin determined by empirical methods. Evidence indicating that latherin is responsible for much of the surface activity of horse sweat was obtained by a simple assay for surface tension and by contact-angle measurements. Latherin adsorbs very readily at hydrophobic surfaces, rendering them wettable. A possible role for latherin in thermoregulation is proposed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHEESMAN D. F., DAVIES J. T. Physicochemical and biological aspects of proteins at interfaces. Adv Protein Chem. 1954;9:439–501. doi: 10.1016/s0065-3233(08)60211-9. [DOI] [PubMed] [Google Scholar]
- CREETH J. M. The use of the Gouy diffusiometer with dilute protein solutions; an assessment of the accuracy of the method. Biochem J. 1952 Apr;51(1):10–17. doi: 10.1042/bj0510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersall P. D., Beeley J. G., Snow D. H., Thomas A. Characterisation of glycoproteins in the sweat of the horse (Equus caballus). Res Vet Sci. 1984 Mar;36(2):231–234. [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. M., Mabon R. M., Manson W. Sweat proteins. Br J Dermatol. 1974 Feb;90(2):175–181. doi: 10.1111/j.1365-2133.1974.tb06382.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macritchie F. Proteins at interfaces. Adv Protein Chem. 1978;32:283–326. doi: 10.1016/s0065-3233(08)60577-x. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Zamyatnin A. A. Protein volume in solution. Prog Biophys Mol Biol. 1972;24:107–123. doi: 10.1016/0079-6107(72)90005-3. [DOI] [PubMed] [Google Scholar]



