Abstract
Cervical cancer remains a leading cause of cancer-related mortality in women in low and middle-income countries despite efforts to improve prevention and standard-of-care interventions. Sub-Saharan Africa (SSA) leads the numbers for global cervical cancer incidence and mortality, with the majority of the incidence diagnosed in the late stage of the malignancy. Although the global cervical cancer death rate has been on the decline for the last two decades owing to advancements in screening and treatment options, the mortality rate in SSA has not declined very much. Chemotherapy has been the treatment of choice for cervical cancer in SSA without meeting the expected survival outcomes in these patients, with the majority having advanced diseases at diagnosis. Immune checkpoint inhibitors have recently shown clinical promise in improving the survival of patients with advanced cervical cancer and have been integrated into the treatment guidelines in most high-income countries, which have helped further reduce the mortality rate of cervical cancer. However, many SSA countries are yet to fully benefit from using immune checkpoint inhibitors in cervical cancer. In this review, we discuss the challenges hindering the effective use of immune checkpoint inhibitors for advanced cervical cancer in Africa and possible solutions.
Keywords: cancer mortality, cervical cancer, immune checkpoint inhibitors, sub-saharan africa, tumor mutational burden
Introduction and background
Cervical cancer remains a major health problem worldwide [1]. It is the fourth most common cancer in women and was estimated to have an incidence of over 1.9 million and over 600,000 deaths globally in 2023 [2]. Although largely preventable through screening programs and vaccination, incidence, and mortality are disproportionately distributed globally. The highest rates are in low- and middle-income countries in sub-Saharan Africa (SSA), Southeast Asia, and Central America. Indeed, these regions accounted for most of the 341,831 deaths in 2020 [3], primarily as a result of differences in access to cervical cancer screening services, human papillomavirus (HPV) vaccination, and social and economic determinants [4].
According to a systematic review, in 28 African countries, only 14% of women between the ages of 30 and 49 had ever had a cervical cancer screening as of 2020, with significant regional and country-level differences [5]. In a different survey involving 23 African nations, the majority of healthcare professionals acknowledged lacking sufficient training and awareness on the HPV vaccine, while only 37.4% of them said they had access to it [6].
HPV, precisely types 16 and 18, is responsible for the majority of precancerous cervical lesions and cervical cancers, accounting for over 70% of all invasive cervical cancer cases [7]. The World Health Organization established a global plan in 2020 to eradicate cervical cancer as a threat to public health by 2030 [3]. This strategy included the 90-70-90 targets, which stipulate that 90% of girls must be vaccinated against HPV by the age of 15, 70% of women must have a high-performance test screening by the age of 35, and again by the age of 45, 90% of pre-cancerous lesions must be treated, and cases of invasive cancer must be managed [7,8]. Currently, there are about six HPV vaccines available worldwide by 2023 [9,10]. Recent data have shown that HPV vaccination significantly reduced cervical cancer cases [11,12]. This underscores the need for more vaccination campaigns with broader and far-reaching coverage.
Low-cure systemic medicines are the cornerstone of treatment for distant metastatic cancer, whereas total hysterectomy combined pelvic lymphadenectomy plus concurrent chemotherapy and radiation therapy is the primary treatment strategy for early-stage and locally invasive disease [13]. Due to the need for more sophisticated treatments with higher cure rates, immunotherapy (currently the most promising anticancer strategy) was assessed for use in cervical cancer. As cancer immunotherapy research advances and gains traction, immune checkpoint inhibitors (ICIs) have emerged as a significant new treatment option for patients with recurring and metastatic cervical malignancies. In many wealthy nations, cervical cancer treatment guidelines now include the use of ICIs [14,15].
The importance of immune checkpoints in SSA cannot be overstated, as up to 80% of cervical cancers in SSA are diagnosed in the late stages of the disease, especially considering the clinical success of ICIs in patients with advanced disease [16-18]. Also, as Africa strives to increase cervical screening per WHO targets, it is expected that more cases of invasive cervical cancer will be detected, mainly in the population that had not previously undergone screening. As a result, cancer management techniques must be implemented and expanded, and any challenges must be identified and resolved. This review aims to identify and discuss the challenges of SSA pertaining to utilizing the immune checkpoint inhibitor option in the management of cervical cancer.
Review
Epidemiology of cervical cancer in SSA
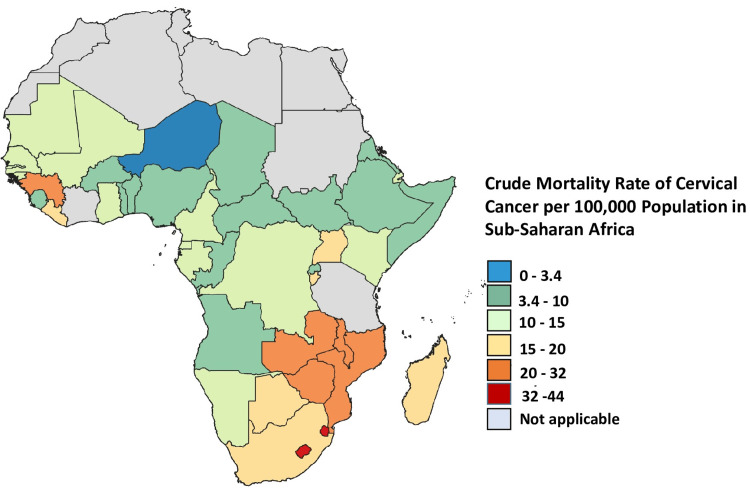
According to recent African cancer data, SSA has the highest prevalence of cervical cancer [19], with greater incidence and fatality rates in eastern Africa (with Malawi having the highest burden), southern Africa, and middle Africa [20]. In contrast to the 27 to 30 cases per 100,000 women in the central and western regions and the 40 to 43 cases per 100,000 women in the eastern and southern African regions, North Africa recorded the fewest cases in 2018. There were approximately seven cases per 100,000 women in North Africa. The majority of North Africa's low incidence rate of cervical cancer is due to advancements in screening technology [19]. Furthermore, in northern Africa, sexual and reproductive health behaviors may be influenced by sociocultural and religious norms, which could reduce the incidence of cervical cancer. Over the past four years, the HPV vaccination campaigns in a few regions of eastern, central, and western Africa have marginally reduced the incidence rate [11]. The cervical cancer mortality rate is higher in southern Africa, as shown in Figure 1. Also, mortality rates are declining only in southern Africa. Over the past four years, the mortality rates in every other African region have increased, except for northern Africa, where the incidence rate is low but the fatality rate is high [19]. In 2022, comparable patterns were observed, with the highest incidence and fatality rates found in eastern Africa [11,20,21]. Furthermore, the highest age-standardized rates (ASR) of incidence and mortality are found in SSA nations [22].
Figure 1. Cervical cancer mortality rate in sub-Saharan Africa.
The crude mortality rate among women of all ages who were diagnosed with cervical cancer in sub-Saharan African countries in 2022. This is an original work of the authors graphed from data collected by the International Cancer Control Partnership (ICCP) [23].
Compared to high-income nations, where the five-year survival is above 50%, women with cervical cancer in SSA countries had a five-year survival rate of only 33% [24-26]. According to a study comprising eight SSA countries (the Gambia, Kenya, Malawi, Seychelles, South Africa, Uganda, and Zimbabwe), there has been an alarming rise in the prevalence of cervical cancer in seven of the eight countries. Nonetheless, throughout 25 years, eight eastern and southern African countries, including Malawi, South Africa, and Kenya, saw rising incidence trends, according to an African study [27].
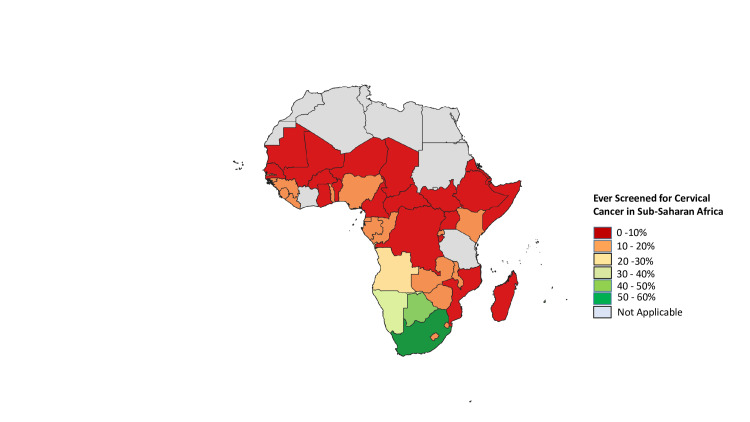
In SSA, the coverage rate for cervical cancer screening was 10% [28]. Because primary (HPV vaccine) and secondary (screening) prevention measures are so successful, cervical cancer is considered nearly avoidable. These laws haven't, however, been enacted consistently between or within nations. National HPV vaccination programs were present in less than 30% of low- and middle-income countries (LMICs) but in more than 80% of high-income countries [29]. With the lowest prevalence among women in SSA [30], just 44% of women in LMICs had ever had a cervical screening, compared to 60% or more in high-income nations [20]. As Figure 2 shows, screening coverage in SSA is still low despite efforts by governmental agencies to screen more women.
Figure 2. Cervical cancer cancer screening rate in sub-Saharan Africa.
This shows the percentage of women screened for cervical cancer at least once in their lifetime in sub-Saharan Africa as of 2019. This figure represents an original work of the authors graphed from data collected by the International Cancer Control Partnership (ICCP) [23].
Cervical screening has been unsuccessful in these areas due to governmental, sociocultural, and financial barriers [31,32]. Some of the systemic challenges that this region faces, which are reflective of their socioeconomic status, include the high cost of Pap smear tests, limited access to service providers, and lengthy wait periods [33]. Cancer patients in SSA are frequently diagnosed after the disease has advanced. Inadequate funding for cancer prevention and early detection is also a contributing factor [34].
If preventative actions like HPV vaccination and screening are not increased, trends in these groups will probably continue to worsen over the coming decades. The global population's aging and growth will also increase the absolute number of cases [35]. Ensuring the implementation of resource-dependent screening and vaccination programs is crucial in these places to improve the situation [36].
Concept of immune checkpoint inhibition
The inhibition of immune checkpoints is an essential aspect of immunotherapy. Immune checkpoints exist to prevent excessive T cell responses on antigen activation, thereby avoiding autoimmunity and supporting peripheral tolerance [37]. An efficient T cell activation requires additional signals (a costimulatory signal and cytokine action) in addition to the first signal provided by the interaction between the T cell receptor (TCR) and an antigen-bounded major histocompatibility complex (MHC) [37,38]. However, when a T cell gets activated, there are also coinhibitory signals delivered by surface proteins to help checkmate the extent of response and maintain immune homeostasis [39]. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4) are two well-studied T cell receptors that negatively regulate T cell activation through interactions with their ligands while the antigen-presenting cells (APCs) express the required ligands for interaction with these inhibitory molecules. CD80 and CD86, present on APCs, can bind to the inhibitory CTLA-4 [40]. Similarly, the PD-1 ligands, PD-L1 and PD-L2, are on APC membranes [41]. Most tumor microenvironments (TME) have high expression of immune checkpoint receptors and their ligands. Precisely, tumors can mimic APCs by expressing and upregulating the ligands to T cell coinhibitory receptors [42]. The implication is that the cytotoxic effect of CD8+ T cells on the tumors is abolished. The characteristic upregulation of ligands by tumors exists in various malignancies, including cervical carcinoma, where PD-L1 was reportedly expressed in up to 50% of the cases [42,43]. Like cancer cells, pathogens can harness immune checkpoints to reduce the extent of the host’s antigen-specific immune responses against them [44]. Evans et al. reported that HPV-positive cervical cancers displayed more significant levels of many immune checkpoint proteins compared to cervical cancers that were HPV-negative [45]. Immune checkpoint blockade therapies mainly consist of monoclonal antibodies used to hamper the coinhibitory molecules or their ligands and improve the function of antitumor T-lymphocytes [38]. A good number of clinical trials exploring the use of immune checkpoint inhibitors against cervical cancer are currently ongoing, with some also involving a combination therapy approach, as seen for other carcinomas [46]. An inhibitor of PD-1, pembrolizumab, already has FDA approval for the treatment of advanced, metastasized, or recurrent cervical cancer [47]. We now look briefly at the signaling mechanisms associated with some immune checkpoint (IC) receptors, focusing on the PD-1/PD-L1 axis and CTLA-4.
PD-1
PD-L1 and PD-L2 expressed on either tumor cells or APCs interact with PD-1 on T cells and initiate the resulting signaling. In some cases, the interaction can occur on the same cell in a cis-fashion, or tumors could generate the ligands as exosomes [48]. Phosphorylation of PD-1 on an ITIM/ITSM motif present in its cytoplasmic domain recruits a protein tyrosine phosphatase SHP2 that can inhibit both TCR signaling and the downstream events following T cell activation [41,48]. A contrasting idea also exists with data showing that the dephosphorylation of the costimulatory CD28 is more preferred to the inhibition of the TCR by the PD-1-recruited SHP2 [49]. A disruption in Ca2+ flux in T cells as the levels of PD-1 expression increased is also reported [50]. This model of PD-1 inhibitory effect of T cells showed that higher numbers of engaged TCRs were needed to start a Ca2+ flux as PD-1 signaling increased. The PD-1-mediated pathways reduce T-cell expansion, cause less cytotoxicity against tumors, and decrease cytokine response [51]. Targeting PD-L1 is another option when an immune checkpoint inhibitor (ICI) is not designed to antagonize PD-1. PD-L1 can internalize itself in cycles and then recycle to the cell surface or degrade in lysosomes. Pathways or molecules preventing PD-L1 ubiquitination and degradation can stabilize its expression in carcinomas, making the ligand available to suppress T cell activation through engagement with PD-1. Palmitoylation of PD-L1 or the expression of COP9 signalosome 5 in cancer cells has been reviewed as some of these pathways [48,52].
CTLA-4
CTLA-4 inhibitory action on T cells is multi-dimensional. Following T-cell activation, CTLA-4 translocates from its intracellular position to the cell surface, leading to engagement with the costimulatory B7 ligands (CD80 and CD86) on an APC [40]. CD28, the costimulatory molecule on T cells for CD80 and CD86, is outcompeted by CTLA-4 based on affinity with outcomes of attenuated effects of PI3K and AKT, which are mediators of CD28 signaling [53]. Furthermore, CTLA-4 can affect TCR-induced RAS activation when CTLA-4’s YVTM motif is phosphorylated to recruit SHP2 as the mediator [48,54]. There are cell extrinsic modulations of T cell activity by CTLA-4. One way is through regulatory T cells (Tregs), which also express CTLA-4. CTLA-4 on Tregs may reduce available B7 ligands for CD28-induced stimulation of proximal effector T cells. In addition, CTLA-4 expression can promote transendocytosis of B7 ligands from APCs to prevent interaction with CD28 [53].
Clinical experience using ICIs in cervical cancer
The mechanism of PD-1-mediated T-cell suppression is the most well-researched when using ICI to treat cervical cancer. As seen in Table 1, ICIs have been studied in numerous clinical trials as a single drug or in combination for cervical cancer. It is expected that the clinical use of ICIs in cervical malignancies will increase in the coming years.
Table 1. Immune checkpoint inhibitors used in the clinical trials of cervical cancer.
AE: adverse event, DCR: disease control rate, DOR: duration of response, DFS: disease-free survival, DLT: dose-limiting toxicity, ICI: immune checkpoint inhibitor, MTD: maximum tolerated dose, NCT: National Clinical Trial, ORR: overall response rate, CRR: cumulative response rate, OS: overall survival, PFS: progression-free survival, R2PD: recommended phase 2 dose, CD8: cluster of differentiation 8, FOXP3: forkhead box P3, VB10.16: vaccine for human papillomavirus type 16.
| NCT number | Drug name | Phase | Primary outcome measure | Status | References |
| NCT05588219 | Tislelizumab | 2 | Tumor regression | Recruiting | [55] |
| NCT05824468 | Zimberelimab | 2 | MTD, ORR, RP2D | Not yet recruiting | [56] |
| NCT05824494 | Cadonilimab | 2 | ORR | Not yet recruiting | [57] |
| NCT03144466 | Pembrolizumab with radiotherapy | 1 | MTD, efficacy | Terminated | [58] |
| NCT04256213 | Nivolumab + ipilimumab | Pilot study (N/A) | CD8+/FOXP3+ relative change of lymphocytes | Active (not recruiting) | [59] |
| NCT03755739 | Pembrolizumab | 2/3 | OS, CRR | Recruiting | [60] |
| NCT03841110 | Nivolumab + pembrolizumab | 1 | MTD | Completed | [61] |
| NCT04652076 | Pembrolizumab+ chemotherapy | 1/2 | DLT occurrence, ORR | Recruiting | [62] |
| NCT06140589 | Cadolinimab | Not applicable | ORR, PFS, DFS, OS | Not yet recruiting | [63] |
| NCT05310383 | Tislelizumab | 2 | ORR | Unknown | [14] |
| NCT06232083 | Cardunizumab | 1/2 | PFS | Recruiting | [64] |
| NCT05187338 | Pembrolizumab + durvalumab + ipilimumab | 1/2 | DCR, PFS, DOR | Recruiting | [65] |
| NCT03556839 | Atezolizumab | 3 | PFS, OS | Active, not recruiting | [66,67] |
| NCT05799469 | Envafolimab+ chemoradiotherapy | 2 | PFS | Not yet recruiting | [57] |
| NCT05492123 | Nivolumab+ iplimumab | 2 | 3-year PFS | Recruiting | [68] |
| NCT04157985 | PD-1/PD-L1 inhibitors | 3 | PFS, time to next treatment | Recruiting | [69] |
| NCT03612791 | Atezolizumab + radiotherapy | 2 | PFS | Active, not recruiting | [70] |
| NCT04405349 | Atezolizumab + VB10.16 vaccine | 2 | AE, ORR | Completed | [71] |
| NCT04800978 | Durvalumab + BVAC-C vaccine | 2 | DLTs, PFS | Not yet recruiting | [72] |
| NCT03228667 | ICIs + immunotherapy | 2 | ORR | Active, not yet recruiting | [73] |
| NCT03527264 | Nivolumab + radiation | 2 | PFS | Terminated | [74] |
| NCT04300647 | Tiragolumab + ICI | 2 | ORR | Active, not yet recruiting | [75] |
| NCT03444376 | Pembrolizumab + vaccination | 1/2 | DLT, ORR | Completed | [76] |
| NCT03786081 | Tisotumab vedotin | 1/2 | DLT, ORR | Active, not yet recruiting | [77] |
| NCT04802876 | Spartalizumab + tislelizumab | 2 | ORR | Recruiting | [78] |
| NCT02628067 (KEYNOTE 158) | Pembrolizumab | 2 | ORR | Recruiting | [79] |
| NCT03635567 (KEYNOTE 826) | Pembrolizumab+ chemotherapy | 3 | PFS, safety, efficacy | Active, not yet recruiting | [80] |
| NCT02488759 (CHECKMATE 358) | Nivolumab monotherapy | 1/2 | ORR | N/A | [81] |
As seen in Table 1, ICIs have been studied in numerous clinical trials as a single drug or in combination for cervical cancer. It is expected that the clinical use of ICIs in cervical malignancies will increase in the coming years.
Clinical trials such as KEYNOTE-158, KEYNOTE-826, and CHECKMATE-358 explored the use of anti-PD1 antibodies like pembrolizumab and nivolumab in cervical cancers that were either advanced, PD-L1 positive, or previously treated with chemotherapy [79-81]. The FDA approved pembrolizumab in treating metastatic or recurrent cervical cancer as a result of these trials [47,82]. The National Comprehensive Cancer Network (NCCN) also suggests nivolumab as a component of second-line therapy for cervical cancer [83]. Antibodies targeting PD-L1 have also been developed and are being tested. Durvalumab and atezolizumab are two such antibodies [46]. The desire to prevent extreme adverse effects, improve patient response, and promote overall survival implies that new molecules, even with the same targets, are continuously being developed for cervical cancer treatment. Ipilimumab was developed as an antagonist to CTLA-4, and the FDA has approved it for treating metastatic melanoma [84]. In the CHECKMATE-358 trial, ipilimumab was used in a combination therapy approach against cervical cancer alongside nivolumab with outcomes of durable clinical activity [85]. Balstilimab plus zalifrelimab are an anti-PD-1/anti-CTLA-4 combination, and both inhibitors yielded longer response duration and manageable safety profiles compared to their use as a monotherapy [86]. Evidence for the expression of PD-L1 in cases of cervical carcinoma in SSA and the high mortality from the disease in the same region supports the case for the incorporation of ICI use in the treatment regimens for cervical cancer in the continent [43,46].
Challenges hindering the usage of immune checkpoint inhibitors in SSA
The Lancet Oncology Commission for Sub-Saharan Africa has projected that by 2040, the number of cancer-related deaths in SSA will have doubled, from about 550,000 to over 1 million annually [87,88]. This calls for strategic approaches aimed at reducing the increasing cancer mortality. ICIs have improved the treatment landscape of advanced tumors. A combination of the ICI, pembrolizumab with chemotherapy improved the progression-free survival (PFS) in patients with advanced cervical cancer compared to those taking chemotherapy with placebo (10.4 months vs. 8.2 months) as seen in the KEYNOTE trial [89,90]. FDA approval of pembrolizumab for advanced cervical cancer underlies the key role immune checkpoint inhibitors can play in reducing the high mortality rate associated with gynecological cancers. The following issues need to be addressed to effectively use ICIs in treating cervical cancer in SSA.
Tumor Mutational Burden (TMB) Genomic Sequencing
Tumor mutational burden represents the number of somatic mutations observed per megabase in a tumor DNA [91-93]. In recent times, TMB has become an essential biomarker predicting the sensitivity of immune checkpoint inhibitors as cancer treatments. Tumors with high mutations (TMB-H) have higher chances of recognition by the immune system. This is because neoantigens found in high mutational tumors are more recognizable by the immune cells. High mutational burden correlates positively with high microsatellite instability (MSI-H) [92]. TMB-H and MSI-H are tumor phenotypes showing evidence as predictive biomarkers of ICI sensitivity [94]. The FDA in 2020 approved ICI drug pembrolizumab for any solid tumor with tumor mutational burden-high [≥10 mutations/megabase (mut/Mb)] [95]. This further shows the importance of robust TMB testing in predicting patients' suitability as candidates for ICI therapy. Tumor analysis from ovarian cancer patients showed that TMB-H was associated with better survival and better immune cell tumor infiltration [96]. TMB-H cervical cell carcinoma development is potentially adversely impacted by the activities of anti-tumor immune cells [97]. Patients with MSI-H tumors in endometrial cancer have shown significant therapeutic benefits from dostarlimab in combination with carboplatin-paclitaxel, according to the results of a clinical trial [98]. This, therefore, supports the idea that patients with gynecological tumors showing TMB-H or MSI-H will benefit from ICI therapy.
It has been established in non-small cell lung cancer (NSCLC) that patients of African ancestry show high TMB, suggesting likely benefits for ICI therapy [99]. Numerous efforts have been geared toward elucidating the TMB outlook in many gynecological tumors, including cervical cancer. Genomic testing in SSA will help establish adequate literature necessary to understand different mutations involved in driving several gynecological tumors and help determine patients who are candidates for immunotherapies. Over 30 genes have been investigated worldwide for their potential to influence the risk of cervical cancer [100,101]. Despite the high death rate from cervical cancer in SSA, less than 30% of these genes have been examined in this group. The epidemiological roles of several genes in cervical cancer have been investigated [102]. These genes include CCR2, Casp8, p53, FASL, HLA, IL10, TGF-beta, and TNF-alpha. Only 12% of cancer immunotherapy centers use genomic sequencing to measure tumor mutational burden as a predictive biomarker of immunotherapy success, according to a recent study carried out in 28 SSA nations [103]. Lack of infrastructure and poor logistics are part of the issues making genomic sequencing difficult and uninteresting to medical practitioners in SSA [104].
Bridging the gap in tumor genomic sequencing in African cervical cancer patients is very important if the current high mortality rate observed in this category of patients is to be reduced. Studies of the tumor mutational burden of SSA cervical cancer patients and comparing it to those of other races can help improve our understanding of predicted ICI sensitivity in SSA cervical patients since clinical trial data of ICI use in cervical cancer in other locations are available. One primary solution to the issues of poor genomic sequencing in SSA is boosting public-private partnership (PPP) in genomic sequencing initiatives. For example, less than 10 SSA countries have been able to conduct robust cervical cancer epidemiological studies [102,105]. Many efficient genomic centers in SSA are championed by for-profit or non-profit private organizations [104]. While these institutions have made tremendous progress in changing the genomic landscape of tumor genomic sequencing in SSA, much work is still needed as Africa contributes to less than 2% of the human genome database [106]. Also, genomic sequencing costs are not yet affordable for most cervical patients in SSA. With PPP, the governments of SSA countries can make commitments that reduce the cost individual cervical cancer patients bear when ordering a genomic study, as they also have to bear the cost of treatment for their cancer [107].
PD-L1 Testing in SSA
Since the last decade, PD-L1 has become a vital biomarker predicting the effectiveness of ICIs in treating cancers [90]. PD-L1 overexpression in cervical cancer has been established with suggestions it is linked to high expressions of cancer stem cell markers [108]. High levels of PD-L1 expression in many malignancies have been linked to a bad prognosis [109], but they have also proven helpful in assisting physicians in determining whether patients with cancer are likely to benefit from ICI therapy, which has grown to be a significant treatment option for advanced cancer patients. For patients whose tumors have a PD-L1 Combined Positive Score (CPS) of ≥1, the FDA approved pembrolizumab, an anti-PD-1 antibody, in 2021 in conjunction with chemotherapy to treat metastatic cervical cancer [110]. To understand the genomic and molecular landscape of cancer, many studies have sought to evaluate racial variations in the expression of PD-L1 in different tumors, which have yielded mixed results so far [109,111]. More studies are needed to fully understand how PD-L1 is influenced by race for each cancer type.
Despite the role of Africa in human evolution and the rich genetic diversity of the zone, Africa remains behind in genetic databases and studies [112]. A study found that less than 50% of the participating clinics in SSA conduct PD-L1 testing before immunotherapy is used in cancer treatment [103]. A global survey of pathologists doing immunohistochemistry testing of PD-L1 found that Africa had a longer turnaround time (TNT), representing the time from testing to when the result becomes available. The survey also found that reporting the results is less standardized in Africa compared to the other continents that participated [113]. Longer TNT is one such problem that can affect the confidence of healthcare providers managing cervical cancer patients in ordering PD-L1 testing, as quick decision-making might be necessary when dealing with an advanced form of the disease. Embracing the benefits of next-generation sequencing (NGS) is one way SSA can improve the current less impressive genomic and diagnostic testing situation. PPPs remain the most viable option in increasing the number of diagnostic centers in SSA capable of performing PD-L1 testing. This will lead to shorter TNT.
Lack of Updated Treatment Guidelines in SSA
A 2019 symposium organized by the Cervical Cancer Research Network (CCRN) assessed the current management of cervical cancer in eleven SSA countries and found that platinum- and taxol-based chemotherapy are the most used therapy for metastatic or recurrent cervical cancer while radiation therapy or chemoradiation was majorly used in palliative care for cases where the cancer was deemed incurable in the participating SSA countries [114]. These strategies are not in line with the latest management used in many high-income countries for the management of advanced disease that has metastasized to multiple sites. In the United States, it is recommended that for those who have recurrent disease or have progressed on chemotherapy, a combination strategy involving bevacizumab should be used [115]. The suggested combinations include cisplatin and paclitaxel with bevacizumab [115]. However, the recent success of the KEYNOTE trial inspired FDA approval of the anti-PD-1 ICI, pembrolizumab to be combined with platinum-based chemotherapy with or without bevacizumab for metastatic cancer with good expression of PDL1 [90]. In the United Kingdom and the European Union, pembrolizumab has been approved and recommended for use, similar to the FDA approval for cervical cancer [116]. Pembrolizumab should be added to chemotherapy with or without bevacizumab in patients with cervical cancer that is chronic, recurring, or metastatic, according to the most recent practice guideline published by the Korean Society of Gynecologic Oncology [117]. To reduce the increasing mortality of cervical cancer in SSA, there is a need to develop efficient clinical guidelines that adopt the latest clinical evidence in the management of advanced cervical cancer. In the event that developing country-specific guidelines proves difficult, regional-based guidelines can be mapped out based on collaboration among multiple SSA countries, which can help speed up the process.
High Cost of ICIs
ICI therapy has advanced the field of clinical oncology, and many positive patient outcomes have been reported. However, the cost of ICIs remains high and out of reach for many patients from LMICs. The average cost of one dose of pembrolizumab is reported to be $8762 [118]. Many late-stage cervical cancer patients would typically require more than one dose. The cost of a single dose of pembrolizumab is more than the yearly minimum wage reported in most SSA countries, making it very challenging for many cervical cancer patients to afford such therapy out of pocket. Yet over 80% of the population of most SSA countries get access to medicine by paying out-of-pocket [119]. In contrast, only about 13% of the population under 65 in the USA is without health insurance [120,121]. Health insurance coverage is an effective way to reduce the financial burden of a costly medical need. Although many SSA countries have introduced universal health insurance (UHI) programs with the hope of bringing cheap and affordable health care to many poor people who lack access to essential health, these schemes have yet to meet the expected need [122]. Challenges like poor funding, administrative bottlenecks, and inadequate primary care facilities are among the major stumbling blocks towards achieving an effective public health coverage scheme that meets the health needs of low-income people who struggle to afford good health care [123,124]. To ensure access to ICIs for cervical cancer patients, the governments of SSA countries should invest in subsidizing the therapy cost so patients can afford it. Information sharing and proper patient referral systems should be built to ensure that patients with advanced cervical cancer are referred to hospitals with adequate facilities to manage their conditions properly.
Conclusions
Since the clinical benefits of immune checkpoint inhibitors (ICIs) in improving patient outcomes in advanced cervical cancer were established, many countries, especially in high-income areas of the world, have outlined guidelines for the effective use of ICI therapy in cervical cancer patients with PD-L1 expression. In recent times, sub-Saharan Africa (SSA) has experienced an increased incidence of cervical cancer with an increasing mortality rate. To benefit from the clinical success of ICIs in cervical cancer, SSA countries need to address the challenges hindering the use of ICIs. To effectively address these issues, public-private partnerships will play a vital role in achieving that. The responsibility of establishing efficient genomic and biomarker testing facilities should not be left in the hands of private firms, as it has recently been in Africa. The governments of the countries of SSA need to provide an enabling environment with facilities aimed at improving logistics and other social amenities needed for biomedical research to thrive. The governments can also coordinate partnerships with countries that have successfully used immunotherapies in cervical cancer to help adopt a framework that can be implemented in Africa. Without an updated guideline that gives authority to ICIs as a beneficial therapy in advanced cervical cancer, getting all health institutions in SSA countries in line with what is obtainable in higher economy countries will be challenging. The health ministries of the various countries of SSA, through their affiliated medical associations and societies, should be tasked with reviewing the current guidelines and ensuring that the current practice is similar to what is currently done in countries where there has been a decrease in cervical cancer mortality in recent years. Finally, more research and clinical trials involving ICIs are encouraged in the SSA countries to help shine a light on how cervical cancer patients with advanced disease respond to ICI therapy compared to the currently-used chemotherapy. This will help decide the best and most efficient way to use these therapies for the patient’s benefit.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Munachiso F. Njoku, Izuchukwu F. Okpalanwaka, Francis I. Anazodo, Zimuzor L. Chike-Aliozor, Kossy M. Ochie, Chika Ekweozor, Onyeka F. Oboh, Faustina C. Okonkwo
Drafting of the manuscript: Munachiso F. Njoku, Izuchukwu F. Okpalanwaka, Francis I. Anazodo, Zimuzor L. Chike-Aliozor, Kossy M. Ochie, Chika Ekweozor, Onyeka F. Oboh, Faustina C. Okonkwo
Critical review of the manuscript for important intellectual content: Munachiso F. Njoku, Izuchukwu F. Okpalanwaka, Francis I. Anazodo, Onyeka F. Oboh, Faustina C. Okonkwo
Supervision: Munachiso F. Njoku
Acquisition, analysis, or interpretation of data: Izuchukwu F. Okpalanwaka
References
- 1.Cancer cervix: epidemiology and disease burden. Pimple S, Mishra G. Cytojournal. 2022;19:21. doi: 10.25259/CMAS_03_02_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer statistics, 2023. Siegel RL, Miller KD, Wagle NS, Jemal A. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Singh D, Vignat J, Lorenzoni V, et al. Lancet Glob Health. 2023;11:0–206. doi: 10.1016/S2214-109X(22)00501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global health equity: cancer care outcome disparities in high-, middle-, and low-income countries. de Souza JA, Hunt B, Asirwa FC, Adebamowo C, Lopes G. J Clin Oncol. 2016;34:6–13. doi: 10.1200/JCO.2015.62.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regional and country-level trends in cervical cancer screening coverage in sub-Saharan Africa: a systematic analysis of population-based surveys (2000-2020) Yang L, Boily MC, Rönn MM, et al. PLoS Med. 2023;20:0. doi: 10.1371/journal.pmed.1004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HPV vaccination in Africa in the COVID-19 era: a cross-sectional survey of healthcare providers' knowledge, training, and recommendation practices. Fokom Domgue J, Dille I, Kapambwe S, et al. Front Public Health. 2024;12:1343064. doi: 10.3389/fpubh.2024.1343064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prevalence of human papillomavirus subtypes 16 and 18 among Yemeni patients with cervical cancer. Ahmed HG, Bensumaidea SH, Alshammari FD, Alenazi FS, ALmutlaq BA, Alturkstani MZ, Aladani IA. Asian Pac J Cancer Prev. 2017;18:1543–1548. doi: 10.22034/APJCP.2017.18.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Canfell K, Kim JJ, Brisson M, et al. Lancet. 2020;395:591–603. doi: 10.1016/S0140-6736(20)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervical cancer: 90-70-90 and palliative care. Cleary JF. JCO Glob Oncol. 2021;7:1426–1428. doi: 10.1200/GO.21.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recent developments in human papillomavirus (HPV) vaccinology. Williamson AL. Viruses. 2023;15 doi: 10.3390/v15071440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prevention of cervical cancer in sub-Saharan Africa: the advantages and challenges of HPV vaccination. Black E, Richmond R. Vaccines (Basel) 2018;6 doi: 10.3390/vaccines6030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barriers and facilitators of HPV vaccination in sub-saharan Africa: a systematic review. Kutz JM, Rausche P, Gheit T, Puradiredja DI, Fusco D. BMC Public Health. 2023;23:974. doi: 10.1186/s12889-023-15842-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cervical cancer immunotherapy: facts and hopes. Ferrall L, Lin KY, Roden RB, Hung CF, Wu TC. Clin Cancer Res. 2021;27:4953–4973. doi: 10.1158/1078-0432.CCR-20-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Immune checkpoint inhibitors in cervical cancer: current status and research progress. Xie Y, Kong W, Zhao X, Zhang H, Luo D, Chen S. Front Oncol. 2022;12:984896. doi: 10.3389/fonc.2022.984896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Advances in immunotherapy for cervical cancer: recent developments and future directions. Sherer MV, Kotha NV, Williamson C, Mayadev J. Int J Gynecol Cancer. 2022;32:281–287. doi: 10.1136/ijgc-2021-002492. [DOI] [PubMed] [Google Scholar]
- 16.Prevalence and determinants of late-stage presentation among cervical cancer patients, a systematic review and meta-analysis. Tekalign T, Teshome M. PLoS One. 2022;17:0. doi: 10.1371/journal.pone.0267571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Population risk factors for late-stage presentation of cervical cancer in sub-Saharan Africa. Stewart TS, Moodley J, Walter FM. Cancer Epidemiol. 2018;53:81–92. doi: 10.1016/j.canep.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Factors associated with late diagnosis of cervical cancer at two national referral hospitals, Kenya 2017: a case control study. Mwenda V, Mwangi M, Gathecha G, Kibachio J, Too R, Gura Z, Temmerman M. Gynecol Oncol Rep. 2024;52:101355. doi: 10.1016/j.gore.2024.101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer in Africa: the untold story. Hamdi Y, Abdeljaoued-Tej I, Zatchi AA, Abdelhak S, Boubaker S, Brown JS, Benkahla A. Front Oncol. 2021;11:650117. doi: 10.3389/fonc.2021.650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 21.Cancer statistics for the year 2020: an overview. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Int J Cancer. 2021 doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 22.Global, regional and national burden, incidence, and mortality of cervical cancer. Momenimovahed Z, Mazidimoradi A, Maroofi P, Allahqoli L, Salehiniya H, Alkatout I. Cancer Rep (Hoboken) 2023;6:0. doi: 10.1002/cnr2.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Cancer Control Partnership: resources. [ May; 2024 ]. 2021. https://www.iccp-portal.org/resources-search?f%5B0%5D=search_api_aggregation_5%3A735&f%5B1%5D=type%3Acountry_profile https://www.iccp-portal.org/resources-search?f%5B0%5D=search_api_aggregation_5%3A735&f%5B1%5D=type%3Acountry_profile
- 24.Cervical cancer survival in sub-Saharan Africa by age, stage at diagnosis and Human Development Index: a population-based registry study. Sengayi-Muchengeti M, Joko-Fru WY, Miranda-Filho A, et al. Int J Cancer. 2020;147:3037–3048. doi: 10.1002/ijc.33120. [DOI] [PubMed] [Google Scholar]
- 25.The path to eliminating cervical cancer in Canada: past, present and future directions. Caird H, Simkin J, Smith L, Van Niekerk D, Ogilvie G. Curr Oncol. 2022;29:1117–1122. doi: 10.3390/curroncol29020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evaluation and prediction analysis of 3- and 5-year relative survival rates of patients with cervical cancer: a model-based period analysis. Fan X, He W, Zhang Q, Zhang B, Dong L, Li L, Liu X. Cancer Control. 2024;31:10732748241232324. doi: 10.1177/10732748241232324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trends in cervical cancer incidence in sub-Saharan Africa. Jedy-Agba E, Joko WY, Liu B, et al. Br J Cancer. 2020;123:148–154. doi: 10.1038/s41416-020-0831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cervical cancer screening uptake in sub-Saharan Africa: a systematic review and meta-analysis. Yimer NB, Mohammed MA, Solomon K, et al. Public Health. 2021;195:105–111. doi: 10.1016/j.puhe.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Global perspectives of determinants influencing HPV vaccine introduction and scale-up in low- and middle-income countries. Guillaume D, Waheed DE, Schleiff M, Muralidharan KK, Vorsters A, Limaye RJ. PLoS One. 2024;19:0. doi: 10.1371/journal.pone.0291990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lifetime prevalence of cervical cancer screening in 55 low- and middle-income countries. Lemp JM, De Neve JW, Bussmann H, et al. JAMA. 2020;324:1532–1542. doi: 10.1001/jama.2020.16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Determinants of sexual activity and its relation to cervical cancer risk among South African women. Cooper D, Hoffman M, Carrara H, et al. BMC Public Health. 2007;7:341. doi: 10.1186/1471-2458-7-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervical cancer: the sub-Saharan African perspective. Anorlu RI. Reprod Health Matters. 2008;16:41–49. doi: 10.1016/S0968-8080(08)32415-X. [DOI] [PubMed] [Google Scholar]
- 33.Integrated review of barriers to cervical cancer screening in sub-Saharan Africa. McFarland DM, Gueldner SM, Mogobe KD. J Nurs Scholarsh. 2016;48:490–498. doi: 10.1111/jnu.12232. [DOI] [PubMed] [Google Scholar]
- 34.Cancer in Africa 2018: the role of infections. Parkin DM, Hämmerl L, Ferlay J, Kantelhardt EJ. Int J Cancer. 2020;146:2089–2103. doi: 10.1002/ijc.32538. [DOI] [PubMed] [Google Scholar]
- 35.Worldwide burden of cervical cancer in 2008. Arbyn M, Castellsagué X, de Sanjosé S, Bruni L, Saraiya M, Bray F, Ferlay J. Ann Oncol. 2011;22:2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 36.Cervical cancer in Africa, Latin America and the Caribbean and Asia: regional inequalities and changing trends. Vaccarella S, Laversanne M, Ferlay J, Bray F. Int J Cancer. 2017;141:1997–2001. doi: 10.1002/ijc.30901. [DOI] [PubMed] [Google Scholar]
- 37.Immune checkpoint inhibitors in cancer therapy. Webb ES, Liu P, Baleeiro R, Lemoine NR, Yuan M, Wang YH. J Biomed Res. 2018;32:317–326. doi: 10.7555/JBR.31.20160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Hargadon KM, Johnson CE, Williams CJ. Int Immunopharmacol. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Current progress and future perspectives of immune checkpoint in cancer and infectious diseases. Cai X, Zhan H, Ye Y, Yang J, Zhang M, Li J, Zhuang Y. Front Genet. 2021;12:785153. doi: 10.3389/fgene.2021.785153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.CTLA-4: a moving target in immunotherapy. Rowshanravan B, Halliday N, Sansom DM. Blood. 2018;131:58–67. doi: 10.1182/blood-2017-06-741033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revisiting the PD-1 pathway. Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Sci Adv. 2020;6 doi: 10.1126/sciadv.abd2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Immune checkpoints in the tumor microenvironment. Toor SM, Sasidharan Nair V, Decock J, Elkord E. Semin Cancer Biol. 2020;65:1–12. doi: 10.1016/j.semcancer.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Programme death ligand 1 expressions as a surrogate for determining immunotherapy in cervical carcinoma patients. Omenai SA, Ajani MA, Okolo CA. PLoS One. 2022;17:0. doi: 10.1371/journal.pone.0263615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Immune checkpoints and their inhibition in cancer and infectious diseases. Dyck L, Mills KH. Eur J Immunol. 2017;47:765–779. doi: 10.1002/eji.201646875. [DOI] [PubMed] [Google Scholar]
- 45.HPV-positive and -negative cervical cancers are immunologically distinct. Evans AM, Salnikov M, Gameiro SF, Maleki Vareki S, Mymryk JS. J Clin Med. 2022;11 doi: 10.3390/jcm11164825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Role of immune checkpoint inhibitors in cervical cancer: from preclinical to clinical data. Duranti S, Pietragalla A, Daniele G, Nero C, Ciccarone F, Scambia G, Lorusso D. Cancers (Basel) 2021;13 doi: 10.3390/cancers13092089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emerging therapeutic strategies of different immunotherapy approaches combined with PD-1/PD-L1 blockade in cervical cancer. Ge Y, Zhang Y, Zhao KN, Zhu H. Drug Des Devel Ther. 2022;16:3055–3070. doi: 10.2147/DDDT.S374672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Immune checkpoint signaling and cancer immunotherapy. He X, Xu C. Cell Res. 2020;30:660–669. doi: 10.1038/s41422-020-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Hui E, Cheung J, Zhu J, et al. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strength of PD-1 signaling differentially affects T-cell effector functions. Wei F, Zhong S, Ma Z, et al. Proc Natl Acad Sci U S A. 2013;110:0–9. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.PD-1 and its ligands are important immune checkpoints in cancer. Dong Y, Sun Q, Zhang X. Oncotarget. 2017;8:2171–2186. doi: 10.18632/oncotarget.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deubiquitination and stabilization of PD-L1 by CSN5. Lim SO, Li CW, Xia W, et al. Cancer Cell. 2016;30:925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fundamental mechanisms of immune checkpoint blockade therapy. Wei SC, Duffy CR, Allison JP. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 54.Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Hwang JR, Byeon Y, Kim D, Park SG. Exp Mol Med. 2020;52:750–761. doi: 10.1038/s12276-020-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tislelizumab combined with concurrent chemoradiotherapy for locally advanced cervical cancer. Cancer: a Prospective, Single-arm, Single-center, Phase II Clinical Study. [ Jul; 2024 ]. 2022. https://clinicaltrials.gov/study/NCT05588219 https://clinicaltrials.gov/study/NCT05588219
- 56.The effectiveness of cancer immune checkpoint inhibitor retreatment and rechallenge-a systematic review. Perdyan A, Sobocki BK, Balihodzic A, Dąbrowska A, Kacperczyk J, Rutkowski J. Cancers (Basel) 2023;15 doi: 10.3390/cancers15133490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Human papillomavirus associated cervical lesion: pathogenesis and therapeutic interventions. Ye J, Zheng L, He Y, Qi X. MedComm (2020) 2023;4:0. doi: 10.1002/mco2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Results of an early safety analysis of a study of the combination of pembrolizumab and pelvic chemoradiation in locally advanced cervical cancer. Duska LR, Scalici JM, Temkin SM, et al. Cancer. 2020;126:4948–4956. doi: 10.1002/cncr.33136. [DOI] [PubMed] [Google Scholar]
- 59.Immune environment and immunotherapy in endometrial carcinoma and cervical tumors. Lainé A, Gonzalez-Lopez AM, Hasan U, Ohkuma R, Ray-Coquard I. Cancers (Basel) 2023;15 doi: 10.3390/cancers15072042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Recent research and clinical progress of CTLA-4-based immunotherapy for breast cancer. Zhang H, Mi J, Xin Q, Cao W, Song C, Zhang N, Yuan C. Front Oncol. 2023;13:1256360. doi: 10.3389/fonc.2023.1256360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.FT500 as monotherapy and in combination with immune checkpoint inhibitors in subjects with advanced solid tumors. [ Jul; 2024 ]. 2019. https://clinicaltrials.gov/study/NCT03841110 https://clinicaltrials.gov/study/NCT03841110
- 62.A comprehensive analysis of immunotherapy in advanced endometrial cancer (review) Wang L, Liu L, Huo D, Zhang Y. Oncol Lett. 2024;27:77. doi: 10.3892/ol.2023.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Exploring the efficacy, safety and cost-effectiveness analysis of cadonilimab in the treatment of cervical cancer. [ Jul; 2024 ]. 2023. https://clinicaltrials.gov/search?id=NCT06140589 https://clinicaltrials.gov/search?id=NCT06140589
- 64.Application of PLDR external irradiation combined with immune checkpoint inhibitors in recurrent cervical cancer. [ Jul; 2024 ]. 2024. https://clinicaltrials.gov/study/NCT06232083 https://clinicaltrials.gov/study/NCT06232083
- 65.MicroRNAs with multiple targets of immune checkpoints, as a potential sensitizer for immune checkpoint inhibitors in breast cancer treatment. Zhou H, Jia W, Lu L, Han R. Cancers (Basel) 2023;15 doi: 10.3390/cancers15030824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.A randomized phase III trial of platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab versus platinum chemotherapy plus paclitaxel and bevacizumab in metastatic (stage IVB), persistent, or recurrent carcinoma of the cervix: the BEATcc study (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030) Grau JF, Farinas-Madrid L, Oaknin A. Int J Gynecol Cancer. 2020;30:139–143. doi: 10.1136/ijgc-2019-000880. [DOI] [PubMed] [Google Scholar]
- 67.Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): a randomised, open-label, phase 3 trial. Oaknin A, Gladieff L, Martinez-Garcia J, et al. Lancet. 2024;403:31–43. doi: 10.1016/S0140-6736(23)02405-4. [DOI] [PubMed] [Google Scholar]
- 68.Cervical cancer treatment update: a Society of Gynecologic Oncology clinical practice statement. Girda E, Randall LM, Chino F, Monk BJ, Farley JH, O'Cearbhaill RE. Gynecol Oncol. 2023;179:115–122. doi: 10.1016/j.ygyno.2023.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: a 2022 update. Boukouris AE, Theochari M, Stefanou D, Papalambros A, Felekouras E, Gogas H, Ziogas DC. Crit Rev Oncol Hematol. 2022;173:103663. doi: 10.1016/j.critrevonc.2022.103663. [DOI] [PubMed] [Google Scholar]
- 70.PD-1/PD-L1 inhibitors for advanced or metastatic cervical cancer: from bench to bed. Huang W, Liu J, Xu K, Chen H, Bian C. Front Oncol. 2022;12:849352. doi: 10.3389/fonc.2022.849352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Advances in immunotherapy for cervical cancer. Grau-Bejar JF, Garcia-Duran C, Garcia-Illescas D, Mirallas O, Oaknin A. Ther Adv Med Oncol. 2023;15:17588359231163836. doi: 10.1177/17588359231163836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi CH, Kim BG, Lee J, et al. Int J Gynecol Cancer . Vol. 33. Seoul National University H, Seoul National University Bundang H, Severance H, National Cancer Center K; 2023. TP003/#1557 An open label, single arm, multicenter trial of durvalumab and BVAC-C, in patients with HPV 16 or 18 positive cervical cancer (DURBAC) p. 0. [Google Scholar]
- 73.QUILT- 3.055: a study of combination immunotherapies in patients who have previously received treatment with PD-1/PD-L1 immune checkpoint inhibitors. [ Jul; 2014 ]. 2017. https://clinicaltrials.gov/study/NCT03228667 https://clinicaltrials.gov/study/NCT03228667
- 74.Current status of clinical trials for cervical and uterine cancer using immunotherapy combined with radiation. Dyer BA, Feng CH, Eskander R, Sharabi AB, Mell LK, McHale M, Mayadev JS. Int J Radiat Oncol Biol Phys. 2021;109:396–412. doi: 10.1016/j.ijrobp.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 75.A non-comparative, randomized, phase II trial of atezolizumab or atezolizumab plus tiragolumab for programmed death-ligand 1-positive recurrent cervical cancer (SKYSCRAPER-04) Salani R, McCormack M, Kim YM, et al. Int J Gynecol Cancer. 2024;34:1140–1148. doi: 10.1136/ijgc-2024-005588. [DOI] [PubMed] [Google Scholar]
- 76.Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: interim results of a single-arm, phase 2 trial. Youn JW, Hur SY, Woo JW, et al. Lancet Oncol. 2020;21:1653–1660. doi: 10.1016/S1470-2045(20)30486-1. [DOI] [PubMed] [Google Scholar]
- 77.Tisotumab vedotin in combination with carboplatin, pembrolizumab, or bevacizumab in recurrent or metastatic cervical cancer: results from the innovaTV 205/GOG-3024/ENGOT-cx8 study. Vergote I, Van Nieuwenhuysen E, O'Cearbhaill RE, et al. J Clin Oncol. 2023;41:5536–5549. doi: 10.1200/JCO.23.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.SOLTI-1904 ACROPOLI TRIAL: efficacy of spartalizumab monotherapy across tumor-types expressing high levels of PD1 mRNA. Prat A, Paz-Ares L, Juan M, et al. Future Oncol. 2022 doi: 10.2217/fon-2022-0660. [DOI] [PubMed] [Google Scholar]
- 79.Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. Chung HC, Ros W, Delord JP, et al. J Clin Oncol. 2019;37:1470–1478. doi: 10.1200/JCO.18.01265. [DOI] [PubMed] [Google Scholar]
- 80.First-line pembrolizumab + chemotherapy versus placebo + chemotherapy for persistent, recurrent, or metastatic cervical cancer: final overall survival results of KEYNOTE-826. Monk BJ, Colombo N, Tewari KS, et al. J Clin Oncol. 2023;41:5505–5511. doi: 10.1200/JCO.23.00914. [DOI] [PubMed] [Google Scholar]
- 81.Nivolumab with or without ipilimumab in patients with recurrent or metastatic cervical cancer (CheckMate 358): a phase 1-2, open-label, multicohort trial. Oaknin A, Moore K, Meyer T, et al. Lancet Oncol. 2024;25:588–602. doi: 10.1016/S1470-2045(24)00088-3. [DOI] [PubMed] [Google Scholar]
- 82.Overview of immune checkpoint inhibitors in gynecological cancer treatment. Pirš B, Škof E, Smrkolj V, Smrkolj Š. Cancers (Basel) 2022;14 doi: 10.3390/cancers14030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.NCCN Guidelines® insights: cervical cancer, version 1.2024. Abu-Rustum NR, Yashar CM, Arend R, et al. J Natl Compr Canc Netw. 2023;21:1224–1233. doi: 10.6004/jnccn.2023.0062. [DOI] [PubMed] [Google Scholar]
- 84.Immune check-point in cervical cancer. De Felice F, Marchetti C, Palaia I, Ostuni R, Muzii L, Tombolini V, Benedetti Panici P. Crit Rev Oncol Hematol. 2018;129:40–43. doi: 10.1016/j.critrevonc.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 85.Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial. Naumann RW, Hollebecque A, Meyer T, et al. J Clin Oncol. 2019;37:2825–2834. doi: 10.1200/JCO.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balstilimab and other immunotherapy for recurrent and metastatic cervical cancer. Bose CK. Med Oncol. 2022;39:47. doi: 10.1007/s12032-022-01646-7. [DOI] [PubMed] [Google Scholar]
- 87.A review of cervical cancer: incidence and disparities. Buskwofie A, David-West G, Clare CA. J Natl Med Assoc. 2020;112:229–232. doi: 10.1016/j.jnma.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 88.Cancer deaths may double by 2030 in sub-Saharan Africa. Larkin HD. JAMA. 2022;327:2280. doi: 10.1001/jama.2022.10019. [DOI] [PubMed] [Google Scholar]
- 89.Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. Colombo N, Dubot C, Lorusso D, et al. N Engl J Med. 2021;385:1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 90.Keynote-158 study, FDA granted accelerated approval of pembrolizumab for the treatment of patients with advanced PD-L1-positive cervical cancer. Borcoman E, Le Tourneau C. Ann Transl Med. 2020;8:1611. doi: 10.21037/atm-20-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Chalmers ZR, Connelly CF, Fabrizio D, et al. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.The challenges of tumor mutational burden as an immunotherapy biomarker. Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R. Cancer Cell. 2021;39:154–173. doi: 10.1016/j.ccell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Martincorena I, Roshan A, Gerstung M, et al. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Real-world application of tumor mutational burden-high (TMB-high) and microsatellite instability (MSI) confirms their utility as immunotherapy biomarkers. Palmeri M, Mehnert J, Silk AW, et al. ESMO Open. 2022;7:100336. doi: 10.1016/j.esmoop.2021.100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Marcus L, Fashoyin-Aje LA, Donoghue M, et al. Clin Cancer Res. 2021;27:4685–4689. doi: 10.1158/1078-0432.CCR-21-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.A novel tumor mutational burden-based risk model predicts prognosis and correlates with immune infiltration in ovarian cancer. Wang H, Liu J, Yang J, et al. Front Immunol. 2022;13:943389. doi: 10.3389/fimmu.2022.943389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Comprehensive analysis of the relationships between tumor mutation burden with immune infiltrates in cervical cell carcinoma. Zhou C, Li C, Peng S, Zhou L, Li H. Front Mol Biosci. 2020;7:582911. doi: 10.3389/fmolb.2020.582911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dostarlimab for primary advanced or recurrent endometrial cancer. Mirza MR, Chase DM, Slomovitz BM, et al. N Engl J Med. 2023;388:2145–2158. doi: 10.1056/NEJMoa2216334. [DOI] [PubMed] [Google Scholar]
- 99.Fewer actionable mutations but higher tumor mutational burden characterizes NSCLC in black patients at an urban academic medical center. Choudhury NJ, Eghtesad M, Kadri S, et al. Oncotarget. 2019;10:5817–5823. doi: 10.18632/oncotarget.27212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Genetic susceptibility of cervical cancer. Chen X, Jiang J, Shen H, Hu Z. J Biomed Res. 2011;25:155–164. doi: 10.1016/S1674-8301(11)60020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Molecular profiles and tumor mutational burden analysis in Chinese patients with gynecologic cancers. Wang M, Fan W, Ye M, et al. Sci Rep. 2018;8:8990. doi: 10.1038/s41598-018-25583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Genetic susceptibility for cervical cancer in African populations: what are the host genetic drivers? Kuguyo O, Tsikai N, Thomford NE, et al. OMICS. 2018;22:468–483. doi: 10.1089/omi.2018.0075. [DOI] [PubMed] [Google Scholar]
- 103.Utilization of cancer immunotherapy in sub-Saharan Africa. Olatunji E, Patel S, Graef K, et al. Front Oncol. 2023;13:1266514. doi: 10.3389/fonc.2023.1266514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bridging the genomic data gap in Africa: implications for global disease burdens. Omotoso OE, Teibo JO, Atiba FA, Oladimeji T, Adebesin AO, Babalghith AO. Global Health. 2022;18:103. doi: 10.1186/s12992-022-00898-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: a systematic review and meta-analysis. Ogembo RK, Gona PN, Seymour AJ, Park HS, Bain PA, Maranda L, Ogembo JG. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0122488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sequence three million genomes across Africa. Wonkam A. Nature. 2021;590:209–211. doi: 10.1038/d41586-021-00313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Genomic-informed pathogen surveillance in Africa: opportunities and challenges. Inzaule SC, Tessema SK, Kebede Y, Ogwell Ouma AE, Nkengasong JN. Lancet Infect Dis. 2021;21:0–9. doi: 10.1016/S1473-3099(20)30939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.PD-L1 is highly expressed in ovarian cancer and associated with cancer stem cells populations expressing CD44 and other stem cell markers. Alwosaibai K, Aalmri S, Mashhour M, et al. BMC Cancer. 2023;23:13. doi: 10.1186/s12885-022-10404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.B7-H3 and PD-L1 expression are prognostic biomarkers in a multi-racial cohort of patients with colorectal cancer. Zhang W, Acuna-Villaorduna A, Kuan K, et al. Clin Colorectal Cancer. 2021;20:161–169. doi: 10.1016/j.clcc.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 110.Review article: immune landscape and immunotherapy options in cervical carcinoma. Kousar K, Ahmad T, Naseer F, Kakar S, Anjum S. Cancers (Basel) 2022;14 doi: 10.3390/cancers14184458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Racial disparities in the molecular landscape of cancer. Heath EI, Lynce F, Xiu J, et al. Anticancer Res. 2018;38:2235–2240. doi: 10.21873/anticanres.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Increasing African genomic data generation and sharing to resolve rare and undiagnosed diseases in Africa: a call-to-action by the H3Africa rare diseases working group. Lumaka A, Carstens N, Devriendt K, et al. Orphanet J Rare Dis. 2022;17:230. doi: 10.1186/s13023-022-02391-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.The International Association for the Study of Lung Cancer global survey on programmed death-ligand 1 testing for NSCLC. Mino-Kenudson M, Le Stang N, Daigneault JB, et al. J Thorac Oncol. 2021;16:686–696. doi: 10.1016/j.jtho.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cervix cancer in sub-Saharan Africa: an assessment of cervical cancer management. Burt LM, McCormak M, Lecuru F, et al. JCO Glob Oncol. 2021;7:173–182. doi: 10.1200/GO.20.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Current management of locally advanced and metastatic cervical cancer in the United States. Pang SS, Murphy M, Markham MJ. JCO Oncol Pract. 2022;18:417–422. doi: 10.1200/OP.21.00795. [DOI] [PubMed] [Google Scholar]
- 116.Immune checkpoint blockades in gynecological cancers: a review of clinical trials. Peng H, He X, Wang Q. Acta Obstet Gynecol Scand. 2022;101:941–951. doi: 10.1111/aogs.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clinical practice guidelines for cervical cancer: the Korean Society of Gynecologic Oncology guidelines. Yoo JG, Lee SJ, Nam EJ, et al. J Gynecol Oncol. 2024;35:0. doi: 10.3802/jgo.2024.35.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. Huang M, Lou Y, Pellissier J, Burke T, Liu FX, Xu R, Velcheti V. Pharmacoeconomics. 2017;35:831–844. doi: 10.1007/s40273-017-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Challenges to the availability and affordability of essential medicines in African countries: a scoping review. Yenet A, Nibret G, Tegegne BA. Clinicoecon Outcomes Res. 2023;15:443–458. doi: 10.2147/CEOR.S413546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Health insurance for people younger than age 65: expiration of temporary policies projected to reshuffle coverage, 2023-33. Hanson C, Hou C, Percy A, Vreeland E, Minicozzi A. Health Aff (Millwood) 2023;42:742–752. doi: 10.1377/hlthaff.2023.00325. [DOI] [PubMed] [Google Scholar]
- 121.The risk of losing health insurance in the United States is large, and remained so after the Affordable Care Act. Einav L, Finkelstein A. Proc Natl Acad Sci U S A. 2023;120:0. doi: 10.1073/pnas.2222100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Universal health insurance in Africa: a narrative review of the literature on institutional models. Ly MS, Bassoum O, Faye A. BMJ Glob Health. 2022;7 doi: 10.1136/bmjgh-2021-008219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Challenges toward achieving universal health coverage in Ghana, Kenya, Nigeria, and Tanzania. Umeh CA. Int J Health Plann Manage. 2018;33:794–805. doi: 10.1002/hpm.2610. [DOI] [PubMed] [Google Scholar]
- 124.Increased investment in universal health coverage in sub-Saharan Africa is crucial to attain the Sustainable Development Goal 3 targets on maternal and child health. Dowou RK, Amu H, Saah FI, Adeagbo O, Bain LE. Arch Public Health. 2023;81:34. doi: 10.1186/s13690-023-01052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]