Abstract
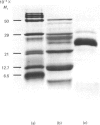
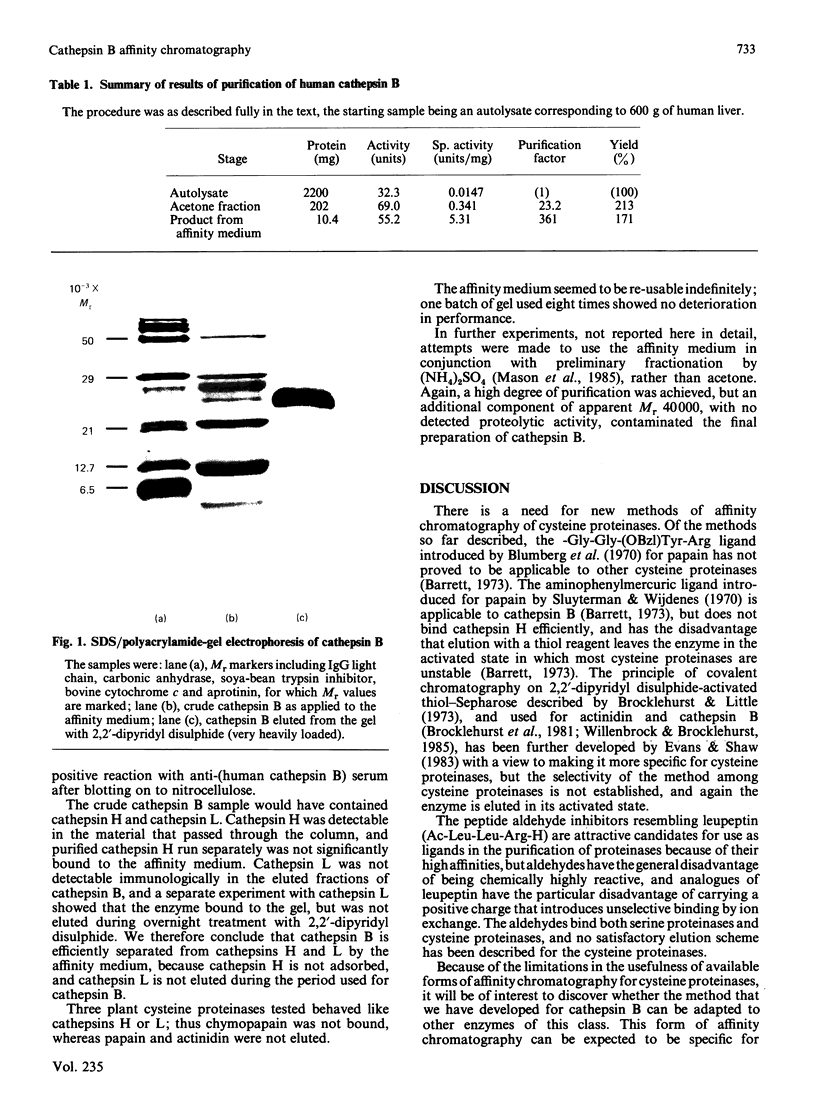
Human cathepsin B was purified by affinity chromatography on the semicarbazone of Gly-Phe-glycinal linked to Sepharose 4B, with elution by 2,2'-dipyridyl disulphide at pH 4.0. The product obtained in high yield by the single step from crude starting material was 80-100% active cathepsin B. The possibility that this new form of affinity chromatography may be of general usefulness in the purification of cysteine proteinases is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. Human cathepsin B1. Purification and some properties of the enzyme. Biochem J. 1973 Apr;131(4):809–822. doi: 10.1042/bj1310809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Blumberg S., Schechter I., Berger A. The purification of papain by affinity chromatography. Eur J Biochem. 1970 Jul;15(1):97–102. doi: 10.1111/j.1432-1033.1970.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Baines B. S., Malthouse J. P. Differences in the interaction of the catalytic groups of the active centres of actinidin and papain. Rapid purification of fully active actinidin by covalent chromatography and characterization of its active centre by use of two-protonic-state reactivity probes. Biochem J. 1981 Sep 1;197(3):739–746. doi: 10.1042/bj1970739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Barrett A. J. Chymopapain. Chromatographic purification and immunological characterization. Biochem J. 1984 Oct 1;223(1):81–88. doi: 10.1042/bj2230081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. E., Barrett A. J. Immunolocalization of human cystatins in neutrophils and lymphocytes. Histochemistry. 1984;80(4):373–377. doi: 10.1007/BF00495420. [DOI] [PubMed] [Google Scholar]
- Evans B., Shaw E. Inactivation of cathepsin B by active site-directed disulfide exchange. Application in covalent affinity chromatography. J Biol Chem. 1983 Sep 10;258(17):10227–10232. [PubMed] [Google Scholar]
- Mason R. W., Green G. D., Barrett A. J. Human liver cathepsin L. Biochem J. 1985 Feb 15;226(1):233–241. doi: 10.1042/bj2260233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J. A., Henes J. B., Fruton J. S. Interaction of papain with derivatives of phenylalanylglycinal. J Biol Chem. 1977 Oct 10;252(19):6776–6782. [PubMed] [Google Scholar]
- Patel A. H., Ahsan A., Suthar B. P., Schultz R. M. Transition-state affinity chromatography of trypsin-like proteinases with dipeptidyl argininal ligands. Biochim Biophys Acta. 1983 Oct 28;748(2):321–330. doi: 10.1016/0167-4838(83)90309-6. [DOI] [PubMed] [Google Scholar]
- Ritonja A., Popovic T., Turk V., Wiedenmann K., Machleidt W. Amino acid sequence of human liver cathepsin B. FEBS Lett. 1985 Feb 11;181(1):169–172. doi: 10.1016/0014-5793(85)81136-4. [DOI] [PubMed] [Google Scholar]
- Schwartz W. N., Barrett A. J. Human cathepsin H. Biochem J. 1980 Nov 1;191(2):487–497. doi: 10.1042/bj1910487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. An agarose mercurial column for the separation of mercaptopapain and nonmercaptopapain. Biochim Biophys Acta. 1970 Mar 31;200(3):593–595. doi: 10.1016/0005-2795(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Willenbrock F., Brocklehurst K. Preparation of cathepsins B and H by covalent chromatography and characterization of their catalytic sites by reaction with a thiol-specific two-protonic-state reactivity probe. Kinetic study of cathepsins B and H extending into alkaline media and a rapid spectroscopic titration of cathepsin H at pH 3-4. Biochem J. 1985 Apr 15;227(2):511–519. doi: 10.1042/bj2270511. [DOI] [PMC free article] [PubMed] [Google Scholar]